Antipsychotic Prescribing
Contact Hours: 3
Author(s):
c
Course Highlights
- In this Antipsychotic Prescribing course, we will learn about how antipsychotics affect the brain and central nervous system.
- You’ll also learn the mechanism of action of first- and second-generation antipsychotics.
- You’ll leave this course with a broader understanding of the importance of holistic care when weighing the risks and benefits in prescribing antipsychotic medications.
Introduction
There are many intricacies surrounding mental health conditions, notably psychosis. Can you imagine being placed into an anti-gravity machine without holding on to any sort of anchor? I imagine this loss of control would be terrifying. Similarly, psychosis can disorient and destabilize patients, as they have lost their grip on reality and their perceptions are no longer reliable. Psychosis is characterized by hallucinations, delusions, disorganized thinking, and impaired social functioning.
The use of antipsychotics has resulted in decreased mortality and improved patients’ quality of life; however, adverse drug reactions from these drugs are major challenges in treating patients with psychotic disorders (2).
In this course, we will review conditions associated with psychosis and explore the crucial role of antipsychotic medications in restoring stability and fostering recovery. Through a comprehensive understanding of antipsychotics and their uses, mechanisms of action, adverse reactions, and contraindications, prescribers can play a pivotal role in administering these treatments with precision and compassion. The ultimate goal is empowering patients to regain their footing and regain control over their lives.
Case Study
Lilly, age 36, presents to the urgent care clinic with complaints of a large and bleeding laceration on her tongue, which she got from biting her tongue while eating earlier this evening. She reports, “I guess you have to take the good with the bad”, which puzzles you, but you decide not to question further what this means right now. While reviewing her chart, you note a diagnosis of schizoaffective disorder.
Medical History: Lilly’s medical history is significant for a family history of mental illness. Her paternal grandmother was diagnosed with schizophrenia. Lilly has been on antipsychotic medication since her diagnosis 10 years ago, with varying degrees of success in managing her symptoms. Lilly has struggled with medication adherence, often discontinuing the drug due to side effects or a belief that she no longer needed treatment.
Current Presentation: At present, Lilly reports strict adherence to her medication regimen with exacerbation of her symptoms for four years, and is grateful that her paranoia and auditory hallucinations have reduced in frequency. She also states, “I cannot remember what the physician told me about the medication side effects, but it cannot be as frightening as my life had become.” She has a history of delusions and hallucinations, accompanied by long-term depression and anxiety.

Overview of Psychosis
Instead of reviewing all mental health conditions, we will examine psychosis, as treatment of this symptom is one of the main goals for antipsychotics. Psychosis can be described as a burden of interruption to an individual’s thoughts and perceptions that makes it difficult for them to recognize what is real and what isn’t. Most people who have experienced psychosis describe it as frightening and confusing.
Psychosis is a symptom, not an illness. As many as 3 in 100 people will have an episode of psychosis at some point in their lives (8). This symptom can be present in different conditions.
Early or first-episode psychosis (FEP) refers to the initial occurrence of the symptom of distortion of reality.
Psychopathology
Mental health professionals need the ability to carefully assess and precisely describe signs and symptoms in a qualified manner. Psychopathology is a discipline that applies systematic methods to study abnormal functioning of psychic activity, with the intent to elaborate subdivisions, classifications, and theories, to identify the causes of mental illnesses.
Psychopathology applies direct observation of the clinical manifestations of mental illnesses to diagnostic systems (e.g., the Diagnostic and Statistical Manual of Mental Disorders-5), which outline the signs, symptoms, and features required to establish a diagnosis.
Psychopathology can be broadly divided into two main subgroups (3):
- Descriptive psychopathology involves the description and denomination of mental states and abnormal behaviors of the patient, carefully removing theoretical assumptions and personal interpretations.
- Interpretative psychopathology – the understanding of a human’s subjective experiences and attempting to explain these features using theoretical models.
Early Warning Signs Before Psychosis
Early psychosis or FEP rarely has a sudden onset. Typically, an individual has gradual, non-specific changes in thoughts and perceptions.
Early warning signs include the following (8):
- Difficulty concentrating
- Suspicion or uneasiness with others
- Poor self-care or personal hygiene
- Isolation
- A worrisome change in job performance, completion of typical duties
- Strong, inappropriate emotions or the absence of response or emotions at all
Signs Of Early or First-Episode Psychosis
It can be challenging to pinpoint the initial episode of psychosis, but these signs and symptoms strongly indicate an episode of psychosis:
- Hearing, seeing, tasting, or believing things that others don’t
- Persistent, unusual thoughts or beliefs that can’t be set aside regardless of what others believe
- Strong and inappropriate emotions or no emotions at all
- Withdrawing from family or friends
- A sudden decline in self-care
- Trouble thinking clearly or concentrating
Such warning signs often indicate a person’s deteriorating health, and a physical and neurological evaluation can help find the problem. A mental health professional performing a psychological evaluation can determine if a mental health condition is involved and discuss next steps. If the psychosis is a symptom of a mental health condition, early action helps to keep lives on track.
Psychosis
Psychosis includes a range of symptoms but typically involves one of these two significant experiences (6):
Hallucinations – seeing, hearing, or feeling things that aren’t there, such as the following:
- Hearing voices (auditory hallucinations)
- Seeing glimpses of objects or people that are not there, or distortions
Delusions – strong beliefs that are not consistent and unlikely to be true, and may seem irrational to others, such as the following (6):
- Believing external forces are controlling thoughts, feelings, and behaviors
- Believing that trivial remarks, events, and objects have personal meaning or significance
- Thinking you have special powers or even that you are God
Causes of Psychosis
The medical and research community is still learning about how and why psychosis develops, but several factors are thought to be involved.
Several factors can contribute to psychosis:
- Genetics
- Many genes can contribute to the development of psychosis, but just because a person has a gene doesn’t mean they will experience psychosis. Ongoing studies will help us better understand which genes play a role in psychosis.
- Trauma
- A traumatic event such as a death, war, or sexual assault can trigger a psychotic episode. The type of trauma—and a person’s age—affect whether a traumatic event will result in psychosis.
- Substance use
- The use of marijuana, LSD, amphetamines, and other substances can increase the risk of psychosis in people who are already vulnerable.
