Course
Nursing Interventions for Sepsis: Fluid Management
Course Highlights
- In this course you will learn about nursing interventions for sepsis.
- You’ll also learn the basics of the crystalloid vs. colloid debate, and how much fluid that patients need per their specific conditions.
- You’ll leave this course with a broader understanding of macrovascular/microvascular end points of resuscitation.
About
Contact Hours Awarded: 2.5

Course By:
Morgan Curry
BSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction – The Importance of Fluid Resuscitation in Nursing Interventions for Sepsis
There are many nursing interventions for sepsis; one of the most important, fluid resuscitation, helps to restore tissue perfusion. However, there is a great deal of variation in the type of fluid, rate of administration, and the total volume of fluid administered that goes into fluid resuscitation protocol in managing a patient with sepsis.
IV fluids should be prescribed like any other drug we give our patients. A critical evaluation related to the indication and contraindication for different fluid types should be done on each patient (1).
Circulatory insufficiency and shock result from inadequate perfusion relative to the tissue demands (2). Of all the nursing interventions for sepsis, early fluid resuscitation is one of the most essential in determining the outcome of patients with circulatory insufficiency and shock.
What can cause circulatory insufficiency?
– Pump failure
– Insufficient vascular tone (the vasodilation we see in sepsis)
– Hypovolemia (2)
Patients with distributive shock (the primary shock seen in sepsis) may experience circulatory insufficiency due to the profound vasodilatation that is associated with the inflammatory reaction to an infection (3).
How Sepsis Affects the Different Organ Systems
Cardiovascular
As shock progresses and the mean arterial pressure (MAP) trends downward, the cerebrocortical functions are the first to be impaired, which often manifests as a change in the level of consciousness or altered mental status (2).
Gastrointestinal & Renal
As perfusion decreases from vasodilation or from decreased cardiac function, tissue beds become globally hypoxic. The GI tract and the kidneys are known to be intolerant of hypotension, and precipitous decrease in MAP, which can lead to impaired mucosal function, bowel integrity, or in extreme cases frank ischemic necrosis (2).
Overall Metabolism
Secondary to developing tissue hypoxia and as a result of anaerobic metabolism in the absence of adequate tissue oxygenation, patients may develop a high lactic acid level as a response to the progressive tissue hypoxia. Serum lactate of 4 or greater is associated with increased severity of illness and poorer outcomes even if hypotension is not present (3).
With this knowledge, providers should be prepared to fluid resuscitate patients who are hypotensive or have a lactate ≥4 mmol/L to expand their circulating blood volume and restore tissue perfusion pressure (3). Fluid resuscitation should be considered as one of the primary nursing interventions for sepsis in these patients.

Self Quiz
Ask yourself...
- How does sepsis affect the cardiovascular system?
- Why is fluid resuscitation one of the primary nursing interventions for sepsis in patients who are hypotensive or have a lactate of ≥4 mmol/L?
The Great Debate: Crystalloid vs. Colloid
Crystalloids
- Low-cost salt solutions that are known to be the go-to, easy to grab, and, often, first choice fluids (4).
- Isotonic crystalloids are the most commonly administered IV fluid internationally.
- Crystalloid solutions were first prepared in response to the cholera pandemic in 1832.
- Only about 20-30% of administered crystalloid fluid will stay in the intravascular space. (5).
Examples: Sodium chloride (Normal saline), Lactated Ringers, or Plasmalyte
Colloids
- They are suspensions of molecules in a carrier fluid with high enough molecular weight to prevent crossing healthy capillary membranes; thus, a larger percentage of the administered fluid will remain intravascular (5).
- Colloids are more expensive fluids and are either man-made (starches, dextrans or gelatins) or naturally occurring.
Examples: Albumin or fresh frozen plasma (4).
The physiologic rationale behind favoring colloid over crystalloid is the thought that colloids may expand intravascular volume more effectively by remaining in the intravascular space and maintaining colloid oncotic pressure (5).

Self Quiz
Ask yourself...
- In what types of situations would you use crystalloids VS colloids?
- How does the cost of colloid factor into the decision-making process, especially when weighed against the negligible potential difference in outcomes?
Choice of Crystalloid Fluid for Resuscitation: Is Normal Saline Really “Normal”?
The use of saline versus a balanced crystalloid solution has been a topic of ongoing debate. Due to the high mortality, the burden to society, and increasing awareness surrounding sepsis, there has been extensive research done to identify the optimal fluid treatment protocols. Ultimately, provider preference, hospital protocol, and regional availability dictate much of choice due to a lack of evidence-based guidelines on specific fluid choice.
