Course
TAVR Nursing Care
Course Highlights
- In this course you will learn about TAVR nursing care, and why it is important for nurses to understand the relevant anatomy of the TAVR procedure.
- You’ll also learn the basics of TAVR contraindications and complications.
- You’ll leave this course with a broader understanding of post-operative care in TAVR nursing care.
About
Contact Hours Awarded: 4

Course By:
Marcie Le
MSN, RN, CRNA
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Aortic stenosis is the second most common valvular heart disease in the Western world, and it is usually diagnosed after the age of sixty-five. With a burgeoning elderly population in the U.S., it is estimated that the number of Americans over the age of sixty-five will double by the year 2060 (2). Therefore, having a full understanding of TAVR nursing care, aortic stenosis, and its effects on the body is essential to provide care for patients undergoing a transaortic valve replacement (TAVR) procedure.
In this course we will discuss the relevant anatomy pertaining to TAVR nursing care and procedure, indications and contra-indications, and the post-operative care of patients undergoing TAVR nursing care and procedure.
Introduction
Aortic stenosis is the most common valvular heart disease in the world. With an aging population, the incidence of aortic stenosis is sure to increase. Over the last fifteen years, the transcatheter aortic valve replacement (TAVR) procedure has evolved. Once offered only when surgical intervention was too risky, this much less invasive procedure is now being performed on lower–risk populations with great results. With the development of newer devices to aid in the TAVR procedure, challenging anatomies, difficult access and poor delivery systems are becoming less of an issue. As these procedures become more common, the experience of the cardiologist and the catheterization lab staff also plays a factor in the success rates of TAVR. This is great news considering that once diagnosed with a critical aortic stenosis, the mortality rate is quite high. TAVR is quickly becoming the procedure of choice to treat aortic stenosis.
Anatomy and Function of the Aortic Valve
The aortic valve works in conjunction with the other three valves of the heart to keep blood moving forward. A brief review of a normally functioning aortic valve helps us understand how the pathology of aortic stenosis causes such profound effects on the rest of the body systems.
The aortic valve is situated between the left ventricle and the aorta. This area is also called the left ventricular outflow tract or LVOT, which simply means the track that flows out of the left ventricle. The opening of the aortic valve, known as the valve area, normally measures 2.6-3.5 2 (5, 7).
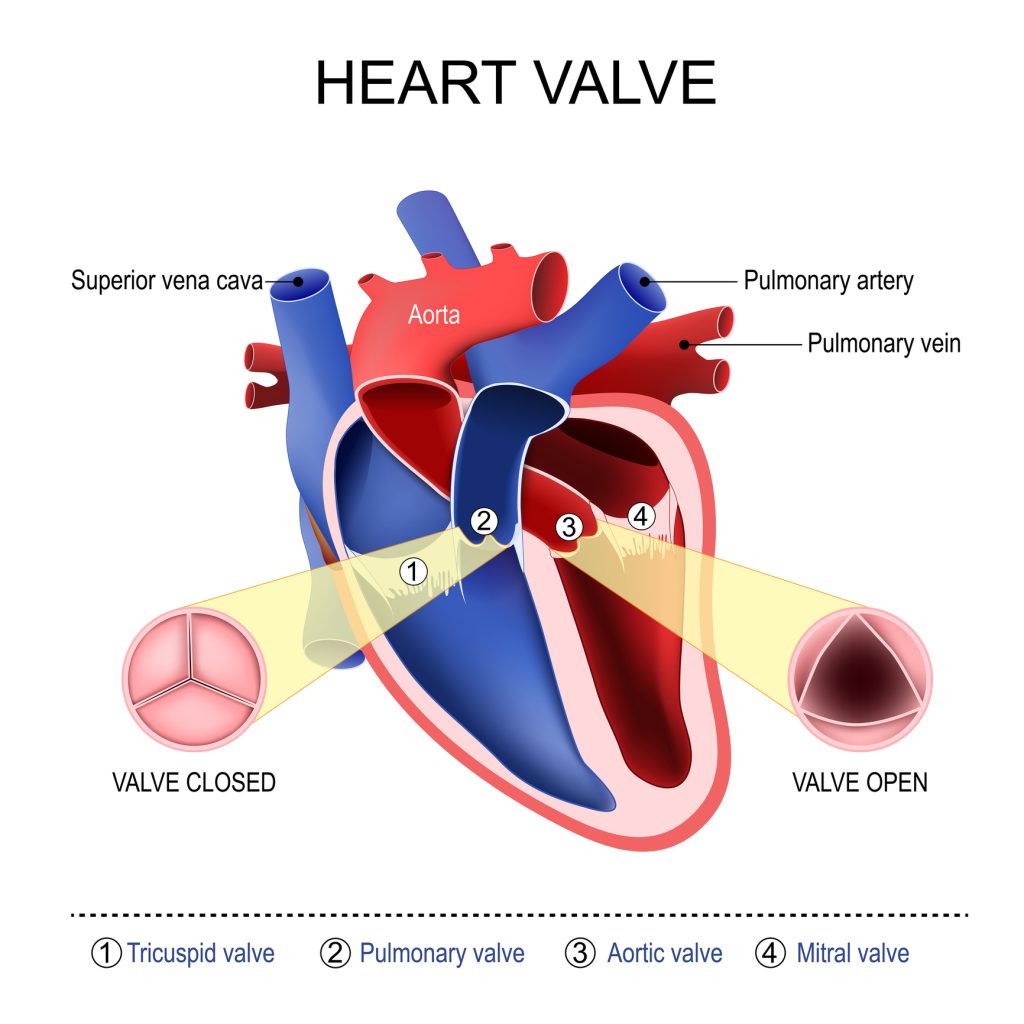
The aortic valve is referred to as a semilunar valve because when closed it looks like three semilunar structures coming together to form a Y-shape. The aortic valve can be divided into four elements and they are as follows:
- One annulus
- Three cusps (leaflets) with four layers
- Three sinuses
- Three commissures
The three semilunar shaped pockets, known as cusps (or leaflets), attach to a fibrous ring known as the annulus. The annulus is a part of the cardiac skeleton, a dense network of connective tissue that lies between the atria and ventricle reinforcing the structure of the heart. The annulus also acts as a shock absorber by transferring the force of the high-pressure circulatory system into the framework of the cardiac skeleton causing less wear and tear on the leaflets.
The cusps are named as the right coronary cusp, the left coronary cusp, and the non-coronary cusp. They are named this way because just behind the cusps, on the ventricle side of the outflow tract, lie three nodules, known as the sinus of Valsalva. The sinus is the area where the left and right main coronaries attach to the aorta. During diastole, the sinuses fill with blood that was just pushed out of the ventricles. As the ventricle begins to relax, the coronary sinuses drain blood into the coronary vessel system, preparing for the next contraction by providing oxygen and nutrients to the heart muscle.
The cusps are pearlescent in appearance and have layers all less than 1mm thick; each layer has a distinct function. The first is a dense collagenous layer which is located close to the aortic surface. This layer is known as the fibrosa. In the middle is the spongiosa, a loose layer of connective tissue that is full of mucopolysaccharides. Lastly is the ventricularis. This is a layer close to the ventricular surface and is rich in elastin. At the ends of these layers, the leaflets are covered by valvular endothelial cells (5, 7).