- Physical illness or injury
- Traumatic brain injuries, brain tumors, strokes, HIV, and some brain diseases, such as Parkinson’s, Alzheimer’s, and dementia, can sometimes cause psychosis.
- Mental health conditions
- Sometimes psychosis is a symptom of a condition like schizophrenia, schizoaffective disorder, bipolar disorder, or depression.
These mental health problems have catastrophic effects on an individual’s life. Schizophrenia (SCZ) and bipolar disorder (BPD) are two of the most relevant mental disorders when focusing on functional impairment (3). There are significant implications for poor long-term functional outcomes, such as lower rates of employment, difficulties in living independently, and harming or preventing stable relationships. The World Psychiatry Association explains that cognition is highly related to functional outcome.
Ask yourself...
- Have you ever cared for a patient experiencing psychosis?
- Can you name some factors that can contribute to the development of psychosis?
- What are some possible long-term outcomes that untreated psychosis can lead to?
- How would you describe the signs of early or first-episode psychosis?
- What additional assessments do you want to complete on this patient?
- What education can you provide to her family regarding her disorder?
- What is the difference between delusions and hallucinations?
Types of Antipsychotics
Antipsychotic drugs tend to fall into one of two categories:
- First Generation Antipsychotics
- Referred to as “Typical” antipsychotics
- Older
- Second Generation Antipsychotics
- Referred to as “Atypical” antipsychotics.
- Newer
- In general, they cause less severe neuromuscular side effects than first-generation antipsychotics.
- Second-generation antipsychotics may be more likely to cause serious metabolic side effects such as rapid weight gain and changes to blood sugar levels.
Both types can potentially work for different people. Remember, the various types of antipsychotics have various side effects.
Indications for Antipsychotic Prescribing
Schizophrenia is the primary indication for antipsychotic agents (2). However, the use of antipsychotics in the treatment of mood disorders such as type 1 bipolar disorder (BD-1), depression with psychosis, and treatment-resistant depression has become widely used in the last decade. The following are descriptions of the indications for antipsychotic use.
Schizophrenia and Schizoaffective Disorders
Schizophrenia is a chronic disorder that affects less than one percent of the U.S. population (1). When schizophrenia is active, symptoms can include delusions, hallucinations, disorganized speech, difficulty thinking, and lack of motivation. Antipsychotic drugs are the mainstay of treatment for this condition.
Antipsychotic drugs are also indicated for schizoaffective disorders, which show characteristics of both schizophrenia and affective disorders. The psychotic aspects of the illness require treatment with antipsychotic drugs, along with other medications such as antidepressants, lithium, or valproic acid (2).
Acute Mania
The manic phase in bipolar affective disorder often requires treatment with antipsychotic agents. First-generation antipsychotics are effective in the treatment of acute mania with psychotic symptoms, and all second-generation antipsychotics (except clozapine) can also be used as a treatment of symptoms of acute mania.
Major Depressive Disorder with Psychotic features
First or second-generation antipsychotics, along with an antidepressant, is typically the treatment of choice for depression with psychotic features.
Personality Disorders
First-generation antipsychotics are indicated in the treatment of paranoia and delusions associated with personality disorders. Borderline personality disorder is a type of personality disorder that can have symptoms of psychosis and paranoia; both first and second-generation antipsychotics are used for the treatment of these symptoms.
Dementia and Delirium
A low dose of high-potency first-generation antipsychotics like haloperidol is recommended for the treatment of agitation in delirium and dementia. It is essential to use caution in elderly patients as the antimuscarinic effects can cause significant adverse effects in this population. Second-generation antipsychotics can also be used for treating behavioral disturbances in dementia. Off-label use of second-generation antipsychotics is associated with acquired immunodeficiency syndrome-related dementia.
Substance-Induced Psychotic Disorder
In cases of severe psychosis secondary to substance use, antipsychotics can be used to control agitation symptoms. Caution is necessary when using first-generation antipsychotics in alcohol withdrawal and phencyclidine intoxication.
Non-Psychiatric Indications
- Antiemetic effect: Older first-generation antipsychotic drugs (except for thioridazine).
- This action is due to dopamine-receptor blockade.
- Some drugs, such as prochlorperazine and benzquinamide, are used solely as antiemetics.
- H1-receptor-blocking
- Phenothiazines have been used for the relief of pruritus or, in the case of promethazine, as preoperative sedatives.

Figure 1. Indications for Antipsychotics (Designed by course author, 2024)
Ask yourself...
- Can you describe the symptoms of schizophrenia?
- What are some conditions for which antipsychotic drugs may be used?
- What are some precautions needed when using antipsychotic drugs to treat substance-induced psychotic disorders?
- Can you describe the antiemetic effect of these drugs?
- What are the different types of antipsychotics?
- What is the difference between schizophrenia and schizoaffective?
- What is the difference between dementia and delirium?
How Do Antipsychotics Work on the Brain?
There are several researched reasons why antipsychotic drugs may help to reduce psychotic symptoms, these include:
Blocking the Action of Dopamine
It is a common perception that many psychotic experiences are caused by the brain producing too much dopamine. Dopamine is a neurotransmitter, which means that it passes messages around in your brain. Most antipsychotic drugs are known to block some of the dopamine receptors in the brain. This reduces the flow of these messages and ultimately helps to reduce your psychotic symptoms.
Dopamine is an important central nervous system neurotransmitter in the body. It belongs to the catecholamine family, which includes dopamine, norepinephrine, and epinephrine. Dopamine is the first catecholamine made in the biosynthetic pathway, produced by the decarboxylation of L-3, 4-dihydroxyphenylalanine (DOPA) by aromatic amino acid decarboxylase (15).
Dopamine is stored in vesicles released into the synaptic cleft; dopamine binds to dopamine receptors at the synapse (15). There are five different types of dopamine receptors (D1, D2, D3, D4, and D5), all of which have different pharmacological, biochemical, and physiological functions.
These receptors are divided into two receptor families (15):
- D1-like receptor family: D1 and D5 receptors
- D2-like receptor family: D2, D3, and D4 receptors
Dopamine pathways are neuronal connections in which dopamine travels to areas of the brain and body to relay messages such as executive thinking, cognition, feelings of reward and pleasure, and voluntary motor movements. Dopamine is responsible for many functions in the brain, including actions and perceptions, voluntary movements, motivation, punishment and reward, sleep, mood, attention, memory, and learning (15).