Fluid composition can have unintended physiological effects, such as altering the pH balance through the metabolism of lactate and acetate, leading to a decrease in bicarbonate, and eventually metabolic acidosis, and the potential for acute kidney injury (6).
Normal Saline vs. Lactated Ringers
The Saline Against Lactated Ringers or Plasma-Lyte in the Emergency Department (SALT-ED) study is a pragmatic, cluster, multiple-crossover trial at a single center evaluating the clinical outcomes of patients treated with 0.9% NS versus balanced crystalloids in the setting of resuscitation in the emergency department (7). The trial included 13,347 patients who received a median of 1 liter of fluid (7). Saline increased the risk of death or renal failure when compared to LR/Plasmalyte (5.6% vs 4.7%, p= 0.02). The subgroup of patients with renal injury at the time of admission was more susceptible to adverse kidney events from saline administration (37.6% vs 28%, p= <0.001) (7). This trial confirmed that, when used in nursing interventions for sepsis, saline increases the risk of renal failure when compared to balanced solutions.
These results were then duplicated at Vanderbilt and included critically ill patients. The SMART trial, a pragmatic, cluster-randomized, multiple – crossover trial, was conducted in five intensive care units at an academic center and included 15,802 adult patients (5). Patients were randomized to receive either 0.9% NS or LR/Plasmalyte. Among the 7,942 patients in the balance crystalloid group, 1,139 (14.3%) had an adverse kidney event, compared to 1,211 of the 7,860 (15.4%) patients in the NS group (p = 0.04) (5). In-hospital mortality at 30 days was higher in the NS group when compared to the balanced crystalloid group, as well (11.1% vs 10.3%, respectively, p = 0.06) (5). The mortality difference in the two groups suggests that NS may not only be causing renal failure but may also be causing harm to patients via additional mechanisms, including increased inflammation. (8).
Normal saline as a resuscitation fluid should not be administered in high amounts as it carries the risk of inducing a hypernatremic hyperchloremic metabolic acidosis (8). Some patients are already extremely acidotic and giving them fluid that will exacerbate their academia is poor practice. The truth is that “normal” saline is not physiologically normal. It is a hypertonic, acidotic fluid that can cause more harm than good, especially in patients who need large volume resuscitation. The development of electrolyte disturbances secondary to fluid administration also depends on the electrolyte status of the patient before resuscitation is initiated.
All of this is not to say that saline is all bad and should never be used, but to point out that just because “it’s what we’ve always used,” “it’s easy to grab,” or “the patient is hyperkalemic!” are not justifiable reasons to use high volumes of NS in the resuscitation of your patients.

Self Quiz
Ask yourself...
- Balanced crystalloids may have an advantage over saline-based solution for IV fluid resuscitation. How will you incorporate this into your practice?
- What types of patients are likely to benefit from a saline-based resuscitation VS balanced crystalloids?
- Some of the unintended effects of saline administration can be high-priority, such as renal failure and a higher risk of death according to some studies. Should we always use balanced solutions in our nursing interventions for sepsis – when electrolytes permit?
How Much Fluid do Septic Patients Need?
The concept of prompt IV fluid administration was first accepted after the 2001 study of early goal-directed therapy (EGDT). The results of this landmark study propelled early and protocolized fluid management to the forefront of sepsis management. Because of this study and future studies that replicated the results, the Surviving Sepsis Campaign (SSC) began promoting EGDT fluid resuscitation as a cornerstone of sepsis and septic shock management (5).
EGDT Study Protocol:
In the study, patients either received standard therapy, which involved arterial and central venous catheterization and a protocol targeting a CVP 8-12 mmHg, mean arterial pressure (MAP) at least 65 mmHg, and urine output of at least 0.5 ml/kg/hr, or the EDGT group (5). The EDGT group included the aforementioned components but also included a catheter to measure central venous oxygen saturation (SvO2), six hours of treatment in the emergency department before admission, and administration of 500 mL of crystalloid fluid every 30 minutes to achieve CVP goals, vasopressors, and vasodilators to maintain MAP goals, and blood transfusions or dobutamine to achieve ScvO2 ≥ 70% (5). Overall, in-hospital mortality was found to be 16% less with EDGT when compared to standard therapy (46.5% vs 30.5%; p= 0.009) (5).