Self-Quiz
Ask Yourself...
- How many heart valves are there?
- What is the function of heart valves?
- Where is the aortic valve located?
- What is another name for the aortic valve?
- As the aortic valve leaflets come together, what shape do they form?
- How many leaflets or cusps does the aortic valve have?
- What are two elements of the aortic valve?
- What function does the annulus perform?
- How are the aortic valve cusps named?
- Can you name two cusp layers?
Aortic Valve’s Role in the Cardiac Cycle
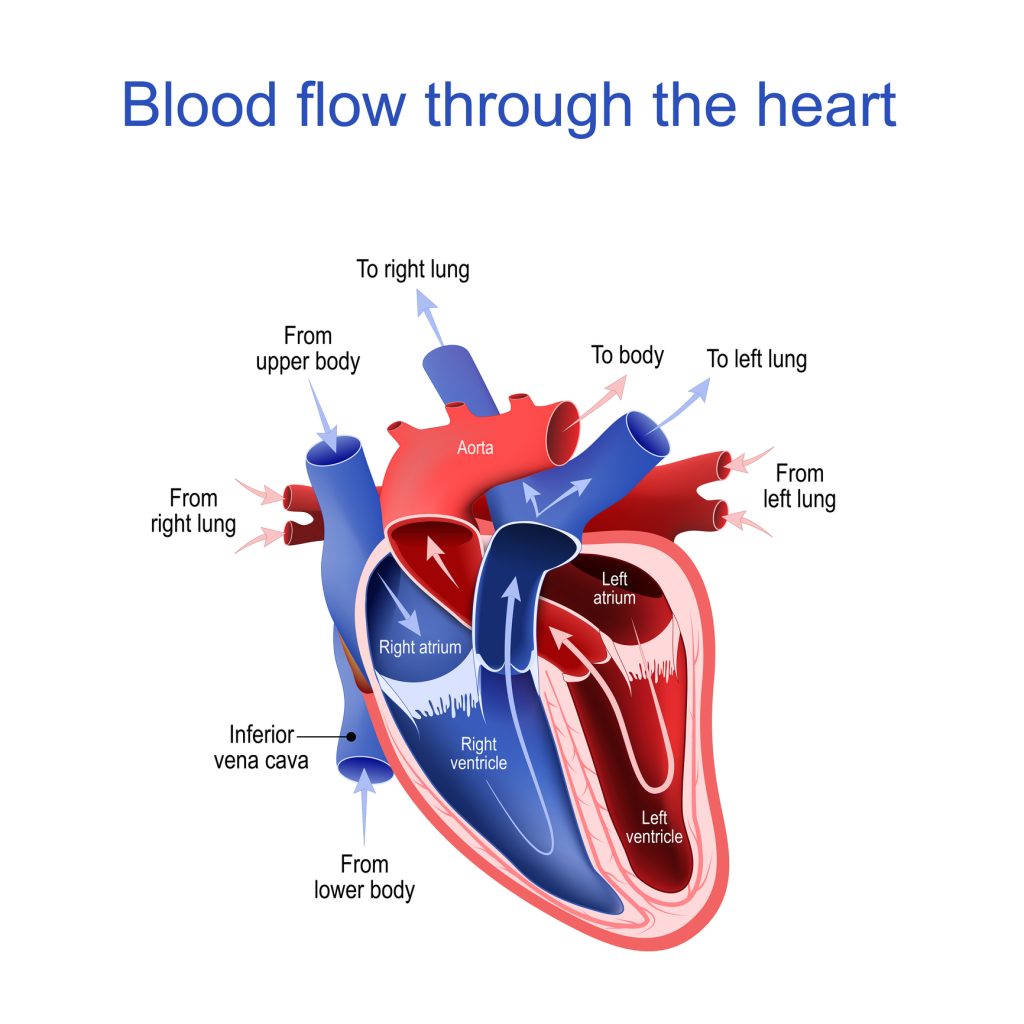
We have looked at the composition of the aortic valve now let’s look at its role in the cardiac cycle, starting with the venous circulation. Deoxygenated blood from the body travels to the heart through the superior and inferior vena cava:
- The superior and inferior vena cava empty into the right atrium of the heart. The atrium contracts, the tricuspid valve opens, and the blood flows into the right ventricle. As the right atrium relaxes, the tricuspid valve closes.
- The right ventricle contracts and opens the pulmonic valve. Blood travels into the pulmonary circulation to eliminate waste and reoxygenate. The oxygen rich blood then travels from the pulmonary circulation into the left atrium of the heart.
- The left atrium contracts, opening the mitral valve and blood flows into the left ventricle. The atrium relaxes and the mitral valve closes. The left ventricle contracts, sending blood out of the aortic valve and to the body through the aorta and carotid arteries. The valve will close when the pressure in the aorta is higher than the pressure in the ventricle.
Closure of the aortic valve is part of the second heart sound, S2, and is heard as the “dub” part of “lub-dub.” Originally, the S1 and S2 heart sounds were thought to be the sounds of the valves snapping shut. However, we now know that the heart sounds occur because of vibrations that happen just after the valve has shut.
As the aortic and pulmonic valves initially close some blood flows back and hits against the valves. The blood hitting against the valves causes vibrations that travel along the corresponding chamber producing the “lub” or “dub” sound as it travels (5, 7, 11).