Affecting Other Brain Chemicals
Most antipsychotics are known to affect other brain chemicals, too. This may include the neurotransmitters serotonin, noradrenaline, and glutamate. These chemicals are thought to be involved in regulating your mood.
Parkinsonism
Some of the medical and research community believe that certain antipsychotics work by causing Parkinsonism, which is a movement disorder. This means they can induce the physical symptoms of Parkinsonism as side effects; they are also known to produce psychological symptoms of Parkinsonism, such as a lack of appropriate emotions or a loss of interest in activities. These effects are more common with first-generation, or ‘typical’, antipsychotics.
Ask yourself...
- How would you describe the role of dopamine in the central nervous system?
- Can you name examples of messages that dopamine transports within the brain?
- Do all dopamine receptors have the same physiological functions?
- What are 2 brain chemicals besides dopamine?
- Do you think elevated dopamine levels could negatively impact mood, attention, sleep, and voluntary movements?
- What interactions have you had with patients with parkinsons and the use of antipsychotics?
- What does blocking dopamine cause in the brain?
Case Study (Continued)
Following the treatment for Lilly’s oral laceration, she asked if this report would be sent to her primary care provider (PCP). She states a psychiatrist does not follow her, but she gets her routine prescription for Chlorpromazine from her PCP.
Previous medical history:
- Schizoaffective disorder, diagnosed in 2013
- Hypothyroidism, diagnosed in 2010
Her medication list includes the following:
- Chlorpromazine 400mg, PO, Twice Daily
- Synthroid 75 mcg, PO, Daily
Lilly reports taking Benadryl every night to help her sleep (which is not on her medication list), as she struggles with ongoing insomnia.
Pharmacokinetics of First-Generation Antipsychotics: Dopamine Receptor Antagonists (DRA)
First-generation antipsychotics are dopamine receptor antagonists (DRA), also known as typical antipsychotics. They work by inhibiting dopaminergic neurotransmission, with the peak effectiveness when 72% of the D2 dopamine receptors in the brain are blocked (4). They also have noradrenergic, cholinergic, and histaminergic blocking actions.
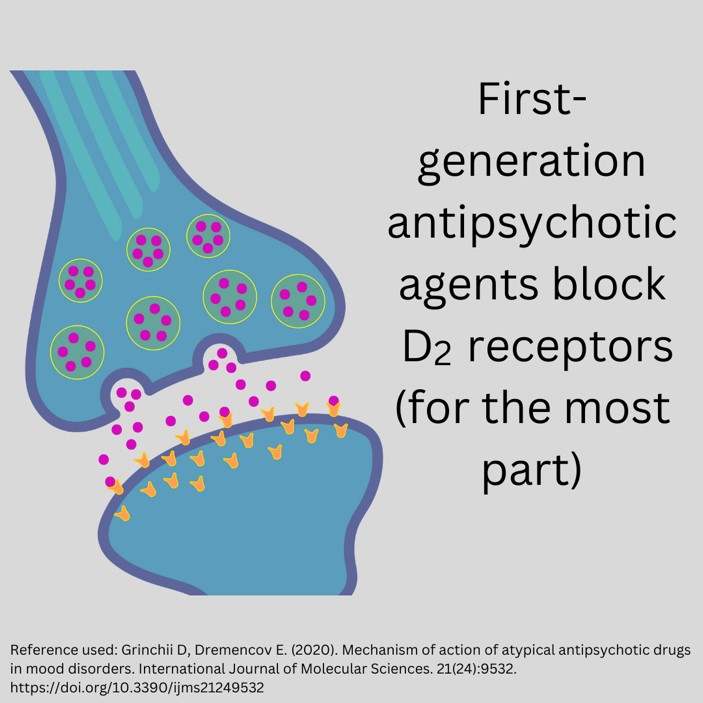
Figure 2. Blocking Action of First-Generation Antipsychotics (Designed by course author, 2024)
First-generation antipsychotics have the following approved uses:
- Psychotic disorders
- Schizophrenia
- Sedation for psychosis
- Acute agitation
- Behavioral disorders
- Schizophrenia and bipolar I agitation
- Non-psychotic anxiety
- Tourette syndrome (a nervous system disorder involving repetitive movements or unwanted sounds)
- Nausea and vomiting
It is important to note that adverse reactions of first-generation antipsychotics are highly prevalent in a significant number of patients. This warrants prescribers to perform risk minimization strategies, including staff training on identification, management, prevention, treatment guidelines and algorithms revision, and laboratory protocol set-up to enable therapeutic and adverse event monitoring (2).
Examples of dopamine receptor antagonists (DRA) include (4):
- Butyrophenones (haloperidol)
- Phenothiazines (trifluoperazine, perphenazine, prochlorperazine, acetophenazine, triflupromazine, mesoridazine)
- Thioxanthenes (thiothixene, chlorprothixene),
- Dibenzoxazepines (loxapine)
- Dihydroindoles (molindone)
- Diphenylbutylpiperidines (pimozide)
Mechanism of Action of Dopamine Receptor Antagonists (DRA)
Five dopaminergic pathways are essential for understanding schizophrenia and the mechanism of action of antipsychotic drugs.
- Mesolimbic-mesocortical pathway
- Projects from cell bodies in the ventral tegmentum in separate bundles of axons to the limbic system and neocortex
- The most closely related to behavior and psychosis.
- Nigrostriatal pathway
- Neurons that project from the substantia nigra to the dorsal striatum
- Involved in the coordination of voluntary movement
- Tuberoinfundibular system
- Occurs in the arcuate nuclei and periventricular neurons and releases dopamine into the pituitary portal circulation.
- Dopamine released by these neurons inhibits prolactin secretion from the anterior pituitary.
- Medullary-periventricular pathway
- Consists of neurons in the motor nucleus of the vagus
- This system is thought to be involved in eating patterns and behavior.
- Incertohypothalamic pathway
- Connections from the medial zona incerta to the hypothalamus and the amygdala
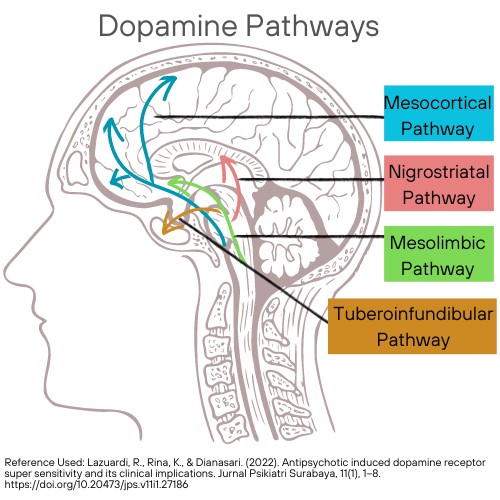
Figure 3. Dopamine Pathways. (This design is copyrighted by Abbie Schmitt, RN, MSN, 2024, and may not be reproduced without permission from Nursing CE Central)
Five dopamine receptors have been described, consisting of two separate families, the D1-like (D1, D5) and D2-like (D2, D3, D4) receptor groups.
The antipsychotic action is thought to be produced mainly by their ability to block the effect of dopamine; D2 receptors inhibit the activity of adenylyl cyclase in the mesolimbic system (4).
The D1-like receptor is coded by a gene on chromosome 5, increases cAMP by Gs-coupled activation of adenylyl cyclase, and is located mainly in the putamen, nucleus accumbens, olfactory tubercle, and cortex (2). D5, coded by a gene on chromosome 4, increases cAMP and is found in the hippocampus and hypothalamus.
The D2 decreases cAMP (by Gi-coupled inhibition of adenylyl cyclase), inhibits calcium channels, and opens potassium channels (2). Both pre- and postsynaptically found on neurons in the caudate-putamen, nucleus accumbens, and olfactory tubercle. A second family member, the D3 receptor, is also thought to decrease cAMP and is located in the frontal cortex, medulla, and midbrain. D4 receptors also decrease cAMP and are concentrated in the cortex (2).
First-generation antipsychotic agents mostly block D2 receptors, and their binding affinity is correlated with clinical antipsychotic and extrapyramidal potency. The degree of this blockade in D2 receptors and its relation to other actions vary considerably among drugs (2).
The first-generation antipsychotics also depress the release of hormones by the hypothalamus and pituitary gland. Loxapine, in addition to D2 receptors, blocks the activity of serotonin 5-HT2A receptors (4).
Ask yourself...
- Can you describe the five dopaminergic pathways?
- Does the degree of D2 receptor blocking vary among first-generation antipsychotic drugs?
- Are there commonly known side effects of these drugs?
- What are some risk minimization strategies that prescribers can use?
Case Study (Continued)
You ask Lilly to describe what happened when she bit her tongue. You note scars from deep lacerations in the mucosa of her tongue, gums, and lips at varying degrees of healing.
Lilly states, “My face and mouth stiffen and jerk for several minutes at a time”. When you ask about the frequency, she reports “almost daily.” She explains that her family gets aggravated at her when she “smacks her lips” or “sticks out her tongue,” but she cannot control these movements. Her elderly mother tells her she is possessed by a demon, which Lilly says causes her significant emotional distress and embarrassment.
Ask yourself...
- What other assessment data would you collect from Lilly?
- How would you assess psychosocial factors, such as coping and support systems?
Drug-Specific Pharmacokinetics of First-Generation Antipsychotics
Butyrophenones (Haloperidol)
The following are the pharmacokinetics of butyrophenones (10):
- Class: Antipsychotic, neuroleptic
- Black Box Warning: Risk of death in dementia-related psychosis
Uses: Psychotic disorders, control of vocal utterances in Gilles de la Tourette’s syndrome, short-term treatment of hyperactive children showing excessive motor activity, prolonged parenteral therapy in chronic schizophrenia, organic mental syndrome with psychotic features, emergency sedation of severely agitated or delirious patients.
- Metabolized by the liver
- Excreted in urine and bile
- Crosses the placenta; enters breast milk
- Protein binding: 92%
- Terminal half-life 12-36 hr. (metabolites)
- PO: Onset erratic, peak 2-6 hr.; half-life 24 hr.
- IM: Onset 15-30 min, peak 15-20 min; half-life: 21 hr.
- IM (Decanoate): Peak 4-11 days; half-life: 3 weeks
Contraindications: Hypersensitivity, coma, Parkinson’s disease
Precautions: Pregnancy, breastfeeding, geriatric patients, seizure disorders, hypertension, pulmonary or cardiac conditions, hepatic disease, QT prolongation, torsades de pointes, prostatic hypertrophy, hyperthyroidism, thyrotoxicosis, pediatrics, blood dyscrasias, neurological injury, bone marrow depression, alcohol and barbiturate withdrawal states, angina, epilepsy, urinary retention, closed-angle glaucoma, and CNS depression.
Phenothiazines
The following are the pharmacokinetics of phenothiazines (7):
Group: Nitrogen and sulfur-containing heterocyclic compounds
Uses: Treatment of schizophrenia, bipolar disorders, control of nausea and vomiting, and other psychotic disorders with delusional manifestations.
Examples of phenothiazines (10):
- Chlorpromazine
- Black Box Warning: Beers Criteria: Avoid in older adults (except in schizophrenia, bipolar disorder, or short-term use as antiemetic in chemotherapy); increased risk for stroke, cognitive decline, and mortality if used in dementia (10).
- Metabolized by the liver, excreted in urine (metabolites)
- Crosses placenta; Enters breast milk
- 95% bound to plasma proteins
- Elimination half-life: 23-37 hr.
- PO: Absorption variable, widely distributed, onset erratic 30-60 min, duration 4-6 hr.
- PO/extended-release (ER): Onset 30-60 min, peak unknown, duration 10-12 hr
- IM: Well absorbed, peak 15-20 min, 4-8 hr duration. IV: Onset 5 min
- Fluphenazine
- Black Box Warning: Increased mortality reported with dementia-related psychosis.
- Metabolized by the liver
- Excreted in urine (metabolites)
- Crosses placenta; Enters breast milk
- Protein binding >90%, not dialyzable
- PO/IM (HCl): Onset 1 hr, peak 90-120 min, duration 6-8 hr, half-life 15 hr
- IM/SUBCUT (decanoate): Onset 1-3 days; peak 1-2 days, duration over 4 wk, single-dose half-life 7-10 days, multiple-dose 14.3 days
Phenothiazines produce the most optimal results when combined with non-pharmacological psychotherapeutic therapy, such as narrative, meta-cognitive, and mindfulness therapy (7)
Thioxanthenes (thiothixene, chlorprothixene)
The following are the pharmacokinetics of thioxanthenes:
Thioxanthenes reduce antipsychotic activity by postsynaptic blocking of central nervous system (CNS) dopamine receptors, inhibiting dopamine-mediated effects; they also have alpha-adrenergic blocking activity.
Example of thioxanthenes:
- Thiothixene
- Thiothixene is not approved for the treatment of patients with dementia-related psychosis (13).
- Absorption: Erratic; high lipophilicity
- Protein binding: 90%
- Metabolism: Hepatic; substrate of CYP1A2
- Half-life elimination: 34 hours
Dibenzoxazepines (Loxapine)
The following are the pharmacokinetics of dibenzoxazepines (10):
Uses: Schizophrenia, bipolar disorder, Unlabeled uses: Anxiety
Black Box Warning: Acute bronchospasm, asthma, chronic obstructive pulmonary disease (COPD), emphysema
Loxapine inhalation must be administered only in a health care facility (14).
- Loxapine
- Onset of action: Oral, IM: Within 30 minutes; Inhalation: 2 minutes
- Peak effect: Oral, IM: 1.5 to 3 hours
- Duration: Oral, IM: ~12 hours
- Absorption: Oral, inhalation, IM: Rapid and complete
- Protein binding: Inhalation: ~97%
- Metabolism: Hepatic to glucuronide conjugates
- Bioavailability: Inhalation: 91% (de Berardis 2017)
- Half-life elimination: Oral: Biphasic: Initial: 5 hours; Terminal: 19 hours; Inhalation: 6 to 8 hours
- Excretion: Urine (as metabolites); feces (as metabolites)
Diphenylbutylpiperidines (Pimozide)
- Pimozide
- Onset of action: Within 1 week; Maximum effect: 4 to 6 weeks
- Duration of action: Variable
- Absorption: ≥50%
- Protein binding: 99%
- Metabolism: Hepatic
- Half-life elimination: Children 6 to 13 years (n=4): Mean ± SD: 66 ± 49 hours; Adults 23 to 39 years (n=7): Mean ± SD: 111 ± 57 hours
- Time to peak, serum: 6 to 8 hours; Range: 4 to 12 hours
- Excretion: Urine
Ask yourself...
- What are the black box warnings for these drugs?
- How do the onset of action and elimination differ among these drugs?
- Are these drugs considered older or newer agents?
- What are some populations that are contraindicated for the use of first-generation antipsychotics?
Adverse Effects of First-Generation Antipsychotics
Possible side effects and adverse effects of first-generation antipsychotics include (1, 2, 5, 6, 7, 8):
- Extrapyramidal symptoms, which are drug-induced movement disorders such as:
- Akathisia
- Dystonia
- Dyskinesia
- Akinesia
- Muscle stiffness
- Tardive dyskinesia
- Anticholinergic effects such as:
- Constipation
- Urinary retention
- Blurred vision
- Sedation
- Confusion
- Drowsiness
- Dizziness
- Headache
- Agitation
- Seizure
- Cerebral edema
- Peripheral edema
- Impaired regulation of body temperature
- Hypotension
- Orthostatic hypotension
- Abnormal ECG results or cardiac abnormalities
- Retinal disorders such as:
- Pigmentary retinopathy
- Retinitis pigmentosa
- Nausea
- Diarrhea
- Hypersensitivity reactions such as:
- Rash
- Pruritus (itching)
- Light sensitivity
- Abnormal liver function test results
- Sexual dysfunction
Neurological Effects
First-generation antipsychotics are associated with significant extrapyramidal (EPS) side effects. The mild to severe EPS, including akathisia, sleepiness, restlessness, and autonomic effects, are unlike any associated sedatives or hypnotics. Prescribers must recognize this possibility and educate patients on these effects.
Tardive dyskinesia, as the name implies, is a late-occurring syndrome of abnormal choreoathetoid movements and is the most important unwanted effect of antipsychotic drugs (2). It is suspected to be caused by cholinergic deficiency secondary to sensitivity of dopamine receptors in the caudate-putamen (2). Tardive dyskinesia is estimated to have occurred in 20–40% of chronically treated patients before the introduction of the newer atypical antipsychotics, which caused the prevalence to vary greatly (11).
The majority of the medical community agrees that the first step after tardive dyskinesia is noted should be to discontinue or slowly reduce the dose of the current antipsychotic agent or switch to one of the newer atypical agents, followed by elimination of all drugs with central anticholinergic action (2)
Neuroleptic malignant syndrome is a life-threatening disorder that occurs in patients who are extremely sensitive to the extrapyramidal effects of antipsychotic agents. This syndrome is believed to result from an excessively rapid blockade of postsynaptic dopamine receptors. The initial symptom is marked muscle rigidity, and then fever may ensue, often reaching dangerous levels (2). The leukocytosis and high fever present with this syndrome may erroneously be considered an infectious process.
Altered blood pressure and pulse rate are also standard (2). Muscle-type creatine kinase levels are usually elevated, reflecting muscle damage.
Switching to an atypical antipsychotic drug following recovery is indicated.
Anticholinergic Effects
Anticholinergic adverse effects like dry mouth, constipation, and urinary retention are common with dopamine receptor antagonists like chlorpromazine and thioridazine (4). The action of H1 histamine blocking causes sedation; chlorpromazine is the most sedating, while fluphenazine, haloperidol, and pimozide are less sedating (4).
Cardiac Effects
Haloperidol can cause abnormal heart rhythm, ventricular arrhythmia, torsades de pointes, and even sudden death if injected intravenously (2). Other adverse effects include prolongation of the QTc interval, prolonged atrial and ventricular contraction, and other cardiac conduction abnormalities (2).
Thioridazine has an FDA-backed warning for sudden cardiac death—low-potency first-generation antipsychotics, like chlorpromazine or thioridazine, commonly cause orthostatic hypotension (2).
Additional Effects
First-generation antipsychotics can also lower the seizure threshold, and chlorpromazine and thioridazine are more epileptogenic than others (2).
Increased serum prolactin concentrations, with breast enlargement, amenorrhea, and changes in sexual function, are known adverse effects due to the action of the dopamine receptor block in the tuberoinfundibular tract (2).
Use In Pregnancy
Although antipsychotic drugs appear to be relatively safe in pregnancy, there is a slight increase in teratogenic risk. If a pregnant woman could manage to be free of antipsychotic medications during pregnancy, this would be desirable because of their effects on the neurotransmitters involved in neurodevelopment.
Drug Interactions for Antipsychotic Drugs
Antipsychotics are contraindicated in combination with other drugs that have sedative effects, including α-adrenoceptor-blocking action, anticholinergic effects, and—for thioridazine and ziprasidone—quinidine-like action (2).
Parenteral Preparations
Parenteral forms of haloperidol and fluphenazine are available for rapid initiation of treatment and maintenance treatment in noncompliant patients (2). Prescribers should closely follow the manufacturer’s literature because the parenterally administered drugs may have much greater bioavailability (2). Fluphenazine decanoate and haloperidol decanoate are appropriate for long-term therapy in patients who cannot or will not take oral medication.
Dosage Schedules
Antipsychotic drugs are often given in divided daily doses, titrating to an adequate dosage (2). The low dosage range is recommended for at least several weeks. After an effective daily dosage has been defined for an individual patient, doses can be given less frequently. Once-daily doses (usually given at night) are feasible for many patients during chronic maintenance treatment. Research shows that simplification of dosage schedules often leads to better compliance.
Ask yourself...
- Can you describe the common adverse effects of these drugs?
- Have you ever witnessed extrapyramidal (EPS) side effects in a patient?
- Are extrapyramidal (EPS) side effects more common in first- or second-generation antipsychotics?
- What are some of the cardiac abnormalities reported with specific first-generation antipsychotic agents?
Pharmacokinetics of Second-Generation Antipsychotics
Second-generation antipsychotics are serotonin-dopamine antagonists and are also known as atypical antipsychotics.
Typical antipsychotics act almost exclusively on the dopamine system; these atypical drugs, however, regulate serotonin (5-HT), norepinephrine, and/or histamine neurotransmission as well (5).
The Food and Drug Administration (FDA) has approved the following atypical antipsychotics:
(* indicates the pharmacokinetics will be discussed in the next section)
- Aripiprazole (marketed as Abilify)*
- Asenapine Maleate (marketed as Saphris)
- Clozapine (marketed as Clozaril)*
- Risperidone (marketed as Risperdal)*
- Quetiapine (marketed as Seroquel)*
- Olanzapine (marketed as Zyprexa)
- Olanzapine/Fluoxetine (marketed as Symbyax)
- Iloperidone (marketed as Fanapt)
- Lurasidone (marketed as Latuda)
- Paliperidone (marketed as Invega)
- Ziprasidone (marketed as Geodon)
Second-generation antipsychotics are approved for treatment of the following:
| Generic Name | Indications |
| Aripiprazole |
|
| Asenapine |
|
| Clozapine |
|
| Iloperidone |
|
| Olanzapine |
|
| Paliperidone |
|
| Quetiapine |
|
| Risperidone |
|
| Ziprasidone |
|
Mechanism of Action of Second-Generation Antipsychotics: Serotonin-Dopamine Antagonists (SDA)
Typical antipsychotics act almost exclusively as blockers of dopamine-2 (D2) receptors. In contrast, second-generation atypical antipsychotic drugs are characterized by serotonin-2A (5-HT2A) antagonistic property (5). Targeting these receptors can lead to altering the excitability of 5-HT neurons.
Some atypical antipsychotics are also potent serotonin-1A (5-HT1A; aripiprazole), serotonin-1C (5-HT1C; clozapine, olanzapine, risperidone), histamine-1 (H1; olanzapine, quetiapine) and α1-(aripiprazole, clozapine, olanzapine, paliperidone, quetiapine) and α2-adrenergic (clozapine, olanzapine, paliperidone, quetiapine, risperidone) receptor blockers (5).
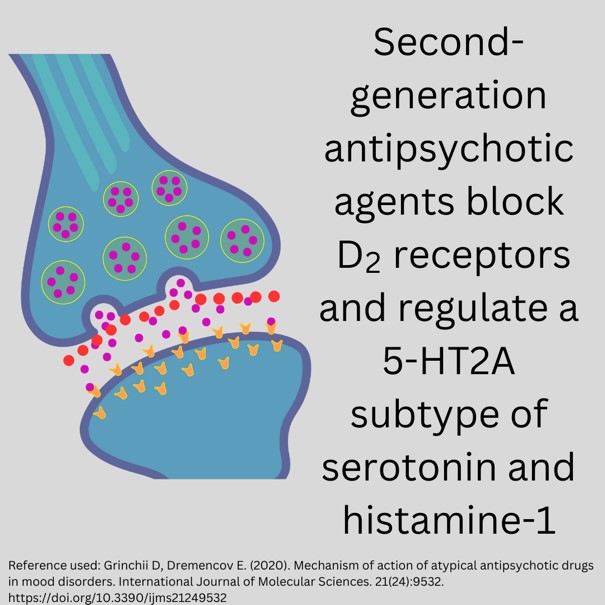
Figure 4. Blocking Action of Second-Generation Antipsychotics (Designed by course author, 2024)
Ask yourself...
- Can you explain the difference in the mechanism of action between first- and second-generation antipsychotic medications?
- Are you familiar with any atypical antipsychotics?
- What would be the benefit of targeting more than one specific neurotransmitter receptor?
- Can you name some uses for second-generation antipsychotics?
Drug-Specific Pharmacokinetics of Second-Generation Antipsychotics
These antipsychotics are identified in the Beers Criteria as potentially inappropriate medications to be avoided in patients 65 years and older due to an increased risk of cerebrovascular accidents (stroke) and a greater rate of cognitive decline and mortality in patients with dementia (12).
Aripiprazole
The following are the pharmacokinetics of aripiprazole (12):
- Onset of action: Initial: 1 to 3 weeks.
- Absorption:
- IM: Extended-release: Slow, prolonged.
- Oral: Well absorbed.
- Distribution: Vd: 4.9 L/kg.
- Protein binding: ≥99%, primarily to albumin.
- Metabolism: Hepatic dehydrogenation, hydroxylation, and N-dealkylation via CYP2D6, CYP3A4
- Bioavailability: Tablet: 87%
- Half-life elimination:
- Aripiprazole: 75 hours; dehydro-aripiprazole: 94 hours
- IM, extended release (terminal): ~30 to 47 days (dose-dependent).
- Time to peak, plasma:
- IM: Extended release (after multiple doses): 4 days (deltoid administration); 5 to 7 days (gluteal administration).
- Tablet: 3 to 5 hours; high-fat meals delay the peak time by 3 hours for aripiprazole and 12 hours for dehydro-aripiprazole.
- Excretion: Feces (55%; 18% of the total dose as unchanged drug; 37% of the total dose as changed drug); urine (25%)
Risperidone
The following are the pharmacokinetics of risperidone (10):
- Black Box Warning: Increased mortality in elderly patients with dementia-related psychosis (10).
- PO: Extensively metabolized by the liver
- Plasma protein binding: 90%
- Peak 1-2 hr.
- Excreted 90% in urine
- Terminal half-life 3-24 hr.
Quetiapine
The following are the pharmacokinetics of quetiapine (10):
- Black Box Warning: Increased risk for mortality in elderly patients with dementia-related psychosis; Increased suicidal ideation in pediatric patients (10)
- Extensively metabolized by the liver
- Half-life: ≥ 6 hr.
- Peak: 1.5 hr.
- Protein binding: 83%
- Excretion: <1% unchanged urine
Clozapine
The following are the pharmacokinetics of clozapine (10):
- Black Box Warning: Bone marrow suppression, hypotension, myocarditis, orthostatic hypotension, geriatric patients with dementia-related psychosis, seizures, syncope (10).
- Patient-specific registration is required before administration
- If WBC <3500 cells/mm³ or ANC <2000 cells/mm³, therapy should not be initiated
- Prescribers can only dispense 7 7-day, 14-day, or 28-day supply upon receipt of a lab report that is within appropriate levels.
- Completely metabolized by liver enzymes
- Bioavailability 27%-47%
- Protein binding: 97%
- Excreted in urine (50%), feces (30%) (metabolites)
- Half-life 8-12 hr.
- Clozapine can cause the following adverse effects:
- Hypersalivation
- Tachycardia
- Hypotension
- Anticholinergic side effects
- Agranulocytosis and leukopenia; therefore, monitoring of white blood cells and absolute neutrophil count is required. The FDA guidelines indicate monitoring absolute neutrophil count weekly for the first six months and, if normal, can be monitored every two weeks after that (10). Clozapine should be discontinued if absolute neutrophil count is below 1000 cells per cubic millimeter or below 500 cells per cubic millimeter in those with benign ethnic neutropenia.
Ask yourself...
- Can you describe the common pharmacokinetics of these second-generation antipsychotic drugs?
- What is a major adverse effect of Clozapine that must be closely monitored before prescribing?
- Can you name the black box warnings for Clozapine?
- How would you compare the pharmacokinetics of these drugs to the first-generation antipsychotics?
Adverse Effects of Second-Generation Antipsychotics
Second-generation antipsychotics (SGAs) have a decreased risk of extrapyramidal side effects as compared to first-generation antipsychotics. However, SGAs are associated with significant weight gain and the development of metabolic syndrome (2).
The FDA recommends that prescribers closely consider the following:
- Diabetes mellitus (personal and family history)
- Dyslipidemia
- BMI
- Blood pressure
- Fasting plasma glucose
- Fasting lipid profile
Risperidone is associated with dizziness, anxiety, sedation, and extrapyramidal side effects.
Paliperidone can cause temperature sensitivity to hot or cold temperatures and QTc prolongation (QTc = corrected QT interval).
Olanzapine has been associated most frequently with weight gain, increased appetite, and somnolence.
Quetiapine is the least likely to cause extrapyramidal side effects. The most common side effects of quetiapine are somnolence, orthostatic hypotension, and dizziness.
Ziprasidone has almost no weight gain but can cause prolongation of QTc. Aripiprazole is associated with the most common side effects of agitation, headache, and akathisia-like restlessness.
Asenapine can cause an increase in serum prolactin concentrations, weight gain, and prolongation of QTc.
Ask yourself...
- What does the FDA recommend monitoring when prescribing second-generation antipsychotics closely?
- What are some adverse effects of Risperidone?
- What is the most common adverse effect of Quetiapine?
- What lab work would help monitor metabolic alterations in patients taking these drugs?
Drug Choice
Choice among antipsychotic drugs is based mainly on differences in adverse effects and possible differences in efficacy. In addition, cost and the availability of a given agent on drug formularies also influence the choice of a specific antipsychotic.
For approximately 70% of patients with psychotic features, first- and second-generation antipsychotic drugs have similar efficacy for treating positive symptoms; however, evidence shows that second-generation drugs may have fewer negative symptoms, poor cognition, risk of tardive dyskinesia, and lesser increases in prolactin levels (2).
Some of the second-generation antipsychotic drugs cause increased weight gain and increased lipid levels compared to some first-generation drugs, and a small percentage of patients develop diabetes mellitus, most often seen with clozapine and olanzapine (2). Thus, these drugs should be considered as second-line drugs unless there is a specific indication.
Uncontrollable behavior may respond equally well to all potent antipsychotics, but is still frequently treated with older drugs that offer intramuscular formulations for acute and chronic treatment. The low cost of the older first-generation antipsychotic drugs contributes to their widespread use despite their risk of adverse EPS effects (2).
The best guide for selecting a drug for an individual patient is the patient’s history of past responses to drugs.
At present, clozapine is limited to patients who have failed to respond to substantial doses of conventional antipsychotic drugs (2). Aripiprazole is one of the most commonly prescribed second-generation antipsychotics in the U.S. due to its lower side effect profile and aggressive marketing (2).

Ask yourself...
- Do both types of antipsychotics (typical and atypical) have similar efficacy for treating positive symptoms?
- Which type of antipsychotics (first or second generation) is more likely to cause tardive dyskinesia?
- Which types of antipsychotics (first or second generation) are more likely to cause an increase in lipid levels?
- How would you explain the benefits and risks of each antipsychotic to a patient who has recently been prescribed one of these drugs?
Case Study (Continued)
After you educate Lilly on the side effects of first-generation antipsychotic medications, you note that Lilly appears anxious. However, you quickly explain that research supports that changing to a second-generation antipsychotic agent can lead to fewer of these involuntary muscle movements. Lilly seems relieved and hopeful.
She agrees to the mental health referral to a psychiatric establishment. She verbalizes understanding of the gradual taper instructions from the psychologist who consulted at your facility, and is thankful for the counseling, outpatient therapy, and community resources. All lab work is regular at this time. The social worker at the hospital is also consulted and begins case management for Lilly, ensuring she has support for housing, financial resources, and employment stability.
Ask yourself...
- What type of antipsychotic agent was Lilly taking?
- What condition do her symptoms align with?
- What resources and referrals would you incorporate if you were involved in her care?
- Do you think the muscle movements and facial twitching are chronic and incurable for Lilly?
Psychosocial Treatment & Cognitive Remediation
Patients with schizophrenia need psychosocial support based around activities of daily living, including housing, social activities, returning to school, obtaining the optimal level of work they may be capable of, and restoring social interactions.
Case management and therapy services are a vital part of the treatment program that should be provided to patients with schizophrenia. First-episode patients may be particularly in need of this support because they may be more likely to deny their illness and display noncompliance with medication.
Ask yourself...
- Why is it important that a case manager be involved with patients experiencing symptoms of psychosis?
- Are you familiar with social determinants of health (SDOH)?
- How can psychosis interfere with an individual’s daily activities?
- Do you think there is a stigma attached to mental health conditions?
Research
Considering that most second-generation and some first-generation antipsychotic agents are at least as potent in inhibiting 5-HT2 receptors as they are in inhibiting D2 receptors, current research is directed toward developing antipsychotic compounds that are more selective for the mesolimbic system (2). This increased selectivity would reduce their effects on the extrapyramidal system and hopefully decrease undesirable side effects.
The differences in receptor effects of various antipsychotics can explain many of their toxicities and are shown to be consistently associated with high D2 potency (2). Essentially, a significant goal of research is to find more effective blockade of dopamine activity with less involuntary movement or toxic effects.
There is also research on non-pharmacological therapies such as psychotherapy, cognitive behavioral therapy, and physical activity in improving these conditions.
Ask yourself...
- What is a significant research goal when considering involuntary movement or toxic effects?
- What are the non-pharmacological areas of research for the treatment of psychosis?
Conclusion
As we have discussed, the use of antipsychotics has resulted in improved quality of life for many patients; however, prescribers must be aware of the adverse drug reactions from these drugs. Treating patients with psychotic disorders requires a holistic care plan for each patient. The pharmacokinetics of each drug, specifically the mechanism of action and side effects, can be used as an effective tool for designing a holistic care plan.
References + Disclaimer
- American Psychiatric Association. (2020). What is schizophrenia? Retrieved from https://www.psychiatry.org/patients-families/schizophrenia/what-is-schizophrenia/
- Bahta, M., Berhe, T., Russom, M., Tesfamariam, E. H., & Ogbaghebriel, A. (2020). Magnitude, nature, and risk factors of adverse drug reactions associated with first generation antipsychotics in outpatients with schizophrenia: a cross-sectional study. Integrated pharmacy research & practice, 9, 205–217. https://doi.org/10.2147/IPRP.S271814
- Cavallaro, R., & Colombo, C. (Eds.). (2022). Fundamentals of psychiatry for health care professionals. Springer. https://doi.org/10.1007/978-3-031-07715-9
- Chokhawala K, Stevens L. Antipsychotic Medications. [Updated 2023 Feb 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519503/
- Grinchii D, Dremencov E. (2020). Mechanism of action of atypical antipsychotic drugs in mood disorders. International Journal of Molecular Sciences. 21(24):9532. https://doi.org/10.3390/ijms21249532
- Katzung B.G., Kruidering-Hall M, Tuan R, Vanderah T.W., & Trevor A.J.(Eds.). (2021) Antipsychotic & bipolar disorder agents. Katzung & Trevor’s Pharmacology: Examination & Board Review, 13e. McGraw-Hill Education. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3058§ionid=255305897
- Kidron A, Nguyen H. Phenothiazine. [Updated 2023 May 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556113/
- National Alliance on Mental Illnesses (NAMI). (2023). Psychosis. Retrieved from https://www.nami.org/About-Mental-Illness/Mental-Health-Conditions/Psychosis?gad_source=1&gclid=Cj0KCQiAw6yuBhDrARIsACf94RUVJ9RgZl6u71jk32H1dD9N67XFuivUjDnIwTqS7TRiyowwk_90kt0aAlwBEALw_wcB
- Rocca, P., & Bellino, S. (Eds.). (2022). Psychosis and personality disorders: unmet needs in early diagnosis and treatment. Springer. https://doi.org/10.1007/978-3-031-09058-5
- Skidmore-Roth, Linda. Mosby’s 2020 Nursing Drug Reference E-Book : Mosby’s 2020 Nursing Drug Reference E-Book, Mosby, 2019. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/liberty/detail.action?docID=5978994.
- Vasan S, Padhy RK. Tardive Dyskinesia. [Updated 2023 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448207/
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Aripiprazole. McGraw HillRetrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426473#monoNumber=426473§ionID=243193191&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Thiothixene. McGraw HillRetrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426942#monoNumber=426942§ionID=243275754&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Loxapine. McGraw Hill; Access Pharmacy.
- Yang S, Boudier-Revéret M, Choo YJ, Chang MC. Association between Chronic Pain, and Alterations in the Mesolimbic Dopaminergic System. Brain Sciences. 2020; 10(10):701. https://doi.org/10.3390/brainsci10100701
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
Complete Survey
Give us your thoughts and feedback!