The SSC 3-hour and 1- hour bundle both recommend the initial administration of 30 mL/kg of crystalloid fluid for hypotension or lactate ≥ 4 mmol/L as a fluid challenge with a target CVP goal ≥ 8 mmHg, ScvO2 of ≥ 70%, and normalization of lactate (9).
A patient may need repeat fluid challenges in the initial phases of sepsis/septic shock. This bolus dose is meant to rapidly expand the patient’s blood volume to allow providers to assess the patient’s response to fluid resuscitation.
A key concept for dosing fluid therapy in the critically ill population is to actively address ongoing losses (drains, stomas, fistulas, or hyperthermia, open wounds, or various causes of polyuria) paired with the frequent reassessment of the need for further hemodynamic support (10). While fluid administration is a critical aspect of resuscitation, excessive fluid accumulation has been associated with worse clinical outcomes- particularly the development of acute kidney injury (AKI), pulmonary edema, pleural effusions, and in some cases, an increase in ventilator days (10).
The idea of interrelated phases of fluid management, coined “ebb and flow,” differentiated according to the patient’s clinical status, with evolving goals for fluid need, is highly individualized, but an important concept in the management of the septic patient. This helps to avoid adverse events related to poor fluid management (10).
Phases of Fluid Resuscitation
Initial Phase
In the initial phase of fluid resuscitation, the objective is the restoration of effective circulating blood volume, organ perfusion, and tissue oxygenation. Fluid accumulation and a positive fluid balance are to be expected here (10).
Second Phase
In the second phase, the goal is a maintenance of intravascular volume homeostasis (10). The goal is to prevent excessive fluid accumulation and to avoid unnecessary fluid loading. By the second phase, the patient should show evidence of adequate tissue perfusion.
Third Phase
In the third and final stage, the objective centers around fluid removal and the concept of “de-resuscitation” as dictated by the state of physiologic stabilization, organ injury recovery, and convalescence (10). During this phase, unnecessary fluid accumulation may add to secondary organ injury and adverse events.
Below is a photo depicting the potential consequences of fluid overload on end-organ function as adapted by Malbrain et al (1).
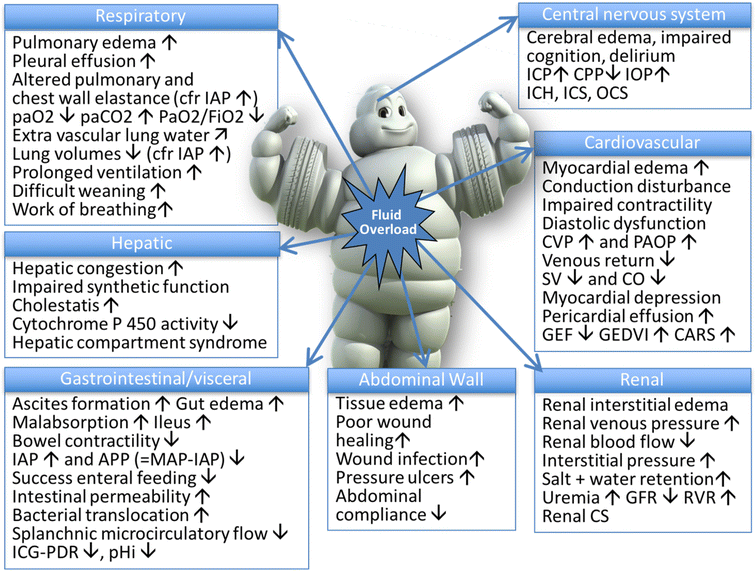
It is important to be aware of theses potential consequences when using fluid resuscitation as on of your nursing interventions for sepsis. Fluid resuscitation needs to be done properly to ensure the safety of the patient.
Macrocirculation End Points of Sepsis Resuscitation
As mentioned previously, resuscitation goals for the septic patient are to return the patient to a physiologic state that promotes adequate organ perfusion and matching metabolic supply and demand.
Ideally, resuscitation end points should assess the adequacy of tissue oxygen delivery (DO2), oxygen consumption (VO2), and should be quantifiable and reproducible. Since there fails to be a single resuscitation endpoint despite years of research, providers must be able to rely on multiple endpoints to determine the patient’s overall response to therapy (11). The SSC focuses their resuscitation guidelines on the original EGDT protocol with an emphasis on macro and microcirculatory endpoints (11):
| Ventilated Patients |
Spontaneously Breathing Patients |
|
| Central Venous Pressure | 12-15 mmHg | 8-12 mmHg |
| Mean Arterial Pressure | ≥ 65 mmHg | ≥ 65 mmHg |
| Urine Output | ≥ 0.5 mL/kg/hr | ≥ 0.5 mL/kg/hr |
| Central Venous O2 Saturation | ≥ 70% | ≥ 70% |
| Mixed Venous O2 Saturation | ≥ 65% | ≥ 65% |

Self Quiz
Ask yourself...