Self-Quiz
Ask Yourself...
- Which two areas are separated by the aortic valve?
- Which heart sound is heard at the closure of the aortic valve?
Pathophysiology and Assessment
In aortic stenosis, the leaflets of the aortic valve become calcified, scarred, and stiff. The calcifications narrow the valve opening from the normal 2.6-3.5 cm2 to ˂1 cm2. The narrow valve obstructs the left ventricular outflow tract (LVOT), increasing the work the heart must do to overcome the obstruction or the afterload. To compensate for this obstruction and increased afterload, the heart begins to squeeze harder, increasing pressure in the left ventricle.
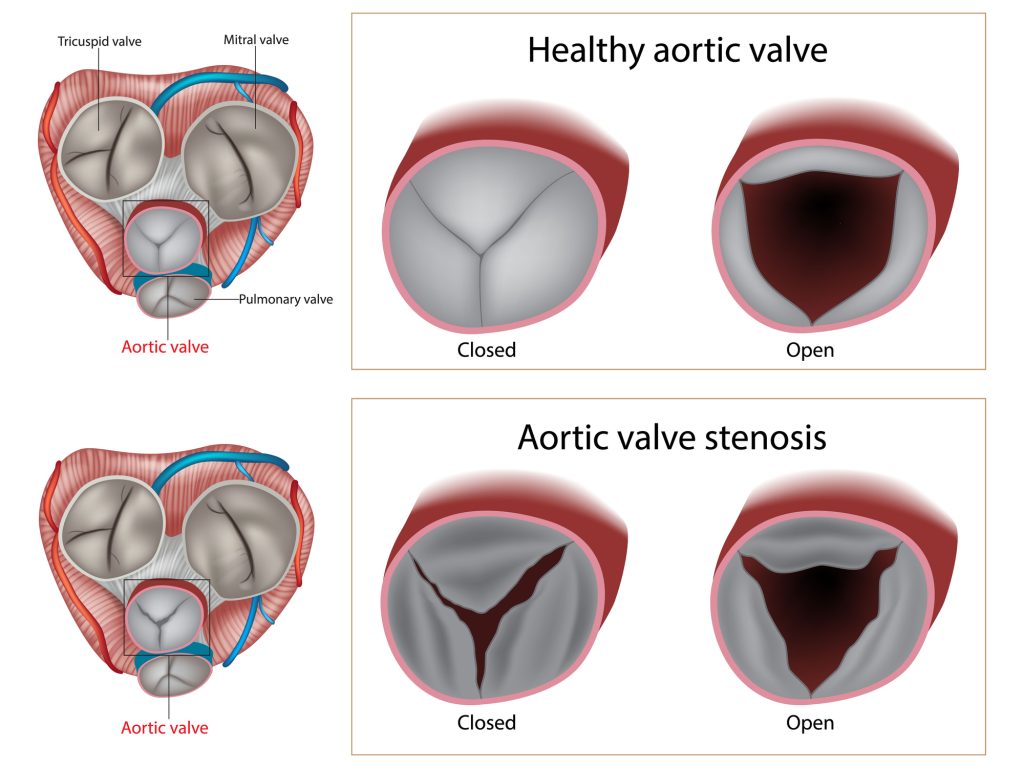
Left ventricle pressures have been reported as high as 300 mmHg in some cases. The constant exposure to high pressure causes the left ventricle to fibrose, thicken, and remodel. The increased muscle mass also demands more oxygen which causes stress on the microvasculature of the heart and contributes to the symptom of angina.
Typically, the left ventricle can relax and to allow for passive filling from the left atrium. The passive filling of the ventricle makes up the 80-85% of the end-diastolic volume, and the remaining 15-20% is provided by the “atrial kick” at the end of diastole. However, clients with aortic stenosis have stiff, non-compliant ventricles that do not relax enough for proper filling during diastole; these clients depend on the atrial kick for 40% of their blood volume.
Therefore, abnormal rhythms such as atrial fibrillation or tachycardia cause clients with aortic stenosis to lose the benefits of the atrial kick, which causes a subsequent decrease in cardiac output to the body. This can lead to syncope and in some cases sudden cardiac death.
Ultimately, in the course of aortic stenosis, the left ventricle will dilate, fail, lead to pulmonary congestion, shortness of breath, and chest pain, which are the hallmark symptoms of aortic stenosis. Clients presenting with the classical triad of aortic stenosis have a 2-5 year prognosis if untreated (2, 11).

Self-Quiz
Ask Yourself...
- What happens to the aortic valve leaflets in aortic stenosis?
- Why does the heart begin to squeeze harder with aortic stenosis?
- Due to increased pressure, what happens to the left ventricle?
- Clients with aortic stenosis depend on the atrial kick for what amount of their blood volume?
- In the presence of arrythmias and aortic stenosis, what happens to the atrial kick?
- A decrease in cardiac output with aortic stenosis can lead to what issues?
- What are the hallmark symptoms of aortic stenosis?
Hallmark Symptoms: Syncope Angina Dyspnea
In severe aortic stenosis, blood from the left ventricle is being pushed out of a tiny calcified and critically stenosed opening at tremendous pressures creating what is called a “nozzle effect”. It is termed the “nozzle effect” because blood is spraying out of the aortic valve like water out of a fire hose.
As the blood hits the sides of the aorta, it causes vibrations that are heard, sometimes even without a stethoscope, and are felt like the thrill of a fistula. The murmur of aortic stenosis is heard and felt loudest in the 2nd intercostal space on the right side this is the area directly over the aorta. The murmur can be heard best during systole since this is when blood is spraying out of the ventricle and creating turbulence in the aortic arch (2, 11).
It is important to be able to identify the murmur of aortic stenosis given that many clients can go decades without any symptoms, and earlier detections can lead to earlier treatment and fewer long-term complications and morbidity. The murmur of aortic stenosis is considered a mid-systolic murmur described as a harsh crescendo-decrescendo murmur. Initially, as blood is pushed out of the ventricle, there is no sound, but as the ventricle squeezes harder the turbulence of the blood flow causes the crescendo and gradually as the ventricle begins to relax the decrescendo ensues.
In the late stages of the disease, S2 may be obscured by the murmur or even lost as the aortic valve becomes less compliant; this is an abnormal finding. The presence of S4 is indicative of left ventricular hypertrophy that develops over time in aortic stenosis. The S4 rhythm is called a gallop rhythm because it sounds like the hoof-beats of a horse.
When listening to a normal heartbeat, during S2 with inspiration, you may be able to distinguish two heart sounds; this is a normal finding called physiological splitting, and it only indicates that the pulmonic valve is taking a bit longer to close due to the inspiration. However, in aortic stenosis, it takes the aortic valve longer to close due to delayed emptying and the ventricles inability to relax. This abnormal finding is called paradoxical splitting, and it is heard during expiration rather than inspiration.
Pulsus tardus is a weakening of the carotid pulse that can be felt with light palpitation of the carotid artery. Pulsus parvus et tardus is when the carotid upstroke is delayed. The carotid upstroke can be assessed by listening to the heart for systole and noting by palpation how long it takes to travel to the carotid artery (2, 11).


Self-Quiz
Ask Yourself...
- What is the “nozzle effect”?
- Where is the murmur of aortic stenosis heard the loudest?
- Why is it important to recognize the murmur of aortic stenosis?
- What is a gallop rhythm?
- What is paradoxical splitting?
- What is pulsus tardus?
Etiology & Risk Factors
There are a few ways in which aortic stenosis can occur. These causes are listed below from most to least common (2, 7, 11).
- Idiopathic calcification
- Congenital bicuspid valve
- Rheumatic heart disease
- Radiation or Endocarditis
- Genetic elevation of lipoprotein
Idiopathic Calcification
Idiopathic calcification occurs mostly in clients over the age of 65; this by far is the most common cause of aortic stenosis and is found in 3 to 5% of the general population. Risk factors for clients in this class to develop aortic stenosis include smoking, male gender, hypertension, hyperlipidemia, and diabetes. They are mostly the same risk factors associated with atherosclerosis (2, 7, 11).
Congenital Bicuspid Aortic Valve Disease
Congenital bicuspid aortic valve disease is a condition where a person is born with only two cusps on the aortic valve. Bicuspid valve disease is the most common congenital heart disease and is found in 1 to 2% of the population. Symptoms usually show up in this group around 40-50 years of age as age-related changes begin to affect the bicuspid aortic valve. Nearly 40% of these clients also have aortic dilatation which can lead to rupture – a medical emergency (2, 7, 11).
Rheumatic Aortic Disease
Rheumatic aortic disease, now a rare cause of aortic stenosis, is caused by an autoimmune response to group A streptococci, which leads to scarring along the commissure of the valve leaflets lessening their pliancy and causing an obstruction to the outflow tract of the left ventricle. Rheumatic aortic stenosis can easily be remedied with antibiotics. With the combination of rapid strep testing and access to antibiotics, this is very rarely encountered in the clinical area (5).
Genetic Elevation of Lipoprotein
Some cases of aortic stenosis are believed to be caused by a genetic variation in the genes that express lipoprotein (a). The elevation of lipoprotein (a) along with low-density-lipids are thought to induce inflammatory changes on the layers of the valve leaflets leading to valve calcification and stenosis.
Other Causes
Other rare causes of aortic stenosis include; endocarditis, radiation therapy, Paget disease, Fabry disease, ochronosis, and end-stage renal disease (2, 7, 11).

Self-Quiz
Ask Yourself...
- What are two ways aortic stenosis can occur?
- What is idiopathic calcification?
- How many leaflets should the aortic valve have?
- With congenital bicuspid aortic valve disease, how many leaflets does the aortic valve have?
- How can rheumatic aortic stenosis be remedied?
Natural History of Aortic Stenosis
In aortic stenosis the aortic valve causes an obstruction of the left ventricular outflow tract. The heart attempts to overcome this obstruction by pushing harder. This causes a tremendous difference in pressure between the left ventricle and the aorta. This difference is measured and termed the transvalvular gradient. A transvalvular gradient higher than 50 mmHg with a small valve area such as 0.8 cm2 is considered to be severe aortic stenosis.
In severe aortic stenosis the left ventricle compensates in two ways: remodeling or thickening. Remodeling happens on a cellular level and is caused by changes to the cellular matrix in response to elevated pressure. Thickening of the heart muscle occurs because of how hard the heart must push against the transvalvular gradient. The more the heart muscle is worked the larger it becomes, just like any other muscle of the body. The thickening, however, has consequences.
The first being that the muscle of the left ventricle has thickened, but the excess tissue leaves no room for blood in the ventricle. Less blood in the ventricle leads to less blood being pushed out to the body. This leads to diastolic heart failure. These clients can appear to have a normal Ejection Fraction (EF), but this can be deceiving. Let’s look at normal ejection fraction vs. the ejection fraction of a client with diastolic dysfunction (2, 5, 7, 11).
Normal ejection fraction is 60%
- The heart fills with 100 ml
- The heart ejects 60 ml
- 60 ml divided by 100 ml = 0.60 or 60%
Ejection fraction of diastolic dysfunction
- The heart can only fill with 50 ml
- The heart ejects 30 ml
- 30 ml divided by 50 ml = 0.60 or 60%
The EF is identical, but the client’s heart (with diastolic dysfunction) is filling up with half the blood and pumping out half the blood amount of the client without dysfunction. The deficit leads to under perfusion to the body, which brings about exertional chest pain. On the other hand, because the ventricle cannot fill up enough or pump out enough, blood begins to back-up from the left ventricle to the left atrium and finally all the way back to the vasculature of the lungs.
The vessels of the lungs begin to leak excess fluid causing pulmonary edema and dyspnea. This dyspnea is especially increased during exertion, because on top of the fact that the heart cannot push out enough blood, now there is tachycardia which decreases the amount of time the heart has to fill up. Clients with aortic stenosis often adjust their activity level over time so that they do not over exert themselves which leads to the chest pain and shortness of breath.
Now let’s look at the other scenario. Clients with aortic stenosis with systolic dysfunction usually have an EF that is lower than normal. In this scenario the left ventricle has become worn out and is unable to pump blood to the rest of the body. The ventricle dilates and thins causing it to lose its ability to pump effectively. Let’s compare the EF to that of a client without dysfunction (2, 5, 7, 11).
Normal ejection fraction is 60%
- The heart fills with 100 ml
- The heart ejects 60 ml
- 60 ml divided by 100 ml = 0.60 or 60%)
Ejection fraction of systolic dysfunction
- The heart can only fill with 100 ml
- The heart ejects 30 ml
- 30 ml divided by 100 ml = 0.30 or 30%
In this case the ventricle has enough room for the normal amount of blood, but the muscle of the ventricle is only able to squeeze out 30 ml to the body. If you notice in both scenarios the client is only pushing 30 ml of blood out of the ventricles; so regardless of whether the client has systolic or diastolic heart failure they end up with the same consequences of chest pain from under-perfusion and shortness of breath from blood backing up into the pulmonary vasculature.
As the aortic stenosis progresses, symptoms such as changes in heart rate, afterload, and vascular resistance mean wide swings may increase. If not corrected this can lead to sudden death. Dyspnea is likely to occur in clients with aortic stenosis who have excessive fluid volume. Dehydration will not allow the ventricle to fill adequately.
Tachycardia does not allow the ventricles to fill while bradycardia will decrease the cardiac output further. Clients with aortic stenosis are best kept close to their baseline vital signs while preparing for the TAVR procedure (2, 5, 7, 11).

Self-Quiz
Ask Yourself...
- What is the transvalvular gradient?
- What classifies aortic stenosis as severe?
- How does the left ventricle compensate in severe aortic stenosis?
- What is remodeling?
- What is thickening?
- What is a normal ejection fraction?
- What happens to the EF in a client with diastolic dysfunction?
- What happens to the EF in a client with systolic dysfunction?
- What are two symptoms of aortic stenosis progression?
- Which symptoms contribute to inadequate diastole?
Overview of Procedure
The TAVR procedure is approved for clients who have severe symptomatic aortic stenosis and who are at high risk for surgical valve replacement. More and more, however, the TAVR procedure is also performed on clients with a low to intermediate risk with favorable results.
After proper imaging has been completed and the optimal sheath insertion site has been established, the TAVR procedure can be scheduled in the catheterization lab. Sheaths are used to make a conduit for the valve deployment device. The valve is round and made of a metal mesh network to which bovine leaflets attach.
The TAVR procedure does not require that the heart is stopped to perform the valve replacement, though the heart is usually paced at a high rate during deployment (1, 4).
Diagnostic Imaging
Diagnostic imaging is crucial if a TAVR is to be planned. Solid imaging of the aortic valve decreases the risk of sub-optimal valve deployment during procedure, which can result in paravalvular regurgitation, aortic injury, heart block, or embolization of the valve prosthesis.
The gold standard of evaluation of aortic stenosis is the transthoracic echocardiogram (TTE). In this section we will look at what a diagnostic TTE assesses. Performing a TTE helps to (10):
- Confirm the diagnosis of aortic stenosis
- Identify the cause of aortic stenosis
- Assess valve morphology
- Identify the severity of the valve lesion
- Note left ventricular remodeling
- Estimate functional capacity of the left ventricle
- Assess for the presence of mitral valve regurgitation
- Assess for concomitant pulmonary hypertension
Assessing Valve Morphology
New generation devices can now be used to treat aortic stenosis in clients with a bicuspid aortic valve (BAV). Previously, BAV could only be treated with open heart surgery, but as TAVR has evolved, devices have been designed to manage this anomaly. It should be noted that different BAV morphologies will impact the outcomes after TAVR. BAV is a term that is used to describe many different aortic valve morphologies. These various morphologies include the location and degree of calcification, the presence or absence of raphe, and the presence of a dilated ascending aorta. This all comes into play post procedure, as unlike with surgery where the valve is excised, clients who’ve undergone TAVR may have complications based on the native aortic valve anatomy.
Traditionally, TTE was used to determine the number of leaflets present in aortic valve anatomy. While this still holds true, a computed tomography (CT) scan can give more detailed information about the bicuspid morphology. Rheumatic valve disease is usually differentiated by the pattern of calcification. While rheumatic heart disease usually shows calcification at the commissure line, aortic stenosis usually affects the base of the leaflet and works in an outward pattern (5, 15).
Identifying Severity of the Valve Lesion
Echocardiographic parameters classify the severity of the valve lesion into mild, moderate, and severe stenosis. The severity of the disease is determined by the valve area, valve gradients, and peak velocity (10).
| Classification | Mild | Moderate | Severe |
| Aortic Valve Area (AVA) cm2 | >1.5 cm2 | 1.1-1.5 cm2 | ≤1 cm2 |
| Mean Gradient mm Hg | ˂20 mmHg | 20-39 mmHg | ≥40 mmHg |
| Peak Velocity (m/sec) | 2.0-2.9 m/sec | 3.0-3.9 m/sec | ≥4 m/sec |
Aortic Valve Area
The aortic valve area (AVA) is a calculation that measures the obstruction of the left ventricular outflow tract, by the stenosed valve. The normal aortic valve area is 2.5-3.5 cm2. The more stenosed the valve, the smaller the aortic valve area.
Valve Gradient
The valve gradient is another calculation that measures the difference in flow across the valve. The gradient can be calculated on echocardiography or performed directly in the catheterization lab. In the cath lab, the pressure in the left ventricle and the pressure in the aorta are measured simultaneously. The difference between the two numbers is the gradient. High pressures in the ventricle with low pressures in the aorta is indicative of aortic stenosis. The normal mean gradient is ˂20 mmHg (10, 12).
Peak Velocity
The peak velocity is a calculation that estimates how fast the blood is traveling across the valve and is measured in meters/second. The standard peak velocity is 2.0-2.9 m/sec. Peak velocity is related to the “nozzle effect,” where the ventricle is trying to push blood out of a narrowed opening. The enormous pressure generated translates into a higher peak velocity (10, 12).

Self-Quiz
Ask Yourself...
- What can solid imaging of the aortic valve prior to TAVR help prevent?
- What is the gold standard of aortic valve stenosis evaluation?
- What are two things that performing a TTE can help assess?
Assessing for Left Ventricular Hypertrophy
Left ventricular hypertrophy (LVH) is abnormal thickening and reshaping of the left ventricle in response to the extreme pressures generated in the ventricle as it pushes against the stenotic valve. LVH is present in approximately 67% of clients diagnosed with asymptomatic severe aortic stenosis. The onset is insidious and can develop long before the onset of symptoms. LVH increases the risk of adverse cardiovascular outcomes up to 4-5 fold. Therefore, echocardiographic evaluation of the left ventricle is useful to estimate the functional capacity and determine the severity of LVH before the TAVR procedure (10, 12).
Assessing for Mitral Regurgitation
The echocardiography is also able to detect concomitant mitral regurgitation (MR). MR is when the blood flows backward from the left ventricle into the atrium. This process can either be acute or chronic. Acute cases of MR usually occur in the setting of myocardial infarction, infective endocarditis, rupture of a chordae tendineae, or malpositioning of the aortic valve during TAVR.
In acute cases, there is an overwhelming back up of blood from the left ventricle to the left atrium and back to the pulmonary circulation. The rapid onset does not allow time for compensation, and it can easily lead to pulmonary congestion, hypoxia, reduced cardiac output, hypotension, or even shock.
Non-acute cases of MR can occur when the left ventricle becomes hypertrophic, and subsequently the annulus of the mitral valve becomes dilated which is common in aortic stenosis.
Regurgitation can lead to pulmonary congestion and edema. The mitral valve can be repaired along with the aortic valve in clients undergoing surgical aortic valve replacement (SAVR), but it is not yet performed in conjunction with TAVR. Ultimately, the decision to perform TAVR vs. SAVR in these clients is based on the heart valve team and the client (10, 12).


Self-Quiz
Ask Yourself...
- What is one type of aortic valve morphology?
- In conjunction with a TTE, what else can give information about aortic valve morphology?
- How many classifications of aortic stenosis are there and what are they?
- What determines the severity of aortic stenosis?
- What does the aortic valve area calculation measure?
- What is valve gradient?
- What is peak velocity?
- How is the peak velocity measured?
- What is left ventricular hypertrophy?
- Why should the severity of left ventricular hypertrophy be determined before TAVR?
- What is mitral valve regurgitation?
- What can mitral valve regurgitation lead to?
Decision to Intervene
The heart valve team is tasked with deciding the best pathway for the client based on current evidence and guidelines. The team typically consists of cardiologists, structural interventional cardiologists, imaging specialists, cardiovascular surgeons, a cardiovascular anesthesiologist, and cardiovascular nursing professionals.
The client and family are involved in each step of the process and may be assigned a heart valve coordinator that works closely with them ensuring high-quality education and information for the decision-making process.
The decision to proceed to intervention is based on several factors (1, 6):
- The client’s goals and beliefs
- The presence or absence of symptoms
- The severity of the lesion
- Remodeling of the ventricles
- Pulmonary or systemic congestion
- A change from baseline heart rhythm
- Risk/benefit based on age
- Co-morbidities and life expectancy
Evaluation of Risk
The Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) is a scoring system that is based on years of data collected by the Society of Thoracic Surgery. The heart valve team takes the STS score and classifies the client into low, intermediate and high risk for surgical intervention.
Before surgery, other factors are also measured such as the frailty index which takes into consideration the client’s ability to perform activities of daily living, assesses any weight loss in the previous year, and tests the client’s ability to rise from a chair. The client may also be asked to perform a six-minute walk test or pass the Mini-Mental State Exam that assesses cognitive function.
Clients are further classified into stages A-D3. These stages describe the severity of stenosis, characteristics of the stenosis, and presence or absence of symptoms.
The American Heart Association (AHA) / American College of Cardiology (ACC) guidelines are made to go along with the STS-PROM assessment. For example, a client at high surgical risk, with symptomatic severe aortic stenosis would fall into the TAVR category, while a client at intermediate risk with severe symptomatic stenosis would be recommended for SAVR. Absolute contraindications for TAVR include:
- Non-calcified aortic valve
- Peripheral vascular or aortic disease
- Coronary artery disease requiring revascularization within 30 days
- End stage renal disease
- Severe left ventricular hypertrophy
- LVEF<20%, severe mitral regurgitation
- Significant neurological disease
- Life expectancy <1 year
Ultimately, SAVR is preferred in clients with low to intermediate surgical risk. However, for clients at high surgical risk measured by STS score>10% TAVR is the preferred intervention. That being said, TAVR in clients with low to intermediate risk is becoming more common as long-term outcomes prove to be positive in clients who’ve undergone TAVR. The evolution of TAVR devices is also playing a role in the decision to perform TAVR on low to intermediate risk clients (1, 6, 8, 12).

Self-Quiz
Ask Yourself...
- Who are two members of the heart valve team?
- What are three factors in the decision to proceed with TAVR?
- What is the STS score?
- Why is the client’s frailty index measured prior to TAVR?
- Which risk severity client type is most common, requiring the TAVR procedure?
Pre-Planning Stage
Choice of Valve
Multi-detector computed tomography (MDCT) or computed angiotomography, is the preferred imaging method to determine the annular size of the aortic valve and facilitates the decision of what valve should be chosen. The 3D data set that MDCT provides produces a more tangible image by which to choose valve size; it is also able to measure the annulus of the aortic valve during systole when the valve is usually wider and fully open.
The MDCT scan measures the aortic root which produces an anatomical image of the sinus of Valsalva, the coronary ostia, and the size of the aorta and sinotubular junction. These views can be used to seat the valve properly since any obstruction to the coronary ostia can lead to ischemia and possible cardiovascular arrest.
MDCT imaging can help determine whether a balloon-expandable, self-expanding, or mechanical deploying valve should be chosen. The balloon-expandable valve fits over a balloon and when the balloon expands it pushes the valve onto the annulus of the aortic valve (3, 10, 12).
Common TAVR Access Sites
A self-expanding valve expands in a spring-like manner without the need for a balloon. The mechanical valves have a seal to reduce paravalvular leak and can be expanded by the cardiologist in a controlled manner.
Regarding preference, a self-expanding valve is a gentler option and may be preferred in clients with “severe calcification of the valve and outflow tract with a risk of rupture, clients with an extremely oval-shaped annulus, or for small transfemoral access”.
If a client requires the transapical approach, as in the case of severe atherosclerosis of the vasculature, the only valve approved for use is the balloon-expandable version (18). Many versions on the market today can be repositioned if malpositioned. Often, however, the choice of valve is merely provider preference (3, 8).
Choice of Access
The outer diameter of the sheaths used for valve deployment range anywhere from 6.9 cm to 8.68 cm depending on the intended valve. Access sites are thoroughly imaged to ensure an entry point that is non-tortuous and mostly free of atherosclerosis which can put the client at risk for cerebral embolization. If entry through the femoral artery is not feasible, other options include transaxillary, transapical, direct aortic, carotid, or transvenous approach (3, 10).

Self-Quiz
Ask Yourself...
- The annular size of the aortic valve is determined by which imaging study?
- Knowing the annular size facilitates which decision in the TAVR procedure?
- What can an obstruction of the coronary ostia lead to?
- What is one type of valve that can be deployed during TAVR?
- How does a self-expanding valve work?
Procedural Considerations
Hybrid Operating Room
Facilities where TAVR procedures are performed have a dedicated hybrid operating room that is a mix between a cath lab and a standard operating room. There is a cardiopulmonary bypass machine available if the procedure is converted to open-chest. Coronary occlusion wires are on hand if coronary embolization occurs intra-procedure, and anesthesia is equipped with advanced airway supplies if necessary. A crash cart and defibrillator are nearby and defibrillator pads are placed on the client per routine (12).
Anesthetic Considerations
Many of these clients have both cardiovascular and non-cardiovascular risk factors. Cardiovascular collapse is a real concern and proper anesthetic management can decrease this risk. Optimal hemodynamics should be maintained through the case, and attention should especially be paid to hypotension. Prompt administration of vasoactive medications aids in the avoidance of hypoperfusion.
While general anesthesia with endotracheal tube is the most common delivery method of anesthesia in clients undergoing TAVR, studies have shown that moderate sedation decreases the need for vasoactive medications. However, an endotracheal tube should be considered if imaging by transesophageal echocardiography (TEE) is expected due to client comfort.
If moderate sedation is to be utilized, the practitioner must be certain that airway securement can be done quickly in the event of respiratory or circulatory collapse. Due to the amount of equipment surrounding the clients head, attention should be paid to the environment and maneuverability in the event of emergent intubation (8).
Intra-Operative Complications
This procedure is most commonly performed on a high-risk population; therefore, complications are not uncommon. There are many different types of complications; however, if recognized promptly most can be managed or reversed.
Some complications are caused by improper placement of the new valve; these complications look different based on whether the valve is deployed too high or too low. Valve placement on the aortic side can cause a blockage of the aorta, injury to the aortic intima, or blockage of the coronary arteries. Valve placement too far into the ventricle can interfere with the mitral valve, causing mitral regurgitation, and subsequent pulmonary edema.
Also, pressure on the atrioventricular node can lead to conduction abnormalities and possible heart block. In these cases, the valve is retrieved and repositioned if possible. A transvenous pacer is inserted at the start of the procedure, which is used if the client experiences complete heart block or any other non-perfusing rhythm.
If the surgical valve fails to cover the entire annulus, a paravalvular leak may occur. The leak can usually be corrected by inflating a balloon inside the valve, effectively pushing it outward to create a better seal, if this fails the valve may need to be recaptured and repositioned or replaced with a larger size.
Some complications are systemic such as cardiovascular collapse, shock, stroke, and myocardial infarction. Shock or hemodynamic collapse is always a risk in unrepaired clients with aortic stenosis. The best management is to keep the client within tight hemodynamic parameters, however, if cardiovascular failure ensues, and is irreversible the client should be placed on coronary bypass.
If the ventricle or annulus is ruptured the procedure should be converted to an open heart. Also, if coronary occlusion and subsequent ischemia ensues the procedure should be converted to an open coronary artery bypass grafting (CABG). In the event of an embolic stroke catheter-based retrieval should be attempted.
In the event of a hemorrhagic stroke anti-coagulation should be reversed and treatment with this type of stroke is conservative. Access site complications such as dissection of the artery may require endovascular or surgical repair. Lastly, bleeding complications can be related to systemic heparinization that may need to be reversed (1, 4, 9).

Self-Quiz
Ask Yourself...
- What are two pieces of emergency equipment available in the hybrid operating room?
- What is the most common type of anesthesia used in TAVR?
- What considerations should be made if moderate sedation is chosen?
- What are two intra-operative complications associated with TAVR?
- When should a TAVR be converted to an open-chest procedure?
Post-Procedure Monitoring: Things to Consider
When obtaining report some items to note are: size and type of valve placed, concern of mal-placement or leak, the type of sheath that was used, and the number of vascular access sites the client has. Other inquiries include:
- Was the repair transapical and if so, were any drains placed?
- Were any irregular arrhythmias noted post-deployment?
- Does the client have a transvenous pacer still in place?
- Is the client pacer dependent, if so, what are the settings, and where is the sheath and control box?
- What type of closure device was used?
- Were there any bleeding incidences in the surgery suite? When was the last activated clotting time (ACT) test and what were the results?
- Does the client have any risk factors for bleeding, such as von Willebrand factor deficiency?
- Are any hematomas noted? If there are hematomas what size and how firm are they? If the client has a femoral access as they are at risk for retroperitoneal hematomas- this may manifest as back pain, hemodynamic instability, and bruising along the flank. It is also advisable to mark the borders of the hematoma as a reference since clients can bleed insidiously.
- Is the client having any pain?
- What type of anesthetic was used local, monitored anesthesia care, or general?
- If it was general anesthesia, what type of airway was used?
- Time and dose of last known pain medication and was local anesthetic used at the access site?
- How much vasoactive medication was necessary during the procedure?
- How much fluid was administered intraoperatively?
- Is there a urinary catheter and how much was the urine output during the procedure?
- How much contrast dye was utilized for the procedure?
TAVR Procedure Complications
Most common TAVR complications include (1, 4, 6):
- Bleeding
- Vascular site complications
- Need for permanent pacemaker
- Significant perivalvular leak
- Stroke
- Death
- Acute kidney injury
- Coronary Occlusion
- Valve embolization
Bleeding and Vascular Site Complications
Before the procedure, clients undergo a series of imaging scans to identify potential vascular access sites. The scans included are a left and right heart catheterization and aortography, TTE and TEE, CT and angiography of the chest, and CT of the abdomen and pelvis.
However, it is still estimated that about 15% of clients experience periprocedural bleeds which outranks all other complications. Many of these bleeds are related to the vascular entry site which carries a complication rate of 10-15%.
Proper monitoring of the access site and the 5 P’s (pain, pallor, pulse, paresthesia, and paralysis) helps to identify any occlusion of the artery which is considered a surgical emergency. Most bleeding can be resolved by proper monitoring and intervention by manual pressure applied to the site. However, for continuous uncontrolled bleeding manual pressure should be applied, an ACT should be assessed, and if manual pressure is not adequate, the client will require surgical stenting of the vessel (1, 4, 6, 9).
Acquired Von Willebrand Syndrome
Clients with aortic stenosis are also at high risk to acquire von Willebrand syndrome. This happens because the stenosed aortic valve causes shearing forces and as blood crosses the valve, clotting factors are destroyed which in some cases can lead to an induced Von Willebrand syndrome.
This condition can subsequently cause Heyde’s syndrome which is a gastrointestinal bleed caused in the setting of absent or defective von Willebrand factor. Replacing the valve may decrease the shearing effect, but it takes time for clotting factors to stabilize. The adenosine diphosphate closure time (CT-ADP) is a bedside test that is run much like an ACT which may be useful in helping to identify paravalvular leaks and clients at risk for bleeding complications (9).
Atrioventricular Blockage
Complete heart block is a condition where the electrical signal of the heart is blocked and cannot travel through the atrioventricular (AV) node. This is characterized by a complete dissociation of p-waves and QRS complexes. Clients can also experience different degrees of AV dissociation which may lead to first- and second-degree AV block.
While it is known that AV blockage can occur after TVAR, it is not well understood why this happens. Some studies have attempted to predict which clients are at risk for AV blockage post-TAVR. Studies have shown that larger valve sizes and the Edwards Sapien 3 Valve in particular have a higher rate of AV block.
In another interesting study, clients with a QRS >120s were found to have a 38% rate of permanent pacemaker placement after TVAR, while none of the clients with a QRS ≤120s had any semblance of heart block. In yet another study they found that longer P-R intervals, QRS duration, history of right bundle branch block (RBBB) and pre-existing first- or second-degree AV block were more common in clients that required permanent pacemakers post procedure.
Most incidences of heart block occur immediately after the procedure while the client is in the critical care unit. A thorough look at the client’s pre-operative EKG and cardiac history may reveal the client at risk of needing a pacer post-operatively, allowing the nurse to prepare for placement of a transvenous pacer or utilization of one that is already in place (1, 4, 9).
Stroke
The mechanism by which stroke occurs after procedure is debatable, but both embolic and hemorrhagic strokes may occur after TAVR. However, more advanced catheters and valves lessen the risk of stroke. The risk of stroke does appear to be the same as clients undergoing SAVR, however, one study found that clients who’ve undergone TAVR who experienced a transient ischemic attack (TIA) post-procedure had a lower 1-year survival rate.
Clients who are immediately post-op and in the ICU are at the highest risk of experiencing a stroke or TIA. However, after the immediate preoperative period, the risk of stroke/TIA decreases dramatically. Since it is postulated that debris from the valve is the cause of many neurological events, studies are examining the use of cerebral protective devices such as a filter to decrease the incidence of embolic stroke. Additionally, the use of antiplatelet therapy does seem to minimize the risk of embolic type strokes. A full neurological exam should be performed upon admission per standard practice.
Clients may still be sleepy from procedure at this time, but it is important to assess for a proper baseline since clients undergoing TAVR are at high risk for stroke especially within the first 24 hours at minimum or per hospital policy. Abbreviated neuro assessments may be performed in between full assessments and they should include at minimum an assessment of balance, dizziness, headache, blurred vision, facial drooping, and speech difficulty. Performing regular neurological checks will aid in early identification and treatment of clients undergoing TAVR (1, 4).
Acute Kidney Injury
The development of acute kidney injury after TAVR is associated with a four-fold increase in mortality. Risk factors for AKI after TAVR are hypertension, COPD, pulmonary disease, and blood transfusions. Some hospitals employ a preoperative infusion of acetylcysteine and IV bicarbonate as a protective measure against the contrast dye, but there is conflicting evidence as to whether this actually decreases risk.
Measuring intake and output for clients at risk for AKI are nursing measures that may help with early diagnosis and treatment. Proper fluid management and avoidance of hypovolemia may reduce the risk of AKI (1).

Pain Management and Early Mobilization
Pain management should be titrated to the client’s needs. Ideally, pain medication should be adjusted so that the client is comfortable during early ambulation and remains alert and oriented. Early mobilization is linked to shorter hospital stays and a decreased risk of venous thrombus. Clients with femoral access sites will usually need to lie with the head of the bed ≤15 degree for a minimum of six hours to ensure hemostasis, then they may resume sitting and walking activities.
Clients with other access sites may be able to ambulate sooner dependent upon hospital policy. Clients should make a goal of walking the unit at least 3-4 times a day if possible (1, 3).

Self-Quiz
Ask Yourself...
- What are five points that must be endorsed during a report on a client who had a TAVR procedure?
- What are five common post-procedure complications associated with TAVR?
- What are the five P’s of access site monitoring?
- What is Heyde’s Syndrome?
- What are two risk factors for AKI post TAVR?
- What are three signs that a client may be having a stroke?
- What early treatment is linked to a shorter hospital stay post TAVR?
- In which situation is bedrest prolonged post TAVR?
Discharge Planning
Respiratory problems, infections, and bleeding events are the main reasons clients who’ve undergone TAVR are readmitted. Proper education throughout the hospital stay may help decrease the incidence of these events.
Most clients will be sent home on a regimen of aspirin and clopidogrel for at least six months to prevent thrombus formation of the newly placed valve. Education should be provided for the client on how to monitor their access site for any signs of bleeding and they should be aware that the clopidogrel will put them at greater risk for bleeding.
The client should be instructed on how to keep the incision site clean and how to apply any dressings if necessary. Educate the client making them aware that ambulation along with coughing and deep breathing will decrease the chance of pneumonia and respiratory infections.
Any medication changes should be discussed and the client should go home with a medication reconciliation form stating what dose should be taken and at what time (1).

Self-Quiz
Ask Yourself...
- What medication regimen will most clients have post TAVR?
- What do these medications predispose the client to?
Conclusion
Over the last few years, TAVR has truly revolutionized aortic valve stenosis treatment. Once only used on clients deemed “not a candidate for surgery” due to their high surgical risk, it is now performed on clients with lower risk. The outcomes of TAVR have been favorable. Though there can be risks and complications associated with TAVR, it is a procedure that has become a staple in may facilities. Specially trained staff have been perfecting the procedure and new valves and deployment devices have made it possible to treat different aortic valve anatomies with confidence.
References + Disclaimer
- Avvedimento, M., & Tang, G. H. (2021). Transcatheter aortic valve replacement (tavr): Recent updates. Progress in Cardiovascular Diseases, 69, 73–83. https://doi.org/10.1016/j.pcad.2021.11.003
- Badiani, S., Bhattacharyya, S., Aziminia, N., Treibel, T. A., & Lloyd, G. (2021). Moderate aortic stenosis: What is it and when should we intervene? Interventional Cardiology Review, 16. https://doi.org/10.15420/icr.2021.04
- Claessen, B. E., Tang, G. L., Kini, A. S., & Sharma, S. K. (2020). Considerations for optimal device selection in transcatheter aortic valve replacement. JAMA Cardiology. https://doi.org/10.1001/jamacardio.2020.3682
- Cormican, D., Jayaraman, A., Villablanca, P., & Ramakrishna, H. (2020). Tavr procedural volumes and patient outcomes: Analysis of recent data. Journal of Cardiothoracic and Vascular Anesthesia, 34(2), 545–550. https://doi.org/10.1053/j.jvca.2019.04.016
- Crawford, P. T., Arbor, T. C., & Bordoni, B. (2023). Anatomy, thorax, aortic valve. http://europepmc.org/books/NBK559384
- Généreux, P., Schwartz, A., Oldemeyer, J., Pibarot, P., Cohen, D. J., Blanke, P., Lindman, B. R., Babaliaros, V., Fearon, W. F., Daniels, D. V., Chhatriwalla, A. K., Kavinsky, C., Gada, H., Shah, P., Szerlip, M., Dahle, T., Goel, K., O’Neill, W., Sheth, T.,…Leon, M. B. (2024). Transcatheter aortic-valve replacement for asymptomatic severe aortic stenosis. New England Journal of Medicine. https://doi.org/10.1056/nejmoa2405880
- Katsi, V., Magkas, N., Antonopoulos, A., Trantalis, G., Toutouzas, K., & Tousoulis, D. (2020). Aortic valve: Anatomy and structure and the role of vasculature in the degenerative process. Acta Cardiologica, 76(4), 335–348. https://doi.org/10.1080/00015385.2020.1746053
- Leclercq, F., Meunier, P., Gandet, T., Macia, J.-C., Delseny, D., Gaudard, P., Mourad, M., Schmutz, L., Robert, P., Roubille, F., Cayla, G., & Akodad, M. (2022). Simplified tavr procedure: How far is it possible to go? Journal of Clinical Medicine, 11(10), 2793. https://doi.org/10.3390/jcm11102793
- Mas-Peiro, S., Fichtlscherer, S., Walther, C., & Vasa-Nicotera, M. (2020). Current issues in transcatheter aortic valve replacement. Journal of Thoracic Disease, 12(4), 1665–1680. https://doi.org/10.21037/jtd.2020.01.10
- Perry, T. E., George, S. A., Lee, B., Wahr, J., Randle, D., & Sigurðsson, G. (2020). A guide for pre-procedural imaging for transcatheter aortic valve replacement patients. Perioperative Medicine, 9(1). https://pubmed.ncbi.nlm.nih.gov/33292498/Pujari SH, Agasthi P. Aortic Stenosis. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2023. PMID: 32491560. https://pubmed.ncbi.nlm.nih.gov/32491560/
- Saadi, R., Tagliari, A., Saadi, E., Miglioranza, M., & Polanczyck, C. (2022). Preoperative tavr planning: How to do it. Journal of Clinical Medicine, 11(9), 2582. https://doi.org/10.3390/jcm11092582
- Spears, J., Al-Saiegh, Y., Goldberg, D., Manthey, S., & Goldberg, S. (2020). Tavr: A review of current practices and considerations in low-risk patients. Journal of Interventional Cardiology, 2020, 1–13. https://doi.org/10.1155/2020/2582938
- Vincent, F., Ternacle, J., Denimal, T., Shen, M., Redfors, B., Delhaye, C., Simonato, M., Debry, N., Verdier, B., Shahim, B., Pamart, T., Spillemaeker, H., Schurtz, G., Pontana, F., Thourani, V. H., Pibarot, P., & Van Belle, E. (2021). Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation, 143(10), 1043–1061. https://doi.org/10.1161/circulationaha.120.048048
- Yoon, S.-H., Kim, W.-K., Dhoble, A., Milhorini Pio, S., Babaliaros, V., Jilaihawi, H., Pilgrim, T., De Backer, O., Bleiziffer, S., Vincent, F., Shmidt, T., Butter, C., Kamioka, N., Eschenbach, L., Renker, M., Asami, M., Lazkani, M., Fujita, B., Birs, A.,…Makkar, R. R. (2020). Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. Journal of the American College of Cardiology, 76(9), 1018–1030. https://doi.org/10.1016/j.jacc.2020.07.005
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