- 30mL/kg can be a large amount of fluid in patients with high body weights. Would you still follow the recommendation of 30mL/kg in these cases?
- There is much debate about the optimal amount of fluid resuscitation. What are some of the concerns with over and under resuscitation.
- Which is likely more detrimental in terms of mortality?
- Has the risk of over or under resuscitation ever worried you when using fluid resuscitation as one of your nursing interventions for sepsis?
Central Venous Pressure
A previously well-established starting point in determining a patient’s need and subsequent responsiveness to fluids is to utilize a static measurement, such as the central venous pressure (CVP) or pulmonary artery occlusion pressure (PAOP) (11). As most providers know, using CVP as an initial resuscitation target and estimate of preload adequacy is fundamentally flawed. Factors such as total blood volume, cardiac output/venous return, pulmonary hypertension, cardiac tamponade, arrhythmias, and human error are all factors that have the potential to impact the central venous pressure (11).
The correct interpretation of a CVP value is as follows: a low CVP value of ≤6 almost always indicates hypovolemia. However, a high value does not exclude hypovolemia, nor does it guarantee hypervolemia. It is important to consider this when using CVP as a measurement to ensure that your nursing interventions for sepsis are the most beneficial and effective.
Lactate
Moving from a “macro” point of view to a “micro” point of view, providers use several clinical and laboratory values to assess the microcirculation. Most commonly, lactate, central venous oxygenation, and capillary refill time.
Lactic acid is one of the most widely accepted biomarkers used to diagnose sepsis-related organ dysfunction. The working theory behind increased lactate in septic shock is that as global tissue hypoxia occurs, oxygenation fails to meet tissue oxygen demand, therefore increasing anaerobic metabolism…and lactic acid level (11). Just like when you show up for that 1st day of spring 5K after spending the last 4 months on your couch watching Netflix documentaries… need... more... oxygen!!!
Unfortunately, this basic explanation fails to consider other contributions to elevated lactate. It continues to be widely accepted and used as a marker of microperfusion, but providers should be aware that there are still limitations.
Elevated lactate can be attributed to 4 broad categories:
- Decreased tissue oxygen delivery – you could see elevated lactate in individuals who have had a tonic-clonic seizure, severe asthma attack, severe anemia, carbon monoxide poisoning, or chose to do one of those Spartan Races in July.
- Underlying disease – you could have increased lactate in patients who have fulminant liver failure, lymphoma/leukemia, small cell lung cancer, pheochromocytoma, or thiamine deficiency (sepsis would also fall under this category) (11).
- Drugs & toxins – Drugs and toxins that can often be responsible for an increased lactate include Biguanides, Linezolid, Cyanide poisoning, NRTIs, and beta 2 agonists (11).
- Inborn errors of metabolism – The rarer inborn metabolism errors are the patients who have enzyme deficiencies such as pyruvate dehydrogenase, pyruvate carboxylase, Fructose-1-6-diphosphatase, and phosphoenolpyruvate carboxykinase (11).
SvO2/ScvO2
Moving past lactate measurement to more technical measurements tissue oxygenation, both mixed venous oxygen saturation (SvO2) and ScvO2 have been considered as important targets because they can be used to estimate a global balance of cellular oxygen demand vs delivery (11).
A ScvO2 <70% is indicative of inadequate oxygen delivery to tissues, increased oxygen extraction, or a combination of the two. It is important to note that a true ScvO2 must be obtained via a central venous catheter with the tip appropriately placed at the junction of the superior vena cava and the right atrium (11).
Assuming it is measured at the correct location, a ScvO2 of 70%-89% is suggestive of a well-balanced VO2/DO2. A ScvO2 ≥90% suggests poor oxygenation utilization at the cellular level, tissue dysoxia, or microcellular shunting (11). Currently, the routine uses of SvO2 and ScvO2 are not supported in the literature, but the role may become more apparent as sepsis end-goal resuscitation research continues to increase in prevalence.
Capillary Refill Time
While technology and invasive tests offer pertinent information, these interventions should be performed in conjunction with frequent clinical examinations to assess the response.
Capillary refill time is a basic examination skill that new literature is examining as a valuable tool to assess regional and global tissue perfusion during septic shock resuscitation.
Capillary refill time is defined as the duration of the time needed for the patient’s fingertip to regain color after direct pressure is applied to cause blanching. In a healthy patient, the refill time should be <3.5 seconds (11). It is important to note that skin temperature, room temperature, age, and use of vasoactive medications can impact capillary refill time and should be taken into consideration. Assuming the patient’s extremities are normothermic, a refill time of >5 seconds suggests the presence of abnormal microcirculatory flow (11).
Serial assessment with normalization within 6 hours is associated with successful resuscitation when compared against traditional resuscitation targets. You must always measure and evaluate to ensure that your nursing interventions in sepsis are successful.
Estimating Fluid Responsiveness with SVV and Bedside Echocardiography
Dynamic indices such as stroke volume variation (SVV), pulse pressure variation, and inferior vena cava variability all have been found to have a better predictive value, sensitivity, and specificity than the static indices (11). In patients who are spontaneously breathing or have arrhythmias, direct measurement tests such as the expiratory occlusion test and passive leg raise may be preferred (11).
As SVV of >12 has an 88% sensitivity and 89% specificity for predicting fluid responsiveness in a patient without cardiac arrhythmias and requiring mechanical ventilation. Some monitoring equipment may calculate SVV with a standard arterial line only; other times, a special arterial line may need to be inserted to measure SVV (15).
Sepsis-induced cardiac dysfunction is well described and often presents as a reduction in left ventricular stroke volume and impaired myocardial performance. Noninvasive ways to measure the cardiac output and cardiac indexes include devices such as the FloTrac or Vigileo systems or basic bedside echocardiography, have become more common. The use of invasive pulmonary artery catheters are associated with more risk than patient benefit, and their use has significantly decreased. The information gained from bedside echocardiography includes a rough estimate of cardiac output, LV and RV function, chamber fluid status, IVC size and variability, and global cardiac function. This information can be invaluable when utilized in real-time, especially to measure the responsiveness of treatments.

Self Quiz
Ask yourself...
- There are multiple types endpoints we can use to measure fluid resuscitation and volume status. Which end points are favored in your clinical practice?
- As an extension to the previous question- will you incorporate any of the information gained in this module into your practice?
Fluid Challenge Without the Fluid
What could be better than determining the effect of a fluid bolus without actually infusing any fluid? Though these techniques are imperfect, they can provide insight into the “fluid responsiveness” of a patient.
Passive Leg Raise
The passive leg raise test is another noninvasive means to assess fluid need by mimicking a fluid bolus. It involves moving a patient from the semi-recumbent position to a position where the legs are lifted at 45 degrees, and the trunk remains horizontal (2). This induces a transfer of 250-350 mL of venous blood from the inferior limbs and the splanchnic compartment towards the thoracic and cardiac cavities, mimics the increase in cardiac preload induced by fluid infusion. The threshold to define fluid responsiveness with a passive leg raise test is a 10% increase in stroke volume or cardiac output (1). Cardiac output changes can be detected 1-2 minutes after the maneuver is performed using either SVV via a noninvasive technology (Flo-Trac) or by utilizing bedside echocardiography to visualize cardiac function changes (12). A positive response may also be noted if blood pressure increases with a decrease in heart rate, though this is less sensitive and specific. Like capillary refill, the passive leg raise can be done regardless of arrhythmia or mechanical ventilation mode (12).
End-Expiration Occlusion Test
The end-expiration occlusion test is another fluid responsive test, but specifically for the subset of patients who require mechanical ventilation. The test consists of stopping mechanical ventilation at end-expiration for 15 seconds and measuring the changes in cardiac output. By pausing mechanical ventilation, there is an increase in cardiac output by stopping the cyclic impediment of venous return that occurs at each ventilator-triggered breath. An increase in the cardiac output above the threshold of 5% indicates fluid responsiveness.
Putting it Together – Performing Nursing Interventions in Sepsis
The best approach is to use multiple techniques to measure the efficacy of fluid resuscitation. Relying on any single parameter is not ideal practice and may lead to under or over-resuscitation. The best way to use this data is to perform interventions that increase perfusion (usually a fluid bolus in sepsis) and re-measure the endpoint. A trend toward better perfusion (lower lactic acid level, faster capillary refill, etc.) indicates a positive response. A negative response can be due to either: 1.) inadequate volume of fluid resuscitation OR 2.) a patient that is no longer fluid responsive. It can be difficult to discern the difference and the passive leg raise or occlusion test may be helpful here. There is no fixed rule, but it is generally thought to be better to over-resuscitate than under-resuscitate.
By systematically using this approach, the aim is to properly resuscitate the patient while avoiding the pitfalls of both over and under-resuscitation. Endpoints should be measured after each intervention.
For example if you measure a lactic acid level of 8 and note delayed capillary refill on the exam, you may determine that fluids will augment cardiac output and increase tissue perfusion. Thus you choose to administer 1L bolus. Once the bolus is complete, you should re-check the lactic acid level and capillary refill. It may not normalize, but there should be an improvement.
Summary – Nursing Interventions for Sepsis
In summary, fluid resuscitation in sepsis is a controversial topic. Nurses should utilize a variety of endpoints to measure fluid status and perfusion status. Newest evidence is suggesting that LR may have a physiologic benefit over NS, and albumin may have a role in the resuscitation of septic patients.
References + Disclaimer
- Malbrain, M. L., Regenmortel, N. V., Saugel, B., Tavernier, B. D., Gaal, P. V., Joannes-Boyau, O., . . . Monnet, X. (2018). Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Annals of Intensive Care,8(1), 1-16. doi:10.1186/s13613-018-0402-x
- Marini, J. J., & Dries, D. J. (2019). Shock and support of the failing circulation. In Critical Care Medicine: The essentials and more(5th ed., pp. 47-67). Philadelphia, PA: Lippincott Williams & Wilkins.
- 3-Hour Bundle. (n.d.). Retrieved March 21, 2019, from http://www.survivingsepsis.org/SiteCollectionDocuments/Bundle-3-Hour-Step4-Fluids.pdf
- Lewis, S. R., Pritchard, M. W., Evans, D. W., Butler, A. R., Alderson, P., Smith, A. F., & Roberts, I. (2018, August 3). Colloids or crystalloids for fluid replacement in critically people. Retrieved March 21, 2019, from https://www.cochrane.org/CD000567 /INJ_colloids-or-crystalloids-fluid-replacement-critically-people
- Semler, M. W., & Rice, T. W. (2016). Sepsis Resuscitation: Fluid Choice and Dose. Clinics in chest medicine, 37(2), 241-50.
- Avila, A. A., Kinberg, E. C., Sherwin, N. K., & Taylor, R. D. (2016). The Use of Fluids in Sepsis. Cureus. doi:10.7759/cureus.528
- Self, W. H., Semler, M. W., Wanderer, J. P., Ehrenfeld, J. M., Byrne, D. W., Wang, L., Atchison, L., Felbinger, M., Jones, I. D., Russ, S., Shaw, A. D., Bernard, G. R., … Rice, T. W. (2017). Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial. Trials, 18(1), 178. doi:10.1186/s13063-017-1923-6
- Farkas, J. (2018, February 27). PulmCrit- Get SMART: Nine reasons to quit using normal saline for resuscitation. Retrieved March 21, 2019, from https://emcrit.org/pulmcrit/smart/
- Levy, M. M., Evans, L. E., & Rhodes, A. (2018). The Surviving Sepsis Campaign Bundle. Critical Care Medicine,46(6), 997-1000. doi:10.1097/ccm.0000000000003119
- Mcdermid, R. C. (2014). Controversies in fluid therapy: Type, dose and toxicity. World Journal of Critical Care Medicine,3(1), 24-33. doi:10.5492/wjccm.v3.i1.24
- Greenwood, J. C., & Orloski, C. J. (2017). End Points of Sepsis Resuscitation. Emergency Medicine Clinics of North America,35(1), 93-107. doi:10.1016/j.emc.2016.09.001
- Boyd, J. H., Sirounis, D., Maizel, J., & Slama, M. (2016). Echocardiography as a guide for fluid management. Critical Care,20(1), 1-7. doi:10.1186/s13054-016-1407-1
- Byrne, L., & Van Haren, F. (2017). Fluid resuscitation in human sepsis: Time to rewrite history?. Annals of intensive care, 7(1), 4.
- De Backer, D., & Vincent, J. L. (2018). Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Critical care (London, England), 22(1), 43. doi:10.1186/s13054-018-1959-3
- Xavier M., Marik, P., Jean-Louis T. (2016) Prediction of fluid responsiveness: an update. Annals of intensive care. doi: 10.1186/s13613-016-0216-7
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate

