Course
Connecticut APRN Bundle Part 2
Course Highlights
In this Connecticut APRN Bundle part 2, we will learn about various topics applicable to APRNs in the state of Connecticut.
About
Contact Hours Awarded: 30 , including 14 pharmacology contact hours.
Course By:
Various Authors
Begin Now
Read Course | Complete Survey | Claim Credit
Flap Surgery: The Basics
Introduction
Flap surgeries can be a critical treatment for various wounds to provide bulk tissue. It is a tad more detailed than skin grafts, as it involves a circulatory supply from a donor site to a recipient site. It is important to recognize what flap surgery entails, the indications, and types of flap surgeries. Nurses should be knowledgeable on care plans and assessment for flap surgery, positioning techniques, patient education topics, and how to identify possible complications such as infection or flap dehiscence. Are you ready to dive into the interesting course topic of flap surgery?
Flap Surgery: What is it?
Flap surgery involves removing healthy, live tissue from one location of the body and transporting it to another area that needs it for healing purposes. Flap surgeries are commonly used to transfer this healthy tissue to areas of lost skin, fat, muscle movement, and/or skeletal support (9). A tissue flap has its own system for vascularization and does not depend on the recipient’s wound bed to perfuse the donor tissue, which differs from non-vascularized skin grafts (8). Essentially, a flap is tissue with a substantiated blood supply that is transferred from a donor site to a recipient site. If the flap surgery was a party, the damaged host site would send out an invite saying “BYOB- Bring Your Own Blood-Supply!”
The flap continues to be fed by the same blood supply from where it was taken, until new blood vessels grow from the recipient site and the wound heals completely. The recipient site is called the primary defect and the wound that is created by cutting, lifting, or sliding the flap to fill the primary defect is called the secondary defect (8). The base, or pedicle, of the flap is the tissue that remains attached to the skin adjacent to the defect, it contains the vascular supply required for initial flap survival (8).
Surgeons have used skin flaps to repair wounds and tissue damage for centuries. The term “flap” was derived from the Dutch word “flappe” during the 16th century (8). Around 700 B.C., the Sushruta Samhita (an ancient text on surgery and medicine) first documented a technique of reconstructing a large nasal tip defect with a flap of cheek tissue (8). New techniques are constantly being developed to meet various needs. Flap surgeries are used for a variety of wounds from pressure ulcers to breast reconstruction following mastectomy.

Self Quiz
Ask yourself...
- Have you ever cared for a patient following a flap surgery?
- Do you recognize the difference between flap surgery and skin grafting?
- Are you familiar with the vascular structure at deeper skin levels?
- Can you discuss how significant improvements could have been made over the past hundreds of years?
Types of Flap Surgeries
Flap surgeries are classified in the following ways: (9)
- Blood supply
- Type/composition of tissue
- Distance of the healthy site from the recipient tissue
- Locations of donor and recipient tissue
- Movement
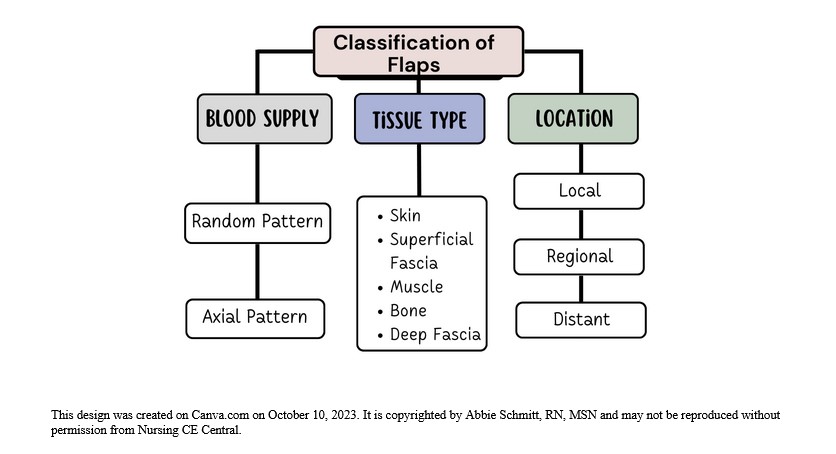
Figure 1: Classification of Flaps
Classification by Blood Supply
Flaps can be named based on the supply of blood. The understanding of the circulation of blood to the donor tissue is critical when describing the type of flap. The terms random and axial are used to categorize the blood supply.
- Random Flaps
- Not based on a specific vessel
- Uses subdermal plexuss (network of blood vessels between the deep reticular portion of the dermis and subcutaneous fat tissue beneath it) (7)
- Axial Flaps
- Single, direct cutaneous artery in the axis of the flap
- Named according to the pathway
Classification by Tissue Type
Flaps can be named according to their composition. The tissue composition may be skin, fascia, muscle, bone, nerve, cartilage, or a combination. Fascia is the thin lining of connective tissue that surrounds and holds each blood vessel, bone, nerve fiber, and muscle in place (7). Cutaneous refers to the layers of skin. Pedicle flaps are those that are still attached to the original site and the other end is moved to cover the recipient area; a free flap is an area of tissue completely removed from one part of the body and surgically placed in another area (8).
Common flaps: (5)
- Skin Flap: Skin and superficial fascia
- Fascio-cutaneous Flap: Skin and deeper layer of deep fascia
- Fascial Flap: Deep fascia only
- Muscle Flap: Muscle only
- Myo-cutaneous Flap: Muscle and skin
- Osteomyocutaneous Flap: Muscle, bone, and skin
- Bone Flaps: Bone (vascularized)
- Innervated Flaps: Flaps that contain a motor or sensory nerve and function
Fascio-cutaneous Flap
This flap includes the skin, subcutaneous tissue, and the underlying deep fascia (5). The musculocutaneous perforators or direct septocutaneous branches of major arteries act as vascular supply (5). Perforator flaps are named based on their location, arterial supply, or the muscle of origin. The indications for fasciocutaneous flaps are based on its advantages of being more simple, reliable, thin, and easily mobilized (8). These flaps can come from many potential donor sites (8).
Muscle Flap
Muscle tissue can be used as donor tissue in flap surgery. Surgeons may utilize the benefits of flap surgery in wound closure following major surgeries. For example, median sternotomy (vertical inline incision through the sternum of the chest) is the most commonly used approach for cardiac surgery (6). Cardiac surgeons face the risk of deep sternal wound infections following surgery, which is associated with significant morbidity and mortality rates. The use of soft tissue flaps for sternal closure is helpful for patients with extensive tissue deficits after debridement (6). It can be used for immediate or delayed closure. Options for donor tissue for sternal flap closure include the pectoralis major, rectus abdominis, and latissimus dorsi muscles, or an omental flap (6).
Remember, flaps are transplanted with blood supply intact, so it’s important to know the supply. For instance, if tissue from the pectoralis major muscle is used, the nurse must recognize that this muscle’s primary and secondary blood supply is the thoracoacromial artery and perforators from the internal mammary artery (6).
Musculocutaneous Flap
This type of flap, which includes muscle and skin layers, is often used when the area to be covered needs more bulk and an increased blood supply. Musculocutaneous flap surgery is frequently used to rebuild a breast after a mastectomy (5).
Bone Flap
A bone flap is comprised of bone with a vascular supply. An example of this flap surgery is for a surgical site infection (SSI) following a craniotomy; in this procedure, operative debridement occurs, and the bone flap is removed, cleaned, and replaced (4). An alternate therapy for this is titanium cranioplasty (implant instead of native bone flap), which has similar outcomes.
Classification by Location and Movement
- Local flap: Donor tissue is located next to the area receiving the tissue; the skin remains attached at one end to allow the blood supply to be left intact (5).
- Regional flap: Donor tissue is a section that is attached by a specific blood vessel.
- Distant flap: Donor and recipient tissues are distally located from each other. This flap surgery involves detaching and reattaching skin and blood vessels from one site of the body to another site; microsurgery is used to connect the blood vessels (5).

Figure 2: Example of Flap Type
The movement of the flap is also used to describe flap surgery. You may hear terms such as advancement, sliding, rotation, and pivotal. Sliding flaps is when the tissue is moved or "slid" directly into the adjacent defect without "jumping" over other tissue (5). Advancement flaps are considered simple movements for local flaps and fall within the group of sliding flaps. Pivotal (geometric) flaps include rotation, transposition, and interpolation (5). Local, random pattern flaps are common for the reconstruction of cutaneous defects.
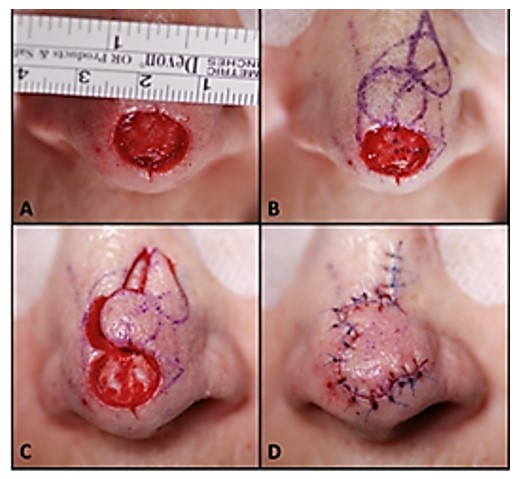
Image 1: Image of a local flap surgical procedure to cover nasal tip defect/wound (9)

Self Quiz
Ask yourself...
- Are you familiar with the differences in skin, muscular, bone, and nerve tissue?
- Can you think of benefits of using a local, pedicle flap over a free flap?
- Can you discuss how fasciocutaneous flaps may have more advantages and more potential donor sites?
- Are you able to recognize the general location and complexity of a surgical note that says “local, random, skin flap with pivotal manipulation at midline of forehead”?
Indications for Surgery
There is an incredible breadth of possibilities for flap reconstructive surgery, from small, skin-only defects to large, multi-tissue defects. There is a wide range of etiologies, such as traumatic, oncological, and congenital (9). The transferred tissue flap can contain multiple types of tissue, including skin, muscle, nerve, fascia, and bone (9). The larger the volume of tissue transferred, the greater the need for perfusion. A common indication is the need for a large bulk of tissue. Flaps are helpful when wounds are large, complex, or need large amounts of tissue for closure.
General Indications:
- Protection of the greater vessels
- Correction of congenital defect
- Abdominal wall reconstruction
- Deep, gaping wounds
- Reconstruction after tumor excision
- Trauma
- Debridement procedure to remove infected or necrotic tissue
- Venous ulcers (non-healing)
- Pressure ulcers (non-healing)
- Breast reconstruction
- Rhinoplasty
- Scar Revision
- Skin Cancer
- Burns
Each type of wound has unique indications. Commonly, skin flap surgery is required when a wound is too big for the edges to be brought together directly, so the flap covers the area and depth of the wound (10).

Self Quiz
Ask yourself...
- Do you have experience in caring for a patient with a deep, healing wound?
- Have you ever cared for a patient following a tumor removal?
- Can you discuss why debridement of the recipient site is essential prior to flap placement?
- Can you name various methods of wound closure? (ex: sutures)
Risks versus Benefits
Flap survival depends on factors of blood flow, angiogenesis (formation of new blood cells), vascularization, edema, wound closure tension, postoperative complications (hematoma/seromas) and infection (8). Before the initial incision, the flap is fully vascularized and viable, but once the flap is raised, it is immediately ischemic. The tissue can survive up to 12 to 13 hours of avascularity at 37°F and many research studies have proven it is viable even longer (8). This time is invaluable to preserve the tissue. Sufficient blood flow through attachment of the base of the flap is essential in the initial 24 to 48 hours after surgery (8). There is a risk for loss of tissue with no meaningful contribution to the needed area, along with a new wound. This risk reminds me of a neighbor who once removed carpet from a closet to patch carpet in a bedroom, only to find the cutting was too small and they were left with two gaping carpet holes.
There is also risk for bleeding, infection, or necrosis at both sites. A recent study found that more than 27% of patients will experience a minor complication (wound dehiscence, infection, fistula, and donor-site problems) after surgery, and 6% of patients will suffer a major complication (flap failure, pneumonia, and cerebrovascular accidents) following surgery (11). Chronic flap complications can also be aesthetic in nature; include scarring, contracture, color/texture mismatch, and lack of hair growth. Patients may experience pain or numbness at the sites on a chronic basis as well (9).
Most flap surgeries are considered safe with a low complication rate, and surgeons report that flap surgery is not avoidable in certain circumstances. However, the surgery preparation itself and anesthesia presents considerations for elderly patients or those with heart disease, uncontrolled diabetes, smokers, or bleeding disorders (2). Nutrition is a key factor in these surgical procedures. Poor nutritional status has been linked with a greater incident of negative outcomes (11). The healthier the patient is before surgery increases, the chances of reduced complications, so glucose control and weight management are examples of risk reduction strategies.

Self Quiz
Ask yourself...
- Do you feel confident with patient education methods for explaining risks versus benefits?
- Can you name reasons informed consent for flap procedures is not only required, but ethical?
- Do you have experience in educating patients on diabetes and the importance of glucose control in wound healing?
- Are you familiar with your facility’s medical literature database?
Preparing for Surgery
Patients undergoing flap surgery need an abundance of education on what to expect throughout this procedure. There are many opportunities to optimize patient outcomes before going to the operating room. Preoperative education, for example, has been suggested to have an important, positive effect on clinical outcomes (11). Many patients are also experiencing other issues, such as cancer diagnoses, poor circulation, comorbidities, bed sores, among others. Taking time to holistically prepare each patient is essential.
Addressing Comorbidities and Other Conditions
Multiple studies have found an increased surgical complication rate in patients with diabetes mellitus, older age, female gender, malnutrition, anemia, and nicotine intake (11). Prior to surgery, the goal is to improve and optimize the modifiable conditions as much as possible, for instance, reduction in nicotine or improvement in glucose control and anemia. Further, patients with advanced cancer can have hypothyroidism affecting postoperative healing if left uncorrected (11). Non-modifiable factors such as a history of radiotherapy, age, advanced cancer stage, or chronic kidney disease, cannot be altered prior to surgery, but can guide care planning and education following the surgery.
Adequate nutrition before and after flap surgery has been demonstrated in numerous studies to improve outcomes. An estimated 35% of patients with head and neck cancers present in a state of malnutrition, and the Enhanced Recovery After Surgery (ERAS) Society recommends that all patients undergo a comprehensive preoperative nutritional assessment and consult with nutritionist (11). Improved nutrition status can hopefully yield enhanced wound healing problems and reduction in risk of infection.
Lab assessment is key to preparation before flap surgery. An example is assessment for anemia prior to surgery. Patients who are anemic at the time of free flap surgery have been found to have poor outcomes (11). Remember, hemoglobin transporting the oxygen to the sites of flap insertion and removal is vital to the survival of the flap. Preoperative hemoglobin values below 10 g/dL have historically been a significant predictor of flap failure and thrombosis (11). A hematocrit level of 30 to 40% with normovolemic hemodilution is ideal to optimize patient outcomes.
Blood transfusions during or following surgery impacts flap success as well. Transfusion can increase blood viscosity and immunosuppression, thus leading to decreased blood flow and flap compromise from poor perfusion (11). Studies also show a link between blood transfusions and increased wound infections (11). Steps and treatments should be taken prior to surgery to improve anemia or blood component abnormalities to give these patients a greater chance for successful flap surgery.
Preoperative considerations should include: (9)
- Patient age
- Diabetes status
- Smoking history
- Atherosclerosis
- Peripheral vascular disease
- Steroid use
- Previous surgeries
- The extent and location of the defect
Providing Preoperative Instructions
A lack of education, difficulty in understanding complex medical information, fear and anxiety about the surgery, and language barriers are some of the challenges a patient for surgery may have. Patients may also have limited access to reliable health resources or be unable to recall important information due to stress or preoperative medications. As a result, they may not be fully informed about the surgical process, potential risks, and postoperative care.
Preoperative education list:
- Assess the patient’s level of understanding.
- Each facility should have a preoperative teaching program with specific content on surgery, but you must assess if the patient understands this information.
- Review specific pathology and anticipated surgical procedure.
- Verify that consent has been obtained / signed.
- Informed surgical choices and consent for the procedure is required, not only a signature.
- Use resource teaching materials, and audiovisuals as available on flap surgery and implement an individualized preoperative teaching plan.
- Preoperative or postoperative procedures and expectations, output (urinary and bowel) changes to expect following surgery, dietary considerations, anticipated intravenous (IV) lines and tubes (nasogastric [NG] tubes, drains, and catheters).
- Preoperative instructions: NPO guidance prior to surgery, shower or skin preparation, medications to take and hold, prophylactic antibiotics or anticoagulants, anesthesia premedication.
- Discuss postoperative pain management plan and options.
- Some patients may expect to be pain-free or are hesitant to take narcotic agents.
- Provide education and encourage practice of coughing and deep breathing.
- Confirm and recheck the surgery schedule, patient identification band, chart, and signed operative consent for the surgical procedure.
- Offer pastoral spiritual care or counseling.

Self Quiz
Ask yourself...
- What are some examples of pertinent laboratory values to assess prior to surgery?
- What are normal values of hemoglobin and hematocrit?
- Do you consider pain management a “one size fits all” care plan?
- Are you familiar with pastoral and spiritual counselors and supportive resources within your organization?
Post-Operative Considerations
This section will cover assessment, drains, positioning, and negative pressure wound therapy during the post-operative period.
Assessment
Frequent monitoring of free flaps in the acute postoperative period is important. It is strongly recommended that flap assessments are performed at least hourly for the first 24 hours postoperatively, then continued at a reduced increment for the duration of the patient's hospitalization (11). Each facility should have standing orders for flap assessment based on the directive of the surgeon or regulatory body.
In addition to conventional assessments of flaps (physical exam of flap warmth, turgor, capillary refill, color, and Doppler assessment of the vascular pedicle), many adjuncts have been developed including implantable Dopplers to assess blood flow (11). Assessments should include a head-to-toe physical assessment and a focused wound assessment. A focused cardiovascular (circulation and perfusion) and integumentary assessment is appropriate. Review of lab work indicative of healing status, hemodynamics, and infection should be a priority. Standard post-operative assessments following anesthesia, such as respiratory and orientation, should be performed according to facility protocol.
Assess circulation of the flap:
- Color of the flap (dusky, blue, pink, pale)
- Warmth
- Dry/Intact? Leaking Fluids?
- Changes in size
- Edema
- Indications of hematoma (sutures over the flap pulling apart, or palpable crepitus beneath the skin)
Assess amount and type of exudate (drainage): (3)
- Amount (scant, small/minimal, moderate, or large/copious)
- Color and thickness: (7)
- Sanguineous: fresh bleeding
- Serous: clear, thin, watery plasma
- Serosanguinous: serous drainage with small amounts of blood noted
- Purulent: thick and opaque; color can be tan, yellow, green, or brown (this is an abnormal finding and should be reported to physician or wound care provider)
Use of Doppler to assess deeper circulation of flap: (11)
- Color duplex ultrasound
- Near-infrared spectroscopy
- LASER Doppler flowmetry
- Implantable Doppler (useful for buried flaps)
Assess circulation distal to the flap:
- Capillary refill
- Color
- Temperature
- Pulses
- Edema
Drains
Drains may be used for removal of fluid around both the donor and recipient surgical areas to enhance healing (7).
Patients may have the following drains after flap surgery:
- Jackson Pratt (JP) drains
- JP drains are closed-suction devices that remove fluids from the surgical sites.
- JP drains contain a flexible bulb that has a plug that can be opened to remove collected fluid.
- Each time fluid is removed from the JP drain, the nurse should squeeze the air out of the bulb and replace the plug before releasing the bulb.
- This suction creates a vacuum that pulls fluid into the drainage tubing and bulb.
- Penrose drains:
- A Penrose drain is a soft, flexible tube inserted into the surgical site that drains fluid away from the wound bed (7).
- Nurses should assess the drain and express fluid when appropriate to prevent accumulation.
Positioning
The goal of positioning following flap surgery is to promote and improve tissue perfusion. Positioning and elevation of the flap recipient will promote venous return and reduce fluid accumulation to improve tissue perfusion. Activity, exercise, and repositioning improve tissue perfusion. Massage of the erythematous area is avoided because damage to the capillaries and deep tissue may occur (10). Patients should never lay on the wound and extremities should never “dangle” (if the donor or recipient site is on the extremities). Positioning may require creativity when there are multiple drains, NG tube, wound therapy devices, IV tubes, and multiple dressings. Positioning should be free of restrictive clothing and flap sites should be visible for assessment of dressings. It is imperative for the surrounding skin to be sanitary and free of debris.
Negative Pressure Wound Therapy
Negative pressure wound therapy (NPWT) is typically used for soft-tissue salvage after the development of complications after flap surgery. For example, NPWT may be applied if an infection occurs in the donor or recipient flap. Immediate postoperative application of NPWT over the flap coverage is not as common (2). However, nurses should be aware of this treatment, the application, and its mechanisms of action.
NPWT is also known as a wound vac. NPWT uses sub-atmospheric pressure to help reduce inflammatory exudate and promote granulation tissue in an effort to enhance wound healing (4). The idea of applying negative pressure therapy is that once the pressure is lower around the wound, the gentle vacuum suction can lift fluid and debris away and give the wound a fighting chance to heal naturally. NPWT systems consist of a sterile foam sponge applied to the wound bed, a semi-occlusive adhesive cover, a fluid collection system or cannister, and the suction pump (1). The foam sponge is applied to the wound and covered. A fenestrated tube is embedded in the foam, the wound is sealed with adhesive tape to make it airtight, and the machine delivers continuous or intermittent suction, ranging from 50 to 125 mmHg (1).
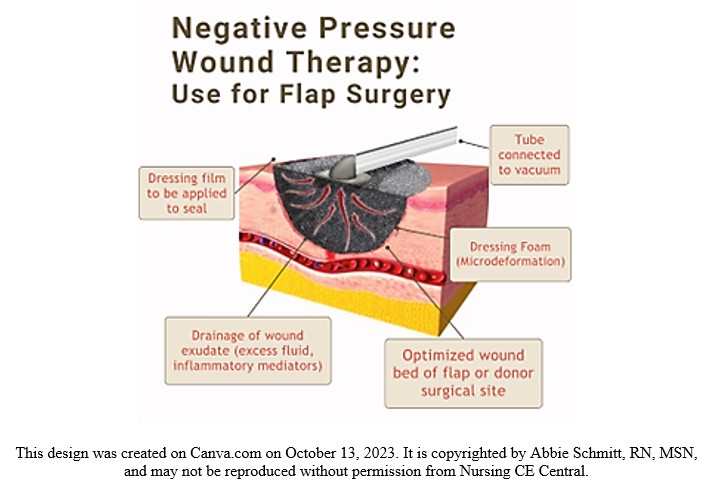
Figure 3. Negative Pressure Wound Therapy Visual

Self Quiz
Ask yourself...
- Have you ever completed a focused wound assessment that resulted in abnormal findings that were anticipated?
- Can you name examples of pertinent laboratory values to assess following general surgery?
- Have you ever used or witnessed the use of a doppler in assessment of proper circulation?
- Can you describe the importance of positioning in tissue perfusion?
Home Care and Patient Education
Educating clients and caregivers about wound care and skin integrity empowers them to actively care for their flap sites. With proper wound cleaning, dressing changes, and preventive measures, individuals can confidently perform their own self-care and enhance the healing process. The use of pamphlets, printouts, websites, and referrals to specialists will give the patients a stronger foundation of knowledge.
- Wound Assessment
- Teach the patient and caregiver about skin and wound assessment, ways to monitor for signs and symptoms of infection, complications, and proper healing.
- Signs of Infection
- Signs of a localized wound infection include redness, warmth, tenderness, and abnormal purulent drainage around the wound.
- Importance of proper nutrition, hydration, and methods to maintain tissue integrity
- Adequate caloric intake and balance of protein and essential vitamins has been shown to improve the healing of flaps (3).
- Dressing changes, wound cleansing, and hand hygiene
- Methods to prevent skin breakdown
- The flap surgery is often for the treatment of a deep pressure wound, so education is needed on impaired skin integrity due to friction.
- Common areas: Sacrum, heels, elbows.
- Avoidance of raising the head of bed often, causing weight to be applied to sacrum.
- Importance of turning, mobility, and ambulation
- Pain management
- Medications
- Heat/cold therapy applications and precautions
- Negative Pressure Wound Therapy devices (wound vac)
- Home healthcare is applicable for these devices and should be changed by a certified individual, but patients should be aware of basic care and troubleshooting.
- Drain maintenance
- Showering restrictions
- Reduction of stress and tension at wound site
- Avoid constipation, strenuous movements, and lifting
- Restrictions vary for location of flaps and per surgeon instructions
- Follow-up appointments

Self Quiz
Ask yourself...
- Can you describe assessment findings that indicate infection after flap surgery?
- Are you familiar with various drains such as JP drains?
- What do you think are some fears among patients going home or to a long-term care facility after flap surgery
Flap Dehiscence or Loss
Flap dehiscence is a complication in which the incision made to either the donor or recipient site reopens. A flap loss refers to the flap not re-establishing blood flow and surviving, leading to necrosis of the tissue. As we mentioned earlier, the survival of the flap is impacted by blood flow, new vascularization and tissue formation, edema, wound closure tension, postoperative complications (hematoma/seromas), and infection (8).
Prevention Strategies
Nurses should apply their basic knowledge on causes of wound dehiscence. Factors that influence dehiscence risk include the ability to synthesize collagen, strength of suture material, closure technique, and stress on the incision, such as coughing, strenuous movement, or obesity (7). Consider areas that have greater stress and tension, such as the abdomen. Dehiscence is most common following abdominal flap surgeries (7). NPWT has been shown to be a great preventative measure for wound dehiscence as studies found a roughly 50% reduction in stress and tension at the incision site with its use (7). This reduction in stress is attributed to the reduction in subcutaneous fluid accumulation and enhanced healing time.
The location of the flap surgical sites will impact prevention. For example, immobilization and stabilization devices are unique to sites such as the abdomen, chest, extremities, and sacral region. Infection prevention measures for wounds are essential for nursing care. Incisions from flap procedures also have a higher chance of opening if the wound becomes infected (7). Prevention of hematomas is also meaningful, including the use of blood thinners. However, the safety precautions for blood thinners is different for each client.
Management and Treatment
It is estimated that 80% of free flaps can be salvaged if dehiscence or compromise is recognized early enough (11). As we mentioned, it is more common for flaps that are removed from (or applied to) the abdomen to open. The following terms describe the depth:
- Superficial dehiscence: the skin wound alone opens, but the rectus sheath remains intact.
- Full thickness dehiscence: the rectus sheath fails to heal and “bursts,” with protrusion of abdominal content.
- This commonly occurs secondarily to intra-abdominal pressure (example: ileus) or poor surgical technique.
The treatments for flap compromise will be determined by the provider once a cause is identified. Treatment may be a return to the operating room for additional surgical intervention or a simple evacuation of hematoma through suctioning.
Recovery
The focus of recovery is the healing and thriving of the flap site and surgical wounds. The time of flap healing varies, and some may heal much quicker than others. There are four phases of wound healing to recognize: hemostasis, inflammatory, proliferative, and maturation (7).
- Hemostasis. This phase begins immediately after surgery when platelets release growth factors that alert various cells to start the repair process.
- Inflammatory. This process involves vasodilation so that white blood cells in the bloodstream can move into the wound to begin cleaning the wound bed. Signs include edema and erythema.
- Proliferative. This phase generally begins a few days after the injury and includes capillary repair and growth, granulation tissue formation, collagen formation, and wound contraction (7).
- Maturation. During this phase, collagen continues to be created to strengthen the wound and fill in the wound gaps.
The healing process can be enhanced in many ways, including nutrition therapy, topical agents, compression therapy, and hyperbaric oxygen therapy (HBOT). The recovery process for flap surgery will include management of pain and discomfort, disturbance of body image, impaired skin integrity, swelling, bruising, and gastrointestinal upset.
Outcomes for recovery should be measurable and achievable. Each patient will have unique recovery goals, integrating comorbidities and psychosocial aspects.
Examples of Patient Outcomes following flap surgery: (3)
- Patient safety: Patient will be able to attain safety by maintaining intra- and extra-cellular environment.
- Healing of wounds: Wounds should heal properly, complications will be prevented or maintained
- Management of pain
- Prevention of further damage or skin breakdown

Self Quiz
Ask yourself...
- How would you describe the process of healing?
- Can you name some underlying causes of flap dehiscence?
- What are some strategies to prevent infection of the surgical site?
Conclusion
As discussed throughout this course on flap surgery, nurses are a key team member in the care and survival of flaps. Hopefully you now understand what flap surgery is, the indications, and types of flap surgeries. Critical knowledge includes pertinent assessment, drain or NPWT management, patient teaching, and prevention and management of possible complications.

Self Quiz
Ask yourself...
- Can you name the various ways to classify flaps?
- Why do you think it’s important to classify flaps according to their blood supply?
- What do you think the reason is for flap surgeries following mastectomies and breast reconstruction?
- Can you name various indications for flap surgery following a burn?
- What are comorbidities that may impact wound healing?
- Can you name common risks of surgeries?
- Can you think of reasons why elderly patients may have poor outcomes following flap surgery?
- Can you identify modifiable and non-modifiable pre-operative considerations?
- What do you think are some common fears and uncertainties among patients prior to flap surgery?
- Can you name teaching topics for a patient who is scheduled for a flap surgery?
Diabetic Ketoacidosis Management
Introduction
Diabetic ketoacidosis is considered one of the most life-threatening complications of diabetes mellitus. More importantly, it is also one of the most preventable complications of diabetes. Through proper education and empowerment of persons with diabetes to self-manage this chronic medication condition, the overall mortality rates associated with this complication have steadily declined in the United States. An interdisciplinary team approach (including medical providers, social workers, case managers, and community resources) has been proven to reduce recurrences of DKA in vulnerable populations. (2)
Definition
DKA, or diabetic ketoacidosis, is defined as the potentially life-threatening medical condition that occurs in people with diabetes. While it usually occurs in persons with type 1 diabetes mellitus, who are dependent on daily insulin injections, it may also occur in individuals with type 2 diabetes for a variety of reasons (underlying physiologic stress, such as an acute infection or trauma, or uncontrolled blood glucose levels and missed routine diabetic medications).
In an acute case of diabetic ketoacidosis, the body is not producing enough insulin to move glucose into the cell for energy, and the liver then begins to break down fat for fuel instead, producing ketones. This buildup of ketones in the body results in ketoacidosis. Left untreated, diabetic ketoacidosis can lead to a diabetic coma and eventual death. (3)

Self Quiz
Ask yourself...
- As you begin this course, think about the diabetic patients you have cared for in your professional career.
- Do you have family or friends in your life that have been diagnosed with diabetes?
- What are your concerns over their self-management ability of this chronic medical condition?
- What areas of diabetes self-management do you consider the highest priority when you are delivering patient discharge instructions?
Epidemiology
Epidemiology is the study of how often a disease process occurs in different populations. By studying the rates of occurrence, epidemiologists are able to evaluate treatment options and develop long term strategies to lower the risk of ongoing or recurrent disease related episodes.
Diabetic ketoacidosis is currently a leading cause of both morbidity and mortality in children with Type 1 diabetes. It usually occurs at the time of the initial diagnosis in as much as 30-40 percent of the children in the United States alone. In children living with a confirmed Type 1 diabetes condition (previously diagnosed), these percentages decrease to average rates of 6-8 percent annually.
The drastic reduction of such occurrences is believed to be directly related to ongoing patient and family education and medication adherence. Diabetic ketoacidosis is potentially life-threatening, but it is for the most part, also preventable. Throughout this educational offering, key components of patient education in diabetic self-management, including reducing the risk of diabetic ketoacidosis, will be discussed (4) (5).
By comparison, other countries, challenged by annual income, healthcare access, cost management, and food insecurity, do not fare so well. Various studies were funded by the Leona M. and Harry B. Helmsley Charitable Trust, and the Juvenile Diabetes Research Foundation Ltd. Several countries included in these studies were deemed “LLMIC” (low and low middle-income countries). Countries, including Haiti, Ethiopia, Senegal, Nepal, and Tanzania, to name a few were found to have inadequate supplies, medications, and equipment to both initially diagnose, and successfully manage diabetes mellitus long term. Critical items necessary for the treatment and stabilization of acute diabetic ketoacidosis were in even shorter supply. These barriers to treatment resulted in delayed or missed diagnosis, increased overall complication rates and premature deaths.
“Evidence from single-center studies suggest that DKA in new-onset T1D is more common in LLMICs compared to upper and upper-middle income countries, with rates ranging from 62.2 to 77.1% in Nigeria, 69.8% in South Africa, and 92.1% in Sudan. In comparison, in upper and upper-middle income countries in North America and Europe the rates range from 14.7% (Denmark) to 42.0% (France”). (6)
Ongoing education of healthcare professionals and patients/families alike, coupled with availability of and easy access to self-management medications, and monitoring equipment, positively affect DKA related health outcomes and quality of health and well-being.
The development of insulin delivery systems (insulin pumps) has further positively impacted the rate of DKA occurrence. Patient comfort, ongoing education, streamlined medication delivery and enhanced monitoring systems have afforded patients with diabetes a better understanding of their condition and empowered them to successfully self-manage their health conditions. While reported rates of DKA in previously diagnosed persons with T1DM were 6.3% in one study, that number decreased to 2.2% at 3 years out.
Ongoing improvements in closed insulin delivery systems medication continues to improve (lower) DKA occurrence rates, when compared to those previously using multiple daily injection therapy. The development of continuous glucose monitoring (CGM) devices, in addition to insulin delivery systems, provides for early detection and treatment of both hypoglycemia and hyperglycemia. The addition of remote app devices further allows constant monitoring and two-way communication between patients, family members, and even healthcare providers.
Sadly, the population identified as being at highest risk for DKA is that of children who are uninsured/underinsured, lacking the insurance coverage for many closed delivery medication systems as well as specialty care (pediatric endocrinology) provider access.
The acute complications associated with DKA account for a high percentage of premature deaths in T1DM patients under the age of 30 years old. (7) Given these statistics of prevalence and incident rates, DKA is an ever-increasing global concern which is best addressed and managed through ongoing, patient specific disease management education.
The prognosis for DKA worsens in the presence of coma, hypotension and in the presence of severe (chronic and acute) comorbidities. Yet, with early identification, ongoing education, and improved glucose monitoring/treatment options, DKA, often life threatening, is also highly preventable. The goal, therefore, is to ensure all patients with diabetes mellitus are given equal opportunity to access both the education and materials necessary to successfully monitor their health condition. (8)
Pathophysiology
Diabetic ketoacidosis occurs when the body is under stress and responds with an increase in catecholamines, cortisol and growth hormones. The release of such hormones decreases the ability of insulin, further increasing insulin resistance and resulting in serum hyperglycemia. Without cellular glucose for energy the body then begins to break down fat and protein for energy, resulting in increased levels of serum ketones. The combination of hyperglycemia and ketosis, as well as dehydration and various electrolyte imbalances, form the basis of diabetic ketoacidosis. (9)
While it is believed that the omission of insulin (nonadherence/ noncompliance, or mechanical failure of insulin delivery systems) accounts for the largest percentage of DKA admissions, other factors may be responsible for the development of this condition. Any disease process that increases insulin resistance, impairs insulin secretion, or interferes with carbohydrate metabolism may contribute to the onset of acute diabetic ketoacidosis in a vulnerable, health compromised patient.
Clinical Signs and Symptoms
Diabetic ketoacidosis is caused by the underlying presence of hyperglycemia, ketoacidosis and ketonuria. Early signs and symptoms may include any of the following:
- Generalized weakness and fatigue.
- Nausea and vomiting
- Diffuse abdominal pain.
- Decreased appetite and anorexia.
- Decreased/ altered levels of consciousness, such as mild disorientation and confusion.
- Dry skin and mucus membranes and decreased perspiration
- Tachycardia (increased heart rate) and tachypnea (increased respiratory rate)
- Acetone/ketone smell on breath
- Significant weight loss (usually a rapid onset in the newly diagnosed Type 1 diabetes mellitus patients)
- A patient history of noncompliance with prescribed insulin therapy (due to coexisting medical issues in which patient may have intentionally stopped insulin due to decreased food/fluid intake), costs factors (unable to afford prescribed therapy) and missed insulin doses (mechanical failure of the patient’s current insulin delivery system).
Additional signs and symptoms may be present, related to the patient’s age. While an adult patient would be able to verbalize symptoms, a child may not be able to do so, especially in cases where the diagnosis of T1DM is done during their initial presentation to an emergency department for suspected DKA.
In all cases, there may be other factors (illness, injury, medication side effects) that cause DKA to occur; thus, thorough examination and diagnostic testing must be done in all cases prior to initiation of treatment. Likewise, discharge planning and ongoing follow-up care must be patient specific to address behaviors and treatments required for optimal health maintenance.
Teens/Young Adults
In the teenager/ young adult population, the following symptoms may occur: (11)
- Increases in urination, thirst, and appetite.
- Unintentional/ unexplained weight loss despite increases in food and fluid intake.
- Changes in energy level (increased fatigue)
- Vision changes
Please note that normal growth and development stages/patterns in a teenager/young adult will influence glucose metabolism (related to hormone levels).
Young Children
In the young children’s population, symptoms usually strike suddenly and, unlike the adult population, are usually not related to a specific lifestyle or dietary practice. Most children present with the following symptoms:
- Increased urination
- Increased thirst
- Fatigue
- Vision problems (blurred vision)
- Acetone/ketone “fruity smell” on breath
- Unexplained weight loss, often despite appearing to eat (and drink) more.
- Changes in mood and behavior
Infants/Toddlers
In the infant/toddler population, symptoms may present as follows:
- Increased food and fluid intake (always appearing thirsty despite normal fluid intake)
- Frequent urination (in the potty-trained child, this may present as a new onset of bed wetting behaviors)
- Increasing fatigue and changes in normal activity levels
- Unexplained weight loss despite increased food and fluid intake
- Increased occurrences in diaper rashes (suspected increase in yeast infection)
- Fruity/acetone smell to breath
- Unusual behavior (child specific)


Self Quiz
Ask yourself...
- Your patient with DKA appears to be “noncompliant” with his prescribed insulin therapy. What factors may be contributing to his failure to take medication as directed?
- What nursing interventions can be done with/for this patient to increase adherence to his current medication regimen?
- Unexplained weight loss in a young adult may indicate diabetes. What other medical conditions could be causing unexplained weight loss in this age group?
- How would you address these concerns with your patient/ their family members?
Etiology
Etiology: Causes of Diabetic Ketoacidosis
Hyperglycemia and low insulin levels lead to diabetic ketoacidosis. Common causes include the following:
- Acute illness, altering a person’s intake of food or drink, makes glucose management more difficult. This is a two-fold situation. The person with diabetes, recognizing the change in their normal food/fluid intake may also choose to intentionally decrease/skip their routine diabetic medications to avoid episodes of hypoglycemia.
- Insufficient levels of insulin due to the demands of normal growth and development patterns in children and young adults.
- Missed insulin doses (intentional decision to take inadequate doses, inadvertently held doses, inaccurate dose amounts, clogged insulin pump tubing).
Other causes of DKA, unrelated to insulin dose administration, are thought to be related to increased stress levels (inflammation/ infection) and normal hormone disruption, physiologic stressors. Persons with Type 2 diabetes may experience DKA due to prolonged, untreated hyperglycemia. (12), (13)
- Myocardial infarction
- Neurological stroke
- Motor vehicle accident with physical injuries (inflammatory response to blunt force/penetrating trauma)
- Abuse of alcohol and illegal drugs
- Medication side effects (diuretic and corticosteroid *use) see below
- Severe or prolonged illness (such as pneumonia and urinary tract infection/ urosepsis/wound infections)

Self Quiz
Ask yourself...
- Why do you think that the number of diabetes cases continues to rise worldwide, despite advances in medication and related treatment options?
- How do you think the healthcare industry can better address diabetic patient education?
- What factors do you think negatively affect the overall health and well-being of persons with diabetes (lack of care, knowledge deficit, health literacy, access to care, costs of care)?
- What can you do as a healthcare professional to improve the health outcomes of patients with diabetes?
Etiology: Precipitating Factors
Common precipitating factors for diabetic ketoacidosis include the following (14):
- Poor compliance with prescribed insulin therapy (intentional, nonintentional)
- Infections (especially T2DM in the elderly/ adult population)
- Newly diagnosed diabetes (especially T1DM in the pediatric/juvenile population)
- Physiologic based stressors, including coronary syndrome, cerebral vascular accidents, ischemic injuries, shock like states, chronic alcoholism, illicit drug use and certain antipsychotic medications.
Etiology: Diabetic Ketoacidosis and Corticosteroid Use
Diabetic ketoacidosis is related to long term corticosteroid usage. yperglycemia has been reported in a large percentage of patients who are using corticosteroids long term, often as high as “64-71%”. The elevated glucose levels combined with the ongoing physiologic stressors warranting use of these medications, increases the risk of DKA. The benefit/risk of using these medications long term must be assessed, especially in patients with pre-existing metabolic risk factors. Ongoing patient monitoring is essential to lower the risk of long-term complications. (15) (16).
Risk factors that “may” increase the likelihood of developing type 2 diabetes after long term steroid usage include the following:
- Overweight (BMI 25.0 -29.9 percent) / obesity (BMI 30 percent or above)
- History of gestational diabetes
- History of polycystic ovarian syndrome
- History of family members with type 2 diabetes

Self Quiz
Ask yourself...
- Your patient, who is recovering from an acute myocardial infarction, has been started on insulin therapy for hyperglycemia. She is adamant that she is “not diabetic” and refuses to take insulin injections. How would you explain to this patient the connection between physiologic stress and hyperglycemia?
- What patient education, regarding insulin and hyperglycemia, would be appropriate for this patient?
- What follow-up care would be appropriate for this patient?
- Would this patient benefit from a referral to a diabetes education/management program at this time?
Treatment
Emergency Treatment
The initial or emergency treatment of diabetic ketoacidosis may require complex, frequent monitoring, often necessitating an intensive care admission. The following generic guidelines refer to intensive care nursing management. Please refer to your specific organization for nursing protocols related to DKA management. Many facilities have strict admission guidelines to ensure the appropriate use of intensive care resources. With respect to patients with DKA, suitable ICU admissions may include the following:
- A newly diagnosed diabetic during an episode of DKA
- Any infectious disease condition that triggers an episode of DKA
- An episode of DKA occurring concurrently with a physiologic stressor event (acute myocardial infarction, cerebrovascular accident/stroke)
The goals of emergency treatment of diabetic ketoacidosis are multifactorial and listed below. Interventions will include, but not be limited to, insulin intravenous infusions, hourly vital sign monitoring (or more frequent), and hourly glucose checks.
- Treatment/correction of dehydration with IV fluids
- Treatment of hyperglycemia with insulin therapy
- Treatment of electrolyte imbalances
- Treatment/correction of acid-base imbalance
Initial/Emergency treatment of DKA includes (20):
- Initial assessment and stabilization ABC airway, breathing and circulation.
- Aggressive fluid therapy to restore circulating volume.
- Isotonic saline IV infusion
- IV with dextrose component once glucose level 200-250mg/dl

Self Quiz
Ask yourself...
- With regards to your current workplace/unit, are there any specific order sets (“standing orders”) for ICU admissions?
- What “standing orders” are currently in place for a suspected diabetic ketoacidosis patient?
- What additional “order sets” would be initiated if a patient with DKA was found to be febrile (102F) with suspected pneumonia?
Laboratory Findings
The following laboratory ranges provide a generic overview of normal ranges and abnormal findings associated with DKA (17) (18) (19). The confirmation of acute diabetic ketoacidosis is dependent on both laboratory findings as well as patient assessment. Please refer to your specific medical organization (unit specific) for further guidance and treatment parameters.
- Serum potassium levels: Normal range (3.5 to 5.0 mEq/L) hyperkalemia range approximately 5.0 to 5.5mEq/L.
- Serum sodium levels: Normal range (137 to 142 mEq/L) severe hyponatremia range approximately 125mEq/L or lower; severe hypernatremia range above 145mE/L
- Serum Amylase level: Normal range (40 to 140 units per liter) (U/L); may be elevated in cases of pancreatitis/ pancreatic inflammation, which may coexist with DKA
- Serum Lipase level: Normal range (0-160 units per liter) (U/L); may be elevated in cases of pancreatitis/ pancreatic inflammation, which may coexist with DKA
- Serum Osmolality level: Normal range 275-295 mOsm/kg: may be elevated to between 300-320 mOsm/kg in DKA
- Arterial blood gas analysis: Arterial ph below 7.3 (normal range 7.35-7.45)
- Anion Gap: Normal 4-12 mEq/L ; levels above > 10 may indicate existing acidosis in DKA
- Serum glucose level (normal fasting below 100mg/dl). Hyperglycemia range above 250mg/dl
- Serum ketone level (normal negative); serum ketones detected in blood; usually greater than 5mEq/L
- Serum bicarbonate level (normal 22-29 mEq/l); usually less than 18mEq/L
- Anion gap level (normal 4-12mmol/L); usually greater than 12 mmol/L)
| Lab Test | Normal Range | DKA | Comment |
| Potassium | 3.5-5.0 mEq/L | >5-5.5 mEq/L and above | |
| Sodium | 137-142mEq/L |
<125mEq/L hyponatremia >145 mEq/L hypernatremia |
|
| Amylase | 40-140 U/L | >140U/L | Elevated with pancreatitis |
| Lipase | 0-160 U/L | >160U/L | Elevated with pancreatitis |
| Arterial PH | 7.35-7.45 | Below 7.3 | |
| Serum Osmolality | 275-295 mOsm/kg | 300-320 mOsm/kg | |
| Anion Gap | 4-12 mEq/L | >10 mEq/L existing DKA |
|
| Glucose | < 100mg/dl | >250mg/dl | |
| Ketone | Negative | >5mEq/L | |
| Bicarbonate | 22-29mEq/L | <18mEq/L | |
| Anion Gap | 4-12mmol/L | >12mmol/L |
To rule out physiologic stressors associated with the development of DKA (systemic infections, acute myocardial infarction, pneumonia, urosepsis), refer to your medical organization (unit specific) guidelines regarding these additional diagnostics:
- Serial blood and wound cultures
- Serial EKG and Troponin levels
- Sputum cultures and sensitivity
- Urinalysis and culture with sensitivity
- Chest Xray
Fluid Resuscitation Guidelines
The American Diabetes Association (ADA) recommends the following initial fluid resuscitation in the adult population; additional boluses may be required after each hourly reassessment: (21). Please refer to your unit specific guidelines regarding fluid boluses, and fluid resuscitation. Caution in use with patients with preexisting heart failure, kidney failure or other medically indicated “fluid restrictions”.
0.9% SC (Sodium Chloride Solution) initially as a 15–20 mL/Kg bolus for hemodynamic resuscitation
- then 250–500 mL/h of fluid until glucose is normalized (usually faster than DKA resolution)
- then 150–250 mL/h until DKA resolution
- For the replenishment, 0.45% SC (Sodium Chloride Solution) unless hyperglycemia-corrected hyponatremia is present.
In the pediatric population, fluid resuscitation boluses are indicated in children who present with the following symptoms: (22)
- Dry mucus membranes
- Poor skin turgor
- Lethargy; altered level of consciousness.
- Nausea and vomiting
- Tachycardia and tachypnea
- Kussmaul type respirations (deep and labored respiratory breathing patterns)
Fluid recommendation: 10–20 mL/kg bolus of isotonic saline given over 30–60 mins.
Insulin Therapy and Acute Diabetic Ketoacidosis
Intravenous use of insulin is preferred in patients with acute diabetic ketoacidosis, as subcutaneous absorption of insulin would most likely be ineffective in light of dehydration.
Intravenous continuous infusion of insulin at a rate of at 0.14 U/kg/hour or
Insulin bolus of 0.1U/kg, followed by insulin continuous infusion at a rate of 0.1U/kg/hour.
Hourly (or more frequent glucose checks) with a decrease in insulin delivery dose when glucose level is 250mg/dl or less. At this time, insulin dose is further decreased to 0.05 or 0.1U/kg hourly until DKA is resolved.
- Patients, once stabilized and deemed able to eat, can be transitioned to subcutaneous insulin administration and routine glucose monitoring (point of care/ POC glucometers)
Laboratory Tests Guidelines Therapy Goals
- Serum glucose levels below 200mg/dl
- Serum bicarbonate level greater than 15mEq/L
- Serum potassium level 4.0 -5.0 mEq/L
- Venous pH greater than 7.30
- Anion gap equal to/less than 12eEq/l. (23)
Electrolyte Imbalance (Hyperkalemia-> Hypokalemia)
Serum potassium levels are usually high/elevated due to the cellular changes occurring as the result of acidosis and decreased insulin. Electrolyte replacement should be monitored very closely in diabetic ketoacidosis. During the rehydration/ volume restoration phase and insulin administration, extracellular potassium shifts back into the intracellular space (causing hypokalemia). In addition, insufficient insulin levels may deplete various serum electrolytes; thus, frequent serum electrolyte levels with appropriate intravenous replacement ensure proper cellular activity.
Treatment-Related Complications
- Hypoglycemia (blood glucose levels below 70mg/dl); treat; accordingly, patient should be transitioned to subcutaneous insulin injections when serum glucose level 200-250mg/dl, and patient is able to tolerate oral intake.
- Hypokalemia (blood potassium levels below 3-3.4 mmol/L); intravenous therapy to include potassium supplements; oral supplements as tolerated once patient transitions to diabetic diet.
- Cerebral edema
Cerebral Edema
Cerebral edema, or brain swelling, occurs for a variety of conditions (brain tumors, blunt trauma, inflammatory conditions, and even infections). Diabetic ketoacidosis and hyponatremia can cause cerebral edema. (24) Cerebral edema is the leading cause of mortality in children. A normal ICP (intracranial pressure) reading is 7-15mmHG; an increased reading in excess of 20-25mmHG, coupled with the following symptoms, may be indicative of cerebral edema.
Initial symptoms of cerebral edema may include the following:
- Headache
- Visual changes (double vision (diplopia) or blurred vision)
- Changes in speech/ ability to talk/ personality.
- Nausea and vomiting
- Changes in level of consciousness (lethargy-> unresponsiveness)
- Changes in respirations/ difficulty breathing
Symptoms that may indicate worsening of cerebral edema.
- decorticate and decerebrate posturing.
- cranial nerve palsies
- fluctuating level of consciousness
- sustained heart rate deceleration,
- increased vomiting, headache, and lethargy
Confirmation Testing:
- CT (Computerized Tomography) scan
- MRI Magnetic Resonance Imaging
Treatment for DKA Related Cerebral Edema
When cerebral edema is confirmed by radiologic testing, the administration of Mannitol (or hypertonic sodium) is recommended as follows (25) (26):
- 0.5-1 g/kg intravenous mannitol may be given over the course of 20 minutes and repeated if no response is seen in 30-120 minutes.
- If no response to mannitol occurs, hypertonic saline (3%) may be given at 5-10 mg/kg over the course of 30 minutes.
- Additional treatments may be warranted, including diuretics, corticosteroids, and possible surgical intervention (to prevent herniation syndrome).
Nursing Care and Management
Nursing Care: Patient Placement
Initial/hourly (or more frequent) assessment to include the following:
Due to the frequency of monitoring and medication administration during the acute phase of DKA, patients are usually placed in the Intensive Care Unit. ICU treatment often includes hourly physical assessments (intake, output, neurological assessment, vital signs; frequent laboratory testing (glucose testing); and rapid identification of complications (cerebral edema, hypoglycemia, hypokalemia).
Transfer to a step-down unit usually occurs when the patient is fully awake, tolerating oral intake (both solid food and liquids), vital signs are stable, and fluid and electrolyte replacements are complete. The average timeline may be 1-2 clinical days. The focus of care now shifts to discharge planning, patient education, and ongoing management.

Self Quiz
Ask yourself...
- What is your current workplace policy of ICU admissions?
- What parameters are used to determine which in-house unit a patient is transferred to?
- Do you feel that patients with acute DKA could be successfully managed on a step-down unit? Why/Why not?
Nursing Care: Acute Phase
- Monitoring of vital signs, level of consciousness/ neurological status, urine output
- Administration of IV fluids as ordered.
- Frequent blood glucose assessment and insulin administration
Nursing Care: Patient Education, Discharge Planning, and Follow-up Care
- Compliance with medications, healthy diet, glucose monitoring, sick day management
- Signs and symptoms of infection
- Importance of follow-up care with primary medical provider/endocrinologist
- Lifestyle behavior changes (smoking cessation, physical activity, healthy diet)
- Medical Alert ID bracelet or wallet insert regarding chronic medical conditions and medication.
- Coordination of follow-up care to ensure ongoing medical support, educational services and financial assistance when appropriate (medical provider, endocrinologist, pharmacist, social worker/ case management services, DSMES classes) (27)
Patient Education
Diabetes Self-Management Education and Support (DSMES)
The Centers for Disease Control and Prevention offer a Diabetes Self-Management Education and Support Toolkit on their website available to the public, designed for various health organizations/ community organizations and others interested in educating persons with diabetes to live a healthier lifestyle. Studies have shown that people who receive such education have better overall health and wellbeing. Despite these studies, a very low percentage of those qualified to receive such services access them. Check out the link below for more information.
- DSMES Program Review: https://www.cdc.gov/diabetes/dsmes/index.html
- DSMES Tooliit: https://www.cdc.gov/diabetes/dsmes-toolkit/index.html
Additional Resources
The following websites are being provided to assist the healthcare professional in accessing appropriate diabetes related information, including insulin coverage, food insecurities, food bank locations, and DSMES information. https://diabetes.org/
The American Diabetes Association provides information on prediabetes, Type 1, and Type 2 diabetes, as well as gestational diabetes. Included on their website are sections on medications, support groups, diet and activity, advocacy efforts, and prevention efforts. https://www.jdrf.org/
The Juvenile Diabetes Research Foundation is a global organization for Type 1 diabetes mellitus. The site offers information on all things T1DM, including sections for those newly diagnosed, those interested in fundraising, research and clinical trials, daily diabetes management, volunteer opportunities, and access to local chapters worldwide. From the healthcare provider perspective, this website offers continuing education programs and pdf downloads for patient specific education. https://getinsulin.org/
The Get Insulin website provides information for persons with diabetes to access affordable insulin coverage. The site also offers information and guidance on health insurance plans, an insulin related newsletter, and external links to food sources (for patients with food insecurity issues) https://www.feedingamerica.org/find-your-local-foodbank
The Feeding America website enables persons with food insecurities to access food banks in their area, according to state location and zip code.

Self Quiz
Ask yourself...
- What community resources are available to your patients, post discharge, regarding access to food and medications?
- If your patient says they simply cannot afford their prescriptions, what is your current facility policy regarding this matter?
- How would you improve your current facility policy regarding patient access to medications for those uninsured/underinsured?
Patient Education and Follow-up Care (DSMES)
DSMES, or Diabetes Self-Management Education and Support, is the gold standard when it comes to patient education on this chronic medical condition. The goal of this education is to educate and empower the patient to successfully manage their medical condition, in efforts to lower the risk of long term, lifetime complications. DSMES is considered an ongoing process, and is recognized as an integral part of patient education at various critical points in their lifetime:
- At time of initial diagnosis
- During all patient medical appointments and routine follow-up care
- At time of onset for newly diagnosed complications
- Anytime a patient expresses concern over current diabetic management challenges.
Medicare and Medicaid
Medicare (Medicare Part B) and Medicaid plans currently offer the following coverage for diabetes related education (28):
- 10 hours of education (combined individual and group training) for an initial diagnosis of diabetes
- 2 hours of follow-up training annually after initial training completion
Qualifying Labs for DSMES
In general, a patient must be diagnosed with type 1, type 2, or gestational diabetes to qualify for DSMES, such as:
- Fasting Blood glucose of 126 mg/dL on 2 separate occasions
- 2-hour Post-Glucose Challenge of ≥200 mg/dL on 2 separate occasions
- Random Glucose Test of >200 mg/dL with symptoms of unmanaged diabetes
DSME Contents Overview
- Diabetes disease process pathophysiology and treatment to increase risk reduction for long term complications.
- Healthy eating includes meal planning, food label reading, carbohydrate counting, and strategies for eating out.
- Physical activity includes the benefits of activity as they related to better weight control, sleep habits and stress reduction.
- Medication usage overview to include medication administration, side effects, storage and cost issues.
- Blood glucose monitoring and management to include proper use of monitoring devices and associated equipment cleaning/repair.
- Prevention of complications (early detection, treatment, acute and chronic complications such as kidney disease and nerve damage; proper foot care)
- Healthy coping strategies to include stress reduction, effective self-management behaviors, and symptom recognition (hypoglycemia/hyperglycemia)
- Sick day management includes intake/output monitoring, over the counter medication usage, carbohydrate counting, ketone assessment, fever control and when to seek emergency services.
- Problem solving to include diabetes management during emergencies (power outages, flooding, tornados, hurricanes)
For more DSMES information visit: https://www.cdc.gov/diabetes/dsmes/dsmes-living-with-diabetes.html

Self Quiz
Ask yourself...
- With respect to DKA, what aspects of DSMES do you think are most important for patient education?
- How do you assess health literacy in your patients?
- What are some nursing interventions that could be done to assess a patient’s ability to correctly use a glucometer (glucose measuring device)?
- What community resources, post hospital discharge, are available for newly diagnosed patients with prediabetes/ type 2 diabetes in your area?
- What aspect of DSMES do you consider most important for ongoing sick day management education for your patients with diabetes?
Safety Considerations (Sick Day Management)
Successful management (prevention) of diabetic ketoacidosis requires patient education and empowerment in managing situations where glucose levels may be elevated and/or insulin levels (doses) are substandard (29).
There are many situations that can put a patient at risk for the development of DKA, including the following (29):
- Illness (acute and chronic), affecting normal food and fluid intake which negatively affects glucose management.
- Missed medication (insulin therapy) due to a clogged insulin pump tubing, a malfunctioning insulin pump, partial doses/skipped doses of insulin (whether related to costs, cognition, or mental health issues {diabetes distress}),
- Medication side effects
- Concurrent use of alcohol or drugs
- Physiologic stress (heart attack, stroke, physical injury)
Patient Education: Sick Day Management
Home treatment/ self-care (30)
The importance of preplanning cannot be understated. All persons with diabetes should have adequate supplies at home, to address an acute illness, including medications to treat basic symptoms before they escalate. These medications may include over the counter medications to treat pain, nausea, vomiting, diarrhea, as well as adequate supplies to manage their diabetes (alcohol prep pads, syringes, prescription medications).
In addition, it is important to stock up on diabetic friendly foods and drinks to maintain nutrition and hydration levels during an acute illness. Such items might include sports drinks, soft drinks, instant cooked cereals, puddings, soups. In the event that a patient cannot eat their regular meals, the goal is to eat or drink 50 grams of carbohydrate every 4 hours to maintain glucose levels.
Sick Day Management Guidelines
- Monitor glucose levels every 4 hours.
- Stay hydrated – 4 to 6 ounces of fluid every ½ hour to prevent dehydration.
- Daily weight
- Temperature checks (rule out underlying infection)
- Current medication compliance- do not stop taking insulin or diabetic oral agents ** notify provider immediately if you choose to stop medications.
Seek emergency care for the following signs/ symptoms:
- Persistent vomiting/diarrhea to the point that you cannot tolerate any food or fluid intake for several hours
- Ongoing glucose levels above 240mg/dl
- The presence of moderate/high levels of ketones in urine
- Unexplained weight loss during an illness
- Any difficulty breathing
- Fruity/acetone smell on breath
- Changes in gait/balance/ vision
Research Findings
Research: Diabetes Distress and Burnout
Diabetes is a 24/7/365 chronic medical condition. Unlike many conditions that are simply managed with lifestyle changes or a single, once a day medication regimen, diabetes mellitus requires lifelong, around the clock commitment. Whether diet, activity, or medication management, a person with diabetes may easily feel overwhelmed by even the basic requirements for self-management. (31)
Ongoing health challenges, comorbid medical conditions, medication and diet cost issues and family dynamics can all affect a person’s ability to successfully manage any health condition. When emotions (sadness, anger, hostility, frustration, and even fear) become overwhelming, diabetic distress (a feeling of defeat) can often occur. Without prompt, patient specific interventions (mental health services, financial assistance, self-management education), these feeling will progress to diabetic burnout, and increase the risk of unhealthy habits (poor medication adherence and overall glycemic control). (32)
Diabetes distress can easily progress to diabetes burnout without appropriate ongoing medical treatment and mental health interventions. When a person with diabetes reaches the point of burnout, they often appear to disconnect from their routine healthcare, exhibiting indifference towards their overall health and well-being. They may become both mentally and physically exhausted from the daily requirements of this chronic medical condition. At this point, it is not uncommon to observe a person’s total disregard for their ongoing medical treatments, daily medications, routine self-care, and more. Missed medications, missed medical appointments, poor dietary intake, and a visible lack of basic hygienic practices are cause for concern.
A multidisciplinary approach to treating suspected diabetic distress and burnout is highly encouraged. From ongoing education, physical and mental health assessments, and enrollment in therapies (individual therapy sessions, and support groups), the person with diabetes needs a supportive environment in which to become empowered in the self-management of their disease progress. In doing so, it is believed that health outcomes are optimal, and the risk of long-term complications is lowered. (33)

Self Quiz
Ask yourself...
- Why do you think diabetes related distress occurs?
- What external factors affect a person’s ability to manage their diabetes successfully?
- What nursing education can you provide to possibly decrease the likelihood of diabetes distress?
- What areas of discharge planning/discharge instructions and follow-up care positively impact a person’s ability to manage their chronic medical condition?
Reserach: Diabulimia
Bulimia nervosa is a potentially life-threatening eating disorder characterized by episodic binge eating of large amounts of food, followed by forced vomiting and possibly laxative use to then “purge” the food. These alternating behaviors are the result of a person fearful of weight gain and willingness to lose weight in unhealthy ways. (34) (35)
Diabulimia is a serious, life threatening eating disorder affecting persons with Type 1 diabetes. Through intentional restricted/ limited use of prescribed insulin, weight loss occurs. This eating disorder is more common in young female adolescents and young adults. (34) (35)
Signs and symptoms may include the following (34) (35):
- Unexplained weight loss
- Hemoglobin A1C > 9
- Multiple episodes of DKA
- Unfilled insulin prescriptions, missed diabetes related medical appointments,
- Expressed fear of insulin related weight gain
- Anxiety related to body image
- Obsessive interest in calories and dieting

Self Quiz
Ask yourself...
- How would you approach patient education with someone you suspect might be suffering from diabulimia?
- What might be some reasons for repeated DKA related incidents, unrelated to intentional restriction of insulin usage?
- How might you encourage a patient to improve compliance with routine medical appointments/ follow-up care?
- How would you respond to a patient’s concerning comment that “insulin is making me gain unwanted weight”?
- What consultations and referrals/resources would be appropriate for discharge planning of patients with suspected diabulimia?

Self Quiz
Ask yourself...
- How would you approach patient education with someone you suspect might be suffering from diabulimia?
- What might be some reasons for repeated DKA related incidents, unrelated to intentional restriction of insulin usage?
- How might you encourage a patient to improve compliance with routine medical appointments/ follow-up care?
- How would you respond to a patient’s concerning comment that “insulin is making me gain unwanted weight”?
- What consultations and referrals/resources would be appropriate for discharge planning of patients with suspected diabulimia?
Research: Insulin Affordability
For many persons with diabetes, the perceived noncompliance with therapy (on behalf of the healthcare professional) is actually that of a cost related issue. Many persons cannot afford ongoing therapies related to management of this chronic medical condition. In attempts to “cut costs”, patients have admitted to skipping certain medications, cutting medications in half, reducing prescribed doses of insulin, and purchasing poorer quality, less expensive foods (that are often lacking in nutritional value). Poorly controlled / uncontrolled diabetes heightens the risk of both acute and chronic complications.
In an attempt to ensure accessibility and affordability of insulin therapy to persons with diabetes, the Inflation Reduction Act of 2022 in part ensures that persons with diabetes on Medicare pay no more than $35 for a month’s supply of insulin product under their prescription drug coverage. Similar drug coverage benefits were also extended to many state-based insurance plans. (36)
In addition, most Medicaid insurance plans, as well as private insurance companies have now enacted reduced insulin costs/ cost sharing programs. Finally, for patients with no insulin costs benefits, many national insurance providers offer free/ reduced cost insulin through their patient assistance program. For a comprehensive list of these resources, please see the following website link (American Diabetes Association): https://diabetes.org/tools-resources/affordable-insulin
Research: Insulin Delivery Systems
With the creation of advanced insulin delivery /monitoring devices (insulin pumps, and continuous glucose monitoring devices), the person with diabetes is afforded a more streamlined process to control their chronic medical condition. Most patients using such devices report better glucose control {“time in range”}, meaning the time their blood glucose levels remained in an acceptable range, ease of portability (of supplies), increased comfort (no more finger sticks), and decreased rates of anxiety, depression and distress.
The following website links represent various insulin delivery devices. Consider making a resource book containing various delivery devices for your specific unit (or hospital organization). Many have 24/7 customer service representatives available if you need to trouble shoot a device suspected of malfunctioning or require additional staff/patient educational resources.
This list contains a variety of websites but is not all inclusive. If you are caring for a patient with an insulin delivery device in place, please contact that specific company for more directions on its usage, removal, replacement parts and more.
Examples of insulin delivery devices:
- Makers of the MiniMed 780G System and the MiniMed 630G system: https://www.medtronic.com/us-en/healthcare-professionals/products/diabetes/insulin-pump-systems.html
- Makers of the Omnipod 5 and the Omnipod DASH Insulin Management System: https://www.omnipod.com/
- Quick overview of various insulin pumps currently on the market: https://consumerguide.diabetes.org/collections/pumps

Self Quiz
Ask yourself...
- What is your facility’s current policy on patient admissions for DKA that want to wear their insulin pumps while in the hospital?
- Would you feel comfortable allowing a Type 1 diabetes patient, admitted for a medical condition unrelated to diabetes, to continue wearing their insulin pump during their hospital stay? Why/Why not?
Case Studies
Case Study #1
A 3-year-old female child is sent, by ambulance, from her local pediatrician’s office with reports of increased lethargy, increased thirst and appetite, and new onset of bedwetting (child had stopped wearing diapers at age 2.5 years). Parents report that the child’s appetite appears increased lately, but pediatrician noted several pounds weight loss since last visit.
- Based on these signs and symptoms alone, what is your initial diagnosis for this child?
- What are your priority nursing interventions for this child?
- How would you assess hydration status on a 3-year-old child?
The child is diagnosed with new onset Type 1 Diabetes and stabilized in the Emergency Department. The parents of this child are visibly distraught over the diagnosis, stating “no one in our family is diabetic; this can’t be happening”.
- What are some of the initial nursing patient/family education areas you could address at this time?
- What are some in hospital consultations that should be considered for this patient and her family?
- What are some community resources you have in your area that you could offer this family?
Case Study #2
A 78-year-old female, with a previous history of CVA, is transferred to your facility from a local nursing home for evaluation of fever and hyperglycemia. The nursing home staff reported the patients’ blood glucose level was 400mg/dl earlier today and her WBC count was 14,500. Upon arrival, the patient is unresponsive, with a temperature 102F , her glucose is 350mg/dl, with +ketones (moderate) and a urinalysis (indwelling catheter) confirms a UTI.
Patient past medical history includes old CVA, T2DM.
- Based on these signs and symptoms alone, what is your initial diagnosis for this patient?
- What diagnostic studies would be warranted in light of hyperglycemia, fever, urinary tract infection, and altered level of consciousness?
- What questions would you ask the nursing home staff, in light of this patient’s initial presentation?
- When this patient is stabilized for transfer back to the nursing home, what information should be included in the discharge instructions/ transfer of care?
Case Study #3
A 60-year-old male present to the Emergency Department with an Acute Myocardial Infarction.
Patient past medical history includes borderline hypertension, and prediabetic HgbA1C 5.8.
The patient was found to have severe coronary artery disease and received Coronary Artery Bypass Graft x 3. During the immediate post operative recovery phase, he receives insulin therapy to control glucose levels >300mg/dl. He is eventually sent home on insulin therapy, pending follow-up with his cardiologist as well as a new consultation for an endocrinologist.
At the time of discharge, the patient is adamant that he was “prediabetic and could easily control my glucose levels with diet alone”; he doesn’t understand how he is now “an insulin diabetic”.
- What are key points in nursing education for this patient, regarding his new “diabetic status”
- How would you explain to the patient the connection between his myocardial infarction, bypass surgery, and currently elevated glucose levels?
- What nursing interventions can you provide PRIOR to discharge to assess his comfort level with insulin injections and glucose monitoring?
- What community resources are available to your patient for a new diagnosis of diabetes?
- The patient wants to know when he can stop the insulin injections. What is your response?
Successful management of acute diabetic ketoacidosis requires resuscitation with intravenous fluids and insulin therapy, replacement of electrolytes and early identification of any events (medical, surgical, and psychological) that contributed to this medical emergency. With the continued increase in diagnosis of diabetes, and ongoing challenges in healthcare costs and coverage, chronic medical conditions will continue to burden the already overwhelmed healthcare arena. By educating and empowering patients to self-manage their disease process, we can lower the risk of long-term complications and improve health outcomes worldwide.
Conclusion
The International Diabetes Federation reports that, in 2021, approximately 10.5 % of the global adult population (ages 20-79 years old) has diabetes, and that nearly 50% of this population are unaware that they are living with this chronic condition. Left untreated, the rates of long-term, nonreversible complications are quite alarming. (37) By the year 2045, it is projected that 1 in every 8 persons (approximately 12.5 %) will be living with diabetes. This will equate to an increase of 46 percent, with nearly 783 million people being affected. This single health condition will represent staggering health expenditures and increased mortality and morbidity associated rates worldwide.
Diabetes ketoacidosis continues to be a potentially life-threatening complication for persons with diabetes. DKA is also, in most cases, a highly preventable condition, with early identification and treatment. The importance of ongoing, patient specific education to address all aspects of diabetes self-management is a key factor in lowering the occurrence of DKA. Dr William Polonsky, a licensed clinical psychologist and certified diabetes educator, is the President of the Behavioral Diabetes Institute in San Diego, California. With regards to the importance of patient education and empowerment, he said the following:
“Well-controlled diabetes is the leading cause of nothing!” Dr William Polonsky. (38)
As healthcare professionals, we have the responsibility to ensure that our patients with diabetes are afforded the education and ongoing support necessary for them to successfully manage their specific disease process. In doing so, we positively impact patient satisfaction, improve medication adherence rates, and lower the risk of long-term complications.

Self Quiz
Ask yourself...
- Why do you think persons with diabetes become “noncompliant” with their diabetes medications?
- What nursing interventions may increase/improve medication compliance rates?
- What community resources are available in your area to assist those who cannot afford their prescribed medications?
- If a patient tells you they simply cannot afford their medications, what resources are available at your place of employment to assist such financial concerns?
- Have you identified any barriers to patient education at your healthcare organization?
- How will your practice change after reading this course?
Heart Disease Prevention & Management
Introduction
Heart disease is an umbrella term encompassing a range of cardiovascular conditions, and stands as the leading cause of death worldwide, claiming millions of lives. Both heart disease and stroke are types of cardiovascular disease. The impact of heart disease transcends individual health, posing a significant economic burden through reduced labor and workforce participation. In 2018, the mean labor income losses were $13,463 for heart disease and $18,716 for stroke. Total labor income losses were estimated at $203.3 billion for heart disease and $63.6 billion for stroke [1].
Heart disease manifests in various forms, each with its unique characteristics and implications. Coronary artery disease, the most common type, arises from the buildup of plaque in the coronary arteries [2]. This narrowing can restrict blood flow to the heart, causing symptoms such as chest pain or shortness of breath [2]. Other prevalent forms include heart failure, rhythm abnormalities, heart valve defects, and congenital heart defects [4]. These conditions can lead to a spectrum of complications, including heart attacks, strokes, and heart failure, significantly impairing quality of life and survival [3]. By understanding the underlying mechanisms, risk factors, and clinical presentations of heart disease, participants will gain a deeper understanding of the disease's impact on the cardiovascular system and overall health.
Prevention, the cornerstone of combating heart disease, will be a central focus. Participants will explore lifestyle modifications that play a pivotal role in reducing the risk of heart disease, including diet, physical activity, weight management, smoking cessation, and stress reduction. Identification and management of risk factors, such as high blood pressure, high cholesterol, and diabetes, is prioritized.

Self Quiz
Ask yourself...
- Why is understanding the underlying mechanisms of heart disease important for prevention and management?
- How can lifestyle modifications, such as diet, physical activity, and stress reduction, play a crucial role in preventing heart disease?
Types of Heart Disease
Heart disease encompasses a spectrum of conditions, each with unique underlying mechanisms and dietary management strategies [5]. Coronary artery disease (CAD), heart failure (HF), hypertensive heart disease (HTN), and arrhythmias constitute a significant portion of these prevalent ailments. Coronary artery disease (CAD) is the most common type of heart disease [6]. The four main types of cardiovascular disease (CVD) include coronary heart disease, stroke, peripheral arterial disease, and aortic disease [7]. Cerebrovascular disease is the second most common type of heart disease [7]. Other forms of heart disease include congenital heart disease, heart valve disease, cardiomyopathy, pericardial disease, arrhythmia, and aortic dissection.
Congenital heart disease (CHD)
Congenital heart disease (CHD) is the most common type of birth defect, affecting about one in 100 babies born in the United States [8]. CHDs can range from mild to severe and can affect the heart's structure, function, or both [8].
Heart Valve Disease
Heart valve disease is a condition in which one or more of the heart valves are damaged or diseased which causes blood to flow backward through the valves, damaging the heart and other organs [9]. Heart valve disease affects 2.5% of the U.S population and can be caused by several factors, including infection, injury, and aging [9].
Heart Failure
Heart failure is a condition in which the heart's weakened pump fails to meet the body's demands for blood and oxygen, leading to a range of debilitating symptoms including fatigue, shortness of breath, swelling, chest pain, palpitations, weight gain, swelling in the ankles, legs, and abdomen, bloated or hard stomach, dry hacking cough, and nausea [10][11]. More than six million adults in the U.S. have heart failure [10].
Cardiomyopathy
Cardiomyopathy represents a collection of diverse conditions of the heart which makes it weaker and less able to pump blood. Cardiomyopathy can be caused by several factors, including infection, toxins, and genetic disorders [12]. Due to the potential for underdiagnosis, estimates of cardiomyopathy prevalence can vary. It is estimated that up to one in 500 adults may be affected by this condition [13].
Pericardial Disease
Pericardial disease is a condition that affects the pericardium, the sac that surrounds the heart [14]. This can cause inflammation, infection, or scarring of the pericardium, which can interfere with the heart's ability to function. Pericardial disease can manifest as acute pericarditis, pericardial effusion, cardiac tamponade, or constrictive pericarditis [14].
Arrhythmia
Arrhythmia is a cardiac disorder characterized by an abnormal heart rhythm, manifesting as tachycardia (excessively rapid heartbeat), bradycardia (abnormally slow heartbeat), or irregular heartbeat patterns [15]. These irregularities can induce symptoms such as palpitations, dizziness, and syncope (fainting episodes) and can arise from various etiologies, including underlying heart conditions, electrolyte imbalances, and adverse effects of certain medications [15].
One in 18 people, or five percent of the U.S. population has an arrhythmia with afib (atrial fibrillation) being the most common [16].
Aortic Dissection
Aortic dissection is a rare, life-threatening condition in which the inner layer of the body’s main artery (aorta), tears [17]. This can cause blood to leak between the layers of the aorta, which can weaken the artery and cause it to rupture. Aortic dissection can be caused by several factors including high blood pressure, atherosclerosis, and connective tissue disorders [17]. Aortic dissection affects about 30 in one million people each year and more than 13,000 die each year [18]. Aortic dissection is most common in individuals over the age of 60 and if not treated, the tear can worsen, ripping the outer layer of the aorta, allowing blood to escape the artery [17] [18]. As many as 40 percent of individuals who suffer from an aortic dissection die, and the risk of death increases by 3-4 percent every hour the condition is left untreated [18].


Self Quiz
Ask yourself...
- Given the diverse range of heart disease conditions, what common underlying mechanisms contribute to their development?
- Considering the various forms of heart disease, what dietary management strategies can be tailored to address specific risk factors and nutritional needs?
- Given the potential for underdiagnosis of certain heart disease conditions, what screening and diagnostic measures can be employed to ensure early detection and intervention?
Epidemiology/Statistical Evidence
Heart disease is a global health crisis, with an estimated 17.9 million deaths attributed to cardiovascular diseases in 2019, representing 32% of all global deaths [19]. This significant number reflects the pervasive nature of heart disease, affecting individuals of all ages, socioeconomic backgrounds, and ethnicities. The distribution and determinants of heart disease plays a pivotal role in understanding the prevalence. Heart disease varies across regions, with higher rates observed in high-income countries compared to low-income countries [20]. However, the burden of heart disease is shifting, with a growing trend in low-income countries due to rapid urbanization, lifestyle changes, and increasing exposure to risk factors [20].
Epidemiology: Modifiable and Non-Modifiable Risk Factors
Epidemiological studies with data from 61 cohort studies, encompassing 12.7 million person-years of follow-up and 56,000 fatalities from coronary heart disease (CHD) and stroke revealed a consistent and graded increase in CVD risk. The risk was associated with higher baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels beyond the usual thresholds of 115 and 75 mmHg. A 20-mmHg elevation in SBP and a 10 mmHg increase in DBP was associated with a two-fold higher risk of CVD [21].
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in individuals with type 2 diabetes mellitus (T2DM). This increased risk stems from a complex interplay of traditional and non-traditional risk factors that contribute to the initiation and progression of atherosclerosis, a pathological process characterized by the buildup of plaque within artery walls [22].
The epidemiology of heart disease involves recognizing the associated risk factors. These can be categorized into modifiable and non-modifiable factors. Modifiable risk factors include hypertension, high cholesterol, smoking, obesity, diabetes, physical inactivity, and poor diet [23]. Non-modifiable risk factors include age, gender, and family history [23]. According to the American Heart Association (AHA), the likelihood of developing CVD in U.S. men and women is 40% between the ages of 40 and 59, 75% between 60 and 79, and 86% for those over the age of 80 [24].
Statistics on Ethnic/Racial Minorities
Patients from minority groups with acute coronary syndrome (ACS) are at an increased risk of heart attack (MI), readmission to the hospital, and death from ACS [25]. Black/African Americans are at 30% increased risk of heart disease and death from heart disease and double the risk of stroke with a higher risk of developing heart failure [25]. Among Asian Americans /Pacific Islanders, coronary artery disease (CAD) manifests earlier in life and affects a larger proportion of the population compared to other ethnic groups [25]. Overall CVD rates are lower among non-White Hispanic individuals. Among Hispanic subgroups, Puerto Rican Americans exhibit the highest HTN-related death rates [25]. Heart failure incidence among Hispanics falls between that of African Americans and non-Hispanic whites [25].
Epidemiology: Genetic and Environmental Factors
The development of heart disease is influenced by a complex interplay of genetic and environmental factors [26]. Major risk factors include sustained high blood pressure placing excessive strain on the heart, increasing the risk of heart failure, stroke, and other complications [27]. Elevated levels of low-density lipoprotein (LDL) cholesterol can accumulate in arteries, narrowing and reducing blood flow to the heart [28].
Diabetes mellitus, characterized by high blood sugar levels that damage blood vessels and nerves throughout the body, increases the risk of heart disease and stroke [29]. Tobacco contributes to atherosclerosis – the buildup of plaque in arteries – through a cascade of mechanisms that lead to atherosclerosis, including thrombosis, insulin resistance, dyslipidemia, vascular inflammation, abnormal vascular growth, angiogenesis, and impaired endothelial repair and regeneration [30] [50].
Obesity and excess abdominal fat can increase the risk of heart disease by elevating blood pressure, cholesterol levels, and blood sugar levels [27]. A lack of regular physical activity contributes to obesity, high blood pressure, and other risk factors for heart disease [31]. A family history of heart disease increases an individual's risk, indicating a genetic predisposition to the condition [32].

Self Quiz
Ask yourself...
- Despite the global prevalence of heart disease, why do we observe variations in its distribution across different regions?
- How do modifiable and non-modifiable risk factors contribute to the development and progression of heart disease?
- How do socioeconomic disparities and minority status influence the prevalence and outcomes of heart disease?
- How does the interplay of genetic and environmental factors contribute to the development of heart disease?
Prevention Strategies
Prevention for heart disease begins with addressing modifiable risk factors with an emphasis on diet, exercise, smoking cessation, and stress management. Prevention also involves regular health screenings.
Key prevention strategies include:
- Adopting a Heart-Healthy Diet consisting of fruits, vegetables, and whole grains, and limiting saturated and trans fats, sodium, and added sugars [33].
- Maintaining a healthy weight through a balanced diet and regular exercise [23].
- Engaging in regular physical activity for at least 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous-intensity aerobic exercise per week [34].
- Participating in smoking cessation, managing high blood pressure, and working with healthcare providers to manage cholesterol levels through lifestyle changes or medication [23].

Self Quiz
Ask yourself...
- Given the emphasis on modifiable risk factors in heart disease prevention, how can individuals make informed and sustainable lifestyle changes to reduce their risk?
- Considering the importance of regular health screenings in heart disease prevention, how can we increase access to and adherence with screening recommendations?
Patient Education for Self-Management
Patient education for self-management plays a vital role in preventing and managing heart disease. Healthcare professionals should begin by conducting a thorough assessment of each patient, considering their unique risk factors, medical history, and lifestyle choices related to heart disease and develop a personalized self-management plan that align with the patient's specific needs and objectives [35].
Provide clear and concise information about heart disease, its risk factors, and prevention strategies, and address individual needs and concerns, with consideration of cultural background, lifestyle, and any underlying health conditions. Effective communication is paramount in this process. Health care professionals should employ strong communication skills to establish a meaningful connection and foster open and honest dialogue, active listening, and demonstration of empathy to gain insight into their preferences [36].
To cater to diverse learning styles, it is essential to offer educational materials in various formats [37]. Tailor the educational approach to each patient's learning style, using plain language, visual aids, and active participation strategies. Involve the patient in setting realistic and achievable self-management goals [38]. These resources may encompass brochures, videos, or online materials. It is imperative that these materials are designed to be comprehensible and sensitive to cultural differences.
Patients must be educated about modifiable risk factors that pertain to heart disease prevention [23]. This includes discussions on dietary choices, physical activity, smoking cessation, and responsible alcohol consumption [23]. Furthermore, healthcare professionals should articulate the significance of adhering to prescribed medications to manage cardiovascular conditions.


Self Quiz
Ask yourself...
- How can healthcare professionals tailor patient education for self-management of heart disease, considering the diverse needs and preferences of their patients?
- How can healthcare professionals communicate the importance of adhering to prescribed medications and lifestyle modifications for managing heart disease and preventing complications?
Medical Management and Treatment
In addition to lifestyle modifications, healthcare providers can prescribe medications to treat heart disease [23]. The common medications used to treat heart disease include antihypertensive medications to lower blood pressure, antihyperlipidemic medications to lower cholesterol levels, and antiplatelet medications to prevent blood clots [40]. Beta-blockers are used to slow the heart rate and lower blood pressure and angiotensin-converting enzyme (ACE) inhibitors are used to lower blood pressure and relax blood vessels. Angiotensin II receptor blockers (ARBs) are prescribed to lower blood pressure and relax blood vessels [41].
In some cases, invasive medical procedures may be required to treat heart disease. The common types of medical procedures used to treat heart disease include coronary angioplasty to open blocked or narrowed coronary arteries, and coronary artery bypass grafting (CABG) to create a new pathway for blood to flow around blocked or narrowed coronary arteries [39][42]. Other common procedures are heart valve surgery to repair or replace damaged heart valves, implantable cardioverter-defibrillators (ICD) to monitor the heart rhythm and deliver electrical shocks to correct abnormal heart rhythms, and implantable pacemakers to help control the heart rhythm [43].

Self Quiz
Ask yourself...
- In what ways do the different pharmacological treatments for heart disease address the underlying mechanisms and risk factors associated with the condition?
- How do invasive medical procedures, such as angioplasty and bypass surgery, address the structural abnormalities and blood flow limitations associated with heart disease?
- What factors are considered when determining whether pharmacological or surgical interventions are the most appropriate treatment approach for heart disease?
Heart Health Resources
Resources for heart disease prevention and management are essential for both healthcare professionals and patients. These resources encompass a wide range of tools, information, and support systems that play a crucial role in reducing the risk and managing heart disease.
Educational Materials/Content
Information is key to prevention and management. Patients and healthcare providers can access pamphlets, brochures, websites, and educational videos that explain the causes, risk factors, symptoms, and treatment options for heart disease [44]. These resources empower individuals to make informed decisions about their heart health. [44].
Patients can benefit from resources that offer guidance on Heart-Healthy diets, including low-sodium meal plans, tips for reducing saturated fats, and strategies for incorporating more fruits and vegetables into their meals.
Published Guidelines
Medical organizations and government health agencies publish guidelines for heart disease prevention. These guidelines provide evidence-based recommendations for diet, exercise, smoking cessation, and medication management, with proper nutrition being a cornerstone of heart disease prevention and management [33].
Support Groups
Support groups provide a platform for individuals with heart disease to connect, share experiences, and receive emotional support [45]. These groups can be in-person or online and offer a sense of community for patients and their families [45].
Technological Advancements
Telehealth has become important in managing chronic conditions such as heart disease [46]. Patients can access virtual consultations with healthcare providers, receive remote monitoring, and access educational materials online. Smartphone apps and wearable devices can help individuals track their health metrics, such as blood pressure, heart rate, and physical activity [47]. Many of these tools provide real-time feedback and reminders to support heart-healthy habits.
Nonprofit Organizations
Nonprofit organizations dedicated to heart health advocacy, such as the American Heart Association, offer a wealth of resources. The American Heart Association (AHA) is a leading organization for heart disease research and education [48]. The National Heart, Lung, and Blood Institute (NHLBI) is a part of the National Institutes of Health (NIH) and offers information on heart disease prevention and management, as well as clinical trials and research studies [49].

Self Quiz
Ask yourself...
- How can healthcare providers utilize available resources to educate and empower patients in making informed decisions about their heart health?
- How can nurses help patients navigate the abundance of resources available and choose those that align with their specific needs and preferences?
Conclusion
Heart disease encompasses a spectrum of conditions with diverse underlying mechanisms and management strategies. Modifiable risk factors, including diet, exercise, smoking cessation, and stress management, play a crucial role in both prevention and management [23]. Resources for heart disease prevention and management empower individuals to take control of their heart health. These resources span from educational materials and support groups to telehealth platforms and smartphone apps, providing comprehensive guidance and support for both patients and healthcare professionals [44][45].

Self Quiz
Ask yourself...
- Why is early detection and intervention crucial for improving outcomes in heart disease?
- How can healthcare professionals advocate for the implementation of preventive measures at the community and population levels to combat the burden of heart disease?
Nutrition for a Healthy Heart
Introduction
Research indicates that a heart-healthy diet is a powerful tool for the prevention and control of cardiovascular disease [1]. A heart-healthy dietary approach to wellness emphasizes the intake of the appropriate proportions of macronutrients, essential vitamins, minerals, and fiber while minimizing the consumption of saturated fats, trans fats, cholesterol, salt (sodium), and the reduction of processed foods, trans-fats, and added sugars [2] [3]. The cornerstone of the diet includes an abundance of fresh fruits and vegetables, whole grains, lean proteins, and healthy fats [2].
For healthcare providers and patients, the goal of adopting a heart-healthy diet is multifaceted. The diet aims for a reduction in overall cardiometabolic risk, improving blood pressure, lowering cholesterol levels, reducing the risk of developing heart disease and stroke, and preserving the overall health of the circulatory system [3].
Nurses play a pivotal role in patient education and promoting heart-healthy lifestyles by empowering patients with knowledge and skills related to nutrition and dietary choices. This course aims to equip nurses with comprehensive knowledge of heart-healthy eating principles, enabling them to guide and educate their patients.
The adoption of healthy lifestyle choices, with nutrition being the most important behavioral factor, is estimated to reduce the risk of myocardial infarction (MI) by 81–94% [5][6][7]. In comparison, treatment with pharmacotherapies alone results in a 20–30% reduction [8].


Self Quiz
Ask yourself...
- Why is nutrition considered the most important behavioral factor in reducing the risk of myocardial infarction (MI) compared to pharmacotherapies alone?
Heart Disease: A Brief Overview
Heart disease remains one of the leading causes of morbidity and mortality worldwide, with diet and nutrition playing a crucial role in both its development and prevention [4]. Cardiovascular diseases, also known as CVDs, are the primary cause of death worldwide, claiming an estimated 17.9 million lives per year [4].
Cardiovascular disorders affect the heart and blood vessels, manifesting in coronary heart disease, cerebrovascular disease, rheumatic heart disease, and others. More than four out of five CVD fatalities stem from heart attacks and strokes, with one-third of these deaths occurring in individuals under the age of 70 [4].
The term "heart disease" encompasses various conditions affecting the heart's structure and function, many of which are influenced by dietary habits [9]. Cardiovascular risk factors, including malnutrition, tobacco and alcohol use, stress, obesity, sedentary lifestyle, hypertension, diabetes, hyperlipidemia, and genetic predisposition, can increase an individual's likelihood of developing cardiovascular diseases [10] [12].
The modifiable risk factors include sedentary lifestyle, smoking, high blood pressure, diabetes, hypercholesterolemia [12]. Almost three quarters of patients (73%) had at least three risk factors compared to 31% of healthy subjects.
Family history of cardiovascular disease (CVD) is a significant independent risk factor for premature coronary heart disease (CHD). The risk of premature CHD increases in a linear fashion as the number of affected family members grows. [11]. Cardiovascular risk factors, including malnutrition, tobacco and alcohol use, stress, obesity, sedentary lifestyle, hypertension, diabetes, hyperlipidemia, and genetic predisposition, can increase an individual's likelihood of developing cardiovascular diseases [10].
The five modifiable risk factors include sedentary lifestyle, smoking, high blood pressure, diabetes, and hypercholesterolemia [10]. A higher proportion of cardiovascular patients (73%) had at least three risk factors compared to healthy individuals, where less than one-third had three or more risk factors [10].

Self Quiz
Ask yourself...
- What are the key dietary factors that contribute to the development of cardiovascular diseases (CVDs)?
- How do modifiable risk factors, such as sedentary lifestyle, smoking, high blood pressure, diabetes, and hypercholesterolemia, increase an individual's likelihood of developing CVDs?
- Why is a family history of CVD considered a significant independent risk factor for premature coronary heart disease (CHD)?
Types of Heart Disease / Statistics
There are multiple types of heart disease, each with distinct mechanisms and implications for dietary management [13]. Coronary artery disease (CAD), heart failure, hypertensive heart disease, and arrhythmias represent a fraction of these conditions. The four main types of CVD include coronary heart disease, stroke, peripheral arterial disease, and aortic disease [15].
Coronary artery disease (CAD) is the most common type of heart disease [14]. Cerebrovascular disease is the second leading cause of heart disease. Other forms of heart disease include congenital heart disease, heart valve disease, cardiomyopathy, pericardial disease, arrhythmia, and aortic dissection.
Congenital heart disease (CHD) is the most common type of birth defect, affecting about 1 in 100 babies born in the United States [16]. CHDs can range from mild to severe and can affect the heart's structure, function, or both [16].
Heart valve disease is a condition in which one or more of the heart valves are damaged or diseased, which causes blood to flow backward through the valves, damaging the heart and other organs [17]. Heart valve disease affects 2.5% of the U.S population and can be caused by several factors, including infection, injury, and aging [17].
Heart failure is the heart's inability to pump blood, leading to symptoms such as fatigue, shortness of breath, swelling, chest pain, palpitations, weight gain, swelling in the ankles, legs, and abdomen, bloated or hard stomach, dry and hacking cough, and nausea [18] [19]. More than 6 million adults in the United States have heart failure [18].
Cardiomyopathy represents a collection of diverse conditions of the heart which makes it weaker and less able to pump blood. Cardiomyopathy can be caused by several factors, including infection, toxins, and genetic disorders [20]. Due to the potential for underdiagnosis, estimates of cardiomyopathy prevalence can vary. It is estimated that up to 1 in 500 adults may be affected by this condition [21].
Pericardial disease is a condition that affects the pericardium, the sac that surrounds the heart [22]. This can cause inflammation, infection, or scarring of the pericardium, which can interfere with the heart's ability to function. Pericardial disease can manifest as acute pericarditis, pericardial effusion, cardiac tamponade, or constrictive pericarditis [22].
Arrhythmia is a cardiac disorder characterized by an abnormal heart rhythm, manifesting as tachycardia (excessively rapid heartbeat), bradycardia (abnormally slow heartbeat), or irregular heartbeat patterns [23]. These irregularities can induce symptoms such as palpitations, dizziness, and syncope (fainting episodes) and can arise from various etiologies, including underlying heart conditions, electrolyte imbalances, and adverse effects of certain medications [23]. One in 18 people, or 5 percent of the U.S. population has an arrhythmia with a-fib (atrial fibrillation) being the most common [24].
Aortic dissection is a rare, life-threatening condition in which the inner layer of the body’s main artery (aorta), tears [25]. This can cause blood to leak between the layers of the aorta, which can weaken the artery and cause it to rupture. Aortic dissection can be caused by several factors, including high blood pressure, atherosclerosis, and connective tissue disorders [25].
Aortic dissection affects about 30 in 1 million people each year and more than 13,000 die each year [26]. Aortic dissection is most common in those individuals over the age of 60 and if not treated, the tear can worsen, ripping the outer layer of the aorta and allowing blood to escape the artery [25] [26]. As many as 40 percent of individuals who suffer from an aortic dissection die, and the risk of death increases by 3-4 percent every hour the condition is left untreated [26].

Self Quiz
Ask yourself...
- What are the distinct mechanisms and implications for dietary management of different types of heart disease?
- How do the etiologies and clinical manifestations of heart valve disease, cardiomyopathy, and pericardial disease differ from each other?
- What are the potential consequences of untreated arrhythmias, and how can lifestyle modifications and pharmacological interventions contribute to their management?
- Why is aortic dissection considered a life-threatening condition, and what are the critical steps involved in its diagnosis and treatment?
Evidence on Diet and Heart Health / Diet Options
When considering the diet options for heart health, the Mediterranean diet, DASH diet, and plant-based diets are among the most researched and recommended. These diets share common elements such as an emphasis on whole foods, minimal intake of red meat, and a high volume of fruits and vegetables. Extensive research has demonstrated that the DASH dietary pattern lowers the risk of cardiovascular disease [37]. Numerous prospective studies have demonstrated the consistent benefits of the Mediterranean diet on cardiovascular health [30].
There is substantial evidence that most vascular events may be prevented by avoiding smoking, participating in regular physical activity, maintaining normal body mass index, and eating a healthy diet [27].
Observational studies have demonstrated that dietary patterns rich in fruits and vegetables, excluding white potatoes, are linked to a lower risk of CVD [28] [29]. Regular consumption of whole grain foods has been linked to a reduced risk of cardiovascular disease (CVD), coronary heart disease (CHD), stroke, metabolic syndrome, and various cardiometabolic risk factors, as evidenced by robust observational studies and clinical trials [28]. Numerous randomized controlled intervention studies have demonstrated that replacing refined grains with whole grains leads to significant improvements in cardiovascular risk factors [28] [31].
Except for a small trial that demonstrated a reduction in arrhythmia recurrences among regular drinkers with atrial fibrillation upon alcohol abstinence, no other studies have reported favorable outcomes associated with alcohol consumption for cardiovascular health [32].
Dietary fiber, abundant in plant-based foods like fruits, vegetables, whole grains, nuts, seeds, beans, and legumes, have shown an inverse association with a lower risk of metabolic syndrome and cardiometabolic risk factors [33].
A systematic review and meta-analysis provided evidence that substituting saturated fat with unsaturated fat can improve cardiovascular outcomes and reduce the risk of CVD [34]. Plant-based diets rich in foods like whole grains, fruits, vegetables, and nuts, have been linked to a reduced risk of cardiovascular events and intermediate risk factors [36].
Current evidence does not support the widespread use of high-dose vitamin and mineral supplements for the prevention of cardiovascular disease (CVD) [28].


Self Quiz
Ask yourself...
- What are the common elements shared by the Mediterranean diet, DASH diet, and plant-based diets that contribute to their positive impact on heart health?
- How does the evidence from observational studies and clinical trials support the link between regular consumption of whole grains and a reduced risk of cardiovascular diseases?
- What are the potential mechanisms by which dietary fiber from plant-based foods exerts its protective effects against metabolic syndrome and cardiometabolic risk factors?
Role of Sodium and Fats
Sodium and fats hold significant impact over heart health. High sodium intake is associated with hypertension, a risk factor for heart disease, while certain types of saturated and trans fats, are linked to an increase in LDL cholesterol and the development of atherosclerosis [28]. A systematic review and meta-analysis revealed the detrimental effects of saturated fat on cardiovascular disease (CVD) outcomes and risk factors compared to unsaturated fat. [36]
A strong body of evidence has documented the adverse effects of trans fatty acids on cardiometabolic risk factors [37]. Adhering to current recommendations to replace saturated fat from meat and dairy with nontropical plant oils also lowers dietary trans fatty acids [38].

Self Quiz
Ask yourself...
- How does the consumption of sodium and certain types of fats contribute to the development of cardiovascular diseases?
- What dietary strategies can be implemented to reduce sodium intake and limit the consumption of saturated and trans fats, thereby promoting heart health?
Healthy Eating Tips and Strategies
Incorporating a heart-healthy diet into a patient’s lifestyle requires practical tips and strategies. First, make gradual changes instead of overhauling the entire diet. Meal planning can help with healthier choices.
Setting realistic goals and collaborating with the patients to set achievable dietary goals. Controlling portion size, using smaller plates and bowls, prioritizing low-calorie, nutrient-rich foods like fruits and vegetables and limit high-calorie, high-sodium foods like refined, processed, or fast foods [39].
Paying attention to the amount of saturated and trans fats, cholesterol, and sodium. Suggest lean protein sources such as fish, poultry, beans, and lentils and limit the intake of unhealthy fats and instead opt for healthy fats like those found in olive oil, avocados, and nuts [39].
Consume at least five servings of fruit and vegetables daily [41]. Choose whole grains over refined grains and limit the intake of added sugars [28]. To have more control over the ingredients, cook and consume meals at home and make gradual changes to diet habits and build on those habits over time.

Self Quiz
Ask yourself...
- How can healthcare providers collaborate with patients to set achievable dietary goals that align with their lifestyle and preferences?
- What are some practical strategies for making gradual changes to a patient's diet, ensuring adherence and long-term success in adopting heart-healthy eating habits?
Patient Education
Nurses are well positioned to provide patient education and counseling on heart-healthy eating. The core of patient education lies in translating knowledge into practice. Nurses must communicate with consideration of cultural, linguistic, and individual patient dietary preferences. Education should be patient-centered, with actionable steps that patients can integrate into their daily lives [40].
Effective patient education strategies can include establishing a rapport, assessing the patient's knowledge, and understanding, tailoring the educational approach, using plain language, incorporating visual aids, encouraging active participation, providing written materials and ongoing support and follow-up, and addressing any barriers and concerns [42].

Self Quiz
Ask yourself...
- How can nurses tailor their patient education strategies to address individual cultural, linguistic, and dietary preferences, ensuring that the information conveyed is relevant, understandable, and actionable for each patient?
Resources
A wealth of resources is available to support nurses in their role as patient educators, from professional organizations like the American Heart Association to local community programs and online platforms [43]. These resources offer educational materials, dietary guidelines, and support tools that nurses can utilize to enhance their teaching.
A heart-healthy diet is one that is low in saturated and trans fats, cholesterol, and sodium. It is also high in fiber, fruits, vegetables, and whole grains [2][3]. Eating a heart-healthy diet can help to lower your blood pressure, cholesterol levels, and risk of heart disease [2].
The National Heart, Lung, and Blood Institute (NHLBI) has a website with information on heart-healthy eating, including recipes, meal plans, and tips for eating out. The American Heart Association (AHA) has a website with information on heart-healthy eating, including recipes, meal plans, and tips for shopping for heart-healthy foods.
The Dietary Approaches to Stop Hypertension (DASH) diet is a heart-healthy eating plan that has been shown to lower blood pressure [45].
The Mediterranean diet is a heart-healthy eating plan that has been shown to have several health benefits, including reducing the risk of heart disease, stroke, and type 2 diabetes [44]. You can find information about the Mediterranean diet on the Mayo Clinic website.
In addition to these websites, there are many cookbooks and other resources available on heart-healthy eating. Patients can also talk to their doctor or a registered dietitian for personalized advice on how to eat a heart-healthy diet.

Self Quiz
Ask yourself...
- How can nurses utilize the wealth of resources available, such as those from professional organizations and online platforms, to enhance their patient education on heart-healthy eating?
- How can nurses convey the key principles of heart-healthy eating, such as limiting saturated and trans fats, cholesterol, and sodium while emphasizing fiber, fruits, vegetables, and whole grains?
- How can nurses guide patients towards reputable and reliable resources, such as those from the National Heart, Lung, and Blood Institute, the American Heart Association, and the Mayo Clinic, to ensure that they have access to accurate and evidence-based information on heart-healthy eating?
Conclusion
In conclusion, adopting a heart-healthy diet is a crucial step towards maintaining cardiovascular well-being and overall health [30]. The nurse's role as a patient educator in promoting a heart-healthy diet is pivotal. By leveraging a position of trust and frequency of contact with patients, nurses can champion dietary choices that can reduce the risk and impact of heart disease.
Research underscores the importance of a balanced intake of nutrients to support the cardiovascular system's health, emphasizing whole foods, lean proteins, healthy fats, and a reduction in sodium, saturated fats, and sugars [3].
Patients adopting a heart healthy diet require a multifaceted approach, combining knowledge with actionable steps that are practical and sustainable. The role of healthcare professional is instrumental in this dietary transition.
A heart-healthy diet offers a multitude of benefits beyond reducing heart disease risk. It can improve blood pressure control, lower cholesterol levels, promote healthy weight management, and enhance overall energy levels [2][3]. A heart-healthy diet can contribute to a stronger immune system, reduced inflammation, and improved mental clarity [46].

Self Quiz
Ask yourself...
- How can nurses bridge the gap between knowledge and practice when educating patients about heart-healthy eating, ensuring that patients understand the rationale behind dietary recommendations and can translate that knowledge into sustainable dietary changes?
- How can nurses emphasize the broader health benefits of a heart-healthy diet beyond reducing heart disease risk, motivating patients to adopt sustainable dietary changes for their overall well-being?
Adverse Childhood Experiences
Introduction
All children should grow and thrive in a caring, nurturing environment that meets their physical, emotional, and social needs. However, many are not so fortunate. Nearly every two in three Americans (64%) have experienced adverse childhood events (ACEs) (1, 2, 4, 5).
ACEs are highly associated with future chronic health conditions, mental illness, premature death, and more (1). In fact, the more ACEs an individual has had, the higher their risk for having poor health outcomes as adults (13).
Fortunately, there are preventive and protective measures that can be taken with the appropriate resources (1). With better awareness of ACEs and the right support in place, healthcare professionals can help improve the quality of life for a myriad of children and the adults they grow up to be.
Definition
Adverse childhood events, or ACEs, can occur from birth until age 18, and are great determinants of future health (1). These are any potentially traumatic events that happened to a child. ACEs are strongly linked to mental and physical disease in adults, contributing to at least half of the leading causes of death in the United States (1).
The ACE score is a 10-item survey to identify any negative occurrences a person had before their 18th birthday (3). The higher a person’s ACE score, the more risk they have for an unhealthy adulthood (1,2).
ACE was a term coined in a large-scale study published in 1998, describing them as childhood abuse and household dysfunction in seven specific areas (1, 2). In subsequent years, three more areas were added (3, 13). Altogether, the ten ACEs are comprised of the following examples of three broad categories:
- Childhood abuse
-
- Psychological
-
- Physical
-
- Sexual
- Household dysfunction
-
- Substance abuse
-
- Mental illness
-
- Parent treated violently.
-
- Family member incarcerated.
- Neglect
-
- Emotional
-
- Physical

Self Quiz
Ask yourself...
- How would you briefly define adverse childhood experiences (ACEs)?
- Would you add or subtract anything from the list above?
- What other examples of ACEs exist?
- Should one’s community and environment factor into ACEs as well? Why or why not?
Statistics and Demographics
The initial adverse childhood experience (ACE) study, conducted in the late 1990s, included over 17,000 adult participants and revealed the following key demographic data (1, 2, 5):
- Nearly 66% of women and 62% of men reported at least one ACE in their lives.
- More than 1 in 6 people (17.3%) said they had experienced four or more types of ACEs.
- Respondents’ races who reported ACEs were: 74.8% white, 11.2% Hispanic, 7.2% Asian/Pacific Islander, 4.5% black, and 2.3% other.
- About 49% of those with college degrees experienced at least one ACE, compared to 44% without a high school diploma.
- The age group who reported the highest incidence of ACEs was those from 35-49, where almost 70% had experienced at least one ACE. The lowest incidence was reported by those aged 65 and older, at 40%.
- Of all women reporting ACEs, 25% said they endured sexual abuse, 30% witnessed substance abuse in the home, and 25% of the respondents’ parents got divorced.
- Of all men reporting ACEs, 30% went through physical abuse, 24% saw substance abuse at home, and 22% of their parents were divorced.
More recent data shows that at least 50% of the top causes of death in the US are associated with ACEs (6). As there is also a high association between ACES and depression, preventing these experiences could reduce adult depression by 44% (6).

Self Quiz
Ask yourself...
- Did any of the statistics surprise you? Why or why not?
- Reflect on your own life. How many of the 10 ACES might you have had, and how might you be able to manage them as an adult?
- What other data gathering might be useful when it comes to identifying and preventing ACEs?
- What would you identify as some of the highest risk factors for the occurrence of ACEs?
Causes and Risk Factors
The causes of adverse childhood events are varied and multifaceted. They can stem from familial or communal challenges, often referred to as social determinants of health. Many of these determinants can cause ACEs, and may include (8):
- Lack of access to healthcare or other resources
- Financial difficulties
- Homelessness or frequent moving
- Discrimination
- Any type of disrupted living situation
As noted earlier, most of the American population has had some sort of adverse childhood experience. However, there are some factors that make it more likely for these to occur. Societal, communal, and familial cultures all play a role in adverse experiences.
The following situations or conditions increase the likelihood of ACEs (7):
Community Risk Factors:
- High rates of poverty, crime, and violence
- Limited educational, economic, and employment opportunities
- Lack of community activities for youth
- Unstable housing and frequent moves by community residents
- Readily accessible alcohol and drugs
- Frequent experiences of food insecurity
Family and Individual Risk Factors:
- Social or developmental disabilities or delays
- Mental or chronic physical illnesses
- A history of abuse experienced by caregivers themselves.
- Youth dating or engaging in sexual activity early.
- Single-parent families, particularly those with young caregivers
- Low income and/or high economic stress
- Low education levels
- Children with few or no friends, or friends who partake in delinquent or aggressive behaviors
- Caregivers with limited understanding of child development
- Use of spanking or other corporal punishment as a form of discipline
- Inconsistent discipline and/or little parental supervision
- Families isolated from extended family, friends, and/or neighbors

Self Quiz
Ask yourself...
- In the community where you live, what might be some factors that contribute to ACEs in those around you?
- What are some ways your community might be able to mitigate some of the risk factors for ACEs?
- Since nearly two in three people have experienced an ACE in their lives, it is likely that you interact with someone affected by this issue. What resources might you recommend to them, and what else might help?
Cultural Considerations
Though many people experience ACEs, some populations are affected disproportionately. This includes women and those in racial or ethnic minorities, who are at greater risk for experiencing 4 or more ACEs (6, 8). Specifically, African Americans, American Indians, and Alaska Natives (AI/AN) are far more likely to have multiple ACEs than any other race or ethnicity (9,10).
The AI/AN community is a relatively young one, with poverty rates up to four times higher than the national average (10). Furthermore, the AI/AN group is often lumped together as “other” as a race category on surveys, making data harder to come by for this population (10).
AI/AN children, when compared to the total US population, are more likely to have:
- Parents who are divorced (33% versus 21%)
- Lived with someone who abused a substance (24% versus 12%)
- Witnessed domestic violence (15% versus 6%)
- Lived with a parent who ultimately died (4% versus 2%)
Lifespan Impact on the Individual
While adverse childhood experiences affect most Americans, having been through four or more puts a person at significantly higher risk of health problems as an adult (1, 2, 9). In general, the more ACEs an individual has experienced, the more likely they will have poor outcomes later in life, with a specifically high correlation to diabetes, heart disease, obesity, depression, substance abuse, smoking, poor academic achievement, and early death (4).
In fact, ACEs can reduce an individual’s life expectancy by as many as 20 years, compared to someone who has had zero ACEs (8).
The original ACEs study was conducted to determine the link between childhood abuse and adult health risk factors (1). The conclusion was overwhelmingly that in the United States, the main causes of morbidity and mortality are related to many of the health behaviors exhibited by those who have experienced ACEs (1). More recent studies find that ACEs contribute significantly to substance abuse, violence, and self-harming behavior (11).
At least 5 of the top 10 leading causes of death in the United States that are strongly related to ACEs include (9,11, 12):
- Heart disease (#1 cause of death)
- Stroke (#5)
- Chronic lower respiratory diseases (#6)
- Diabetes (#8)
- Chronic liver disease and cirrhosis (#9)
As far as mental health goes, the link with ACEs is clear: The higher one’s ACE score, the more likely they are to be depressed, experience impaired work performance, and have suicidal tendencies (13). Harmful behaviors associated with high ACE scores include smoking, drinking, and drug abuse, as these methods are often used to cope with past traumatic experiences (13).

Self Quiz
Ask yourself...
- In light of the correlation between ACEs and some of the top causes of mortality in the US, what interventions could possibly be made in childhood to prevent these deaths from occurring?
- Though the top 10 causes of death in the US have a variety of causes, the link between ACEs has been well-established for nearly three decades. Why do you think more funding and attention aren’t given to this matter?
- What other physical or mental health concerns not listed above might a person with a high ACE score have?
Societal Implications
Adverse childhood events are preventable and have been shown to contribute to at least half of the leading causes of death in the United States (1, 9, 11). Thus, the impact of ACEs on public health is vast. Since the CDC considers ACEs a public health concern, it is of utmost importance to decrease their incidence (1). Through the reduction and prevention of ACEs, general public health would improve markedly, drastically cutting down on healthcare costs and resources (1, 5, 6, 9).
The societal implications for reducing ACEs are manifold:
- Dramatic reduction of preventable causes of death
- Longer lifespans for the general population
- Better mental health
- More cost-effective physical and mental healthcare
- Lower rates of disease and depression in the general population

Self Quiz
Ask yourself...
- What other societal impacts might ACEs have?
- How could you go about preventing some ACEs in your own life or community?
- What are some local, national, or global resources to which you could introduce those in need?
Ways to Mitigate ACEs
Though ACEs are widely pervasive in American society, they need not be so. With proper resources and support systems, many -if not all- ACEs may be eradicated. The best way to mitigate ACEs would be to prevent them in the first place (4). This requires creating safe, stable, nurturing environments for children and their families (9). Furthermore, as ACEs can occur for a wide array of reasons, they need to be addressed at both the family and communal levels.
With individuals, a first step would be prevention of ACEs in the home. This could involve a vast array of mental health, education, and social work services such as: home visits, parenting classes, therapy sessions, and more (8, 9). Different emotion management techniques and child development concepts could be taught to at-risk families, promoting bonding and trust between children and their caregivers.
High-quality childcare and after-school programs with trusted adults can help mitigate ACEs as well (9). Screening for ACEs at regular intervals would also be helpful. This could include annual check-ups at the doctor’s office, visits with the school nurse or counselor, or a consultation with a home health provider. Medical management of physical and mental health conditions would be made available, including routine health screenings and necessary medications (4, 8, 9).
At the community level, prevention could take the form of free education for family members, food and housing assistance, adequate access to healthcare services, and fostering safe neighborhoods (4, 9). Economic support for families could include family-friendly work policies, earned income tax credits, and so forth. Children could partake in community events like after-school clubs and sports, helping them build bonds with trusted and supportive adults.
Three principles are the most helpful preventing long-term effects of ACEs, (4):
- Reducing stress by ensuring basic needs are met, as well as tending to abusive situations, community crime, substance abuse, discrimination, and poverty.
- Strengthening life skills can strengthen the resilience of children and their family members by practicing planning, focus, and self-control.
- Building responsive relationships by having adults listen to their children and respond adequately to their needs, thereby fostering a safe, trusting relationship.

Self Quiz
Ask yourself...
- What specific local resources are available where you live or work that could help prevent ACEs from occurring in the first place?
- What types of secondary and tertiary prevention resources can you identify for ACEs?
- What are other ways ACEs can be mitigated or prevented at the state or federal level?
Reporting ACEs
Considering there are various types of ACEs, they can be difficult to identify and thus report. However, some are clear-cut. In situations where child abuse or neglect is occurring or suspected, a mandated reporter (e.g., nurse, teacher, counselor) would need to report it as soon as possible. If the child is in imminent danger, take them to a safe place and make a report.
To report child abuse in the United States, call or text 1-800-4-A-CHILD (1-800-422-4453). The website is https://www.childhelphotline.org/.
If there is suspicion that a child is being sexually exploited, the phone number to call is 1-800-THE-LOST (1-800-843-5678), and the website is https://report.cybertip.org/.
To report human trafficking, call 1-888-373-7888, use TTY at 711, text BEFREE (233733), or visit the website at https://humantraffickinghotline.org/.
For anyone experiencing feelings of self-harm or suicide, 988 is the national Suicide and Crisis Lifeline that can be called or texted. The website is https://988lifeline.org/.
Support and Additional Resources
While there is no single way to prevent ACEs from occurring, there is a plethora of resources available to help mitigate the effects and to educate others. Visting a healthcare provider can connect a patient to mental health professionals, support groups, or specialty services like social work and support groups (8).
The Substance Abuse and Mental Health Services Administration (SAMHSA) has an abundance of resources on topics like trauma-informed care, early childhood mental health programs, Native Connections for the AI/AN population, school, and campus health, and much more. More information can be found at https://www.samhsa.gov/programs.
Healthy Outcomes from Positive Experiences (HOPE) is a national resource center offering research, training, and technical assistance in order to better the lives of children and their families. Their website is https://positiveexperience.org/.
The Centers for Disease Control and Prevention (CDC) offers ACE-specific information at this website: https://www.cdc.gov/violenceprevention/aces/resources.html.
Conclusion
Adverse childhood events, or ACES, affect the majority of the American population. Strongly correlated with many leading causes of death, ACEs are preventable and warrant attention from healthcare providers and the general public alike. Fortunately, many resources exist at the individual, community, and national levels to provide care and prevention for these experiences.
Though ACEs tend to lead to negative health outcomes, they need not condemn a person to lifelong problems. Through screening tools, community food and housing programs, mental health assistance, education, and adequate medical care, ACEs can be mitigated, well managed, and prevented.
Despite the misfortune in a child’s life, there is still much hope available when they reach adulthood.
Conclusion
Adverse childhood events, or ACES, affect the majority of the American population. Strongly correlated with many leading causes of death, ACEs are preventable and warrant attention from healthcare providers and the general public alike. Fortunately, many resources exist at the individual, community, and national levels to provide care and prevention for these experiences.
Though ACEs tend to lead to negative health outcomes, they need not condemn a person to lifelong problems. Through screening tools, community food and housing programs, mental health assistance, education, and adequate medical care, ACEs can be mitigated, well managed, and prevented.
Despite the misfortune in a child’s life, there is still much hope available when they reach adulthood.
Trauma Informed Care in Nursing
Introduction
As nurses, we have been trained to perform ongoing assessments on patients with every encounter. Many patients have gone through traumatic events that often go undiscovered by the healthcare team.
A trauma informed care approach will help healthcare providers uncover past trauma. This will allow them to tailor a plan of care that helps alleviate patient suffering by decreasing overall stress and anxiety. When the healthcare team is aware of past trauma, they can avoid conversations and situations that could potentially re-traumatize the patient.
Trauma Informed Care
Trauma is a human experience that can affect people from all different backgrounds and walks of life. There is no race, gender, sexual orientation, or social class that is immune to the far-reaching clutches of trauma. A traumatic event is an event that is marked by sexual violence, severe injury and/or death. These events can be first-hand accounts where the people themselves were the victims of the event.
They can also be indirectly experienced by witnessing the event take place on another person. The event can also produce vicarious trauma when it happens to a close friend or loved one. No matter how traumatic an event was experienced, it can have severe and long-lasting effects (1).
Despite the rising accounts of traumatic events, trauma informed care is an underused skill in the healthcare field. Those who support and use trauma informed care understand that there is a large population of people seeking healthcare services who have had past traumatic experiences. They are also aware that just by seeking out care, they have the potential of being re-traumatized.
Empowering the patient to have control over their care through collaboration will help decrease the chances of re-traumatization.
There are six principles of trauma informed care:
- Safety
- Trustworthiness and transparency
- Peer support
- Collaboration and mutual support
- Cultural and historical sensitivity
- Empowerment of voice and choice
Trauma informed care runs parallel to ethics in the healthcare setting. It endeavors to ease the patient’s suffering by preventing re-traumatization and empowering the patient. (3)

Self Quiz
Ask yourself...
- What are two principles of trauma informed care?
- What is a past traumatic event?
- Who is affected by trauma?
Principles of Trauma Informed Care
It is meaningful to explore each of the six principles of trauma informed care
Safety
In trauma informed care, safety pertains to both the psychological and physical safety of the patient and their family throughout their encounter with the healthcare organization. The goal is to prevent any form of re-traumatization. This is accomplished by creating safe spaces, access to services, and engagement between the patient and the healthcare team.
One of the greatest aspects of safety in trauma informed care is environmental. This demands that the healthcare teams create an environment that is both welcoming and accommodating to all patients regardless of their diverse backgrounds. We must understand that patients who have undergone traumatic events can be hypersensitive to their environmental safety.
The trauma informed care plan must be individually tailored and include communication initiatives that promote the patient’s self-identity and how they would like to be addressed (1).

Self Quiz
Ask yourself...
- In trauma informed care, what kind of safety is discussed?
- How does the environment play a part in safety?
Trustworthiness and Transparency
Nursing has long been recognized as the nation’s most trusted profession. This has been accomplished through the intentional focus of patient advocacy. Nation-wide policies and safety goals have promoted diversity, equity, and inclusion. Initiatives to make sure that the patient understands and agrees with their plan of care, such as using their preferred language in plain and clear terms, is an example of how nurses build that trust.
Using the concept of patient-centered care helps demonstrate transparency, which is a way nurses further build trust. True transparency can be accomplished through encouraging patients to be involved in their care. Asking patients if it is acceptable that we document their experiences and even share our notes or computer screens to demonstrate our desire to be transparent.
Educating patients using the teach-back method also indicates our transparency as our knowledge becomes theirs (1).

Self Quiz
Ask yourself...
- How can the healthcare team build trust using trauma informed care?
- How can transparency be accomplished?
Peer Support
Contrary to popular beliefs, the goal of peer support in trauma informed care is not fixing the individual. Rather, the goal is to assist the individual to empower themselves by connecting them with supportive groups of peers that have similar experiences, culture, beliefs, and religion. Empowerment of the patient should be the goal of peer support. Traumatic stress is often accompanied by a re-traumatization cycle that can be interrupted and ultimately stopped through individual empowerment of the patient.
These peer support groups often contain members who themselves have survived traumatic events. To have the best probability of success, the patient should leave behind former friends who enable the re-traumatization cycle and gravitate to the peer support group members. This can leave the patient with further feelings of loss and grief. This has to be considered when developing the plan of care for the patient (1).
Identifying Past Trauma
Trauma informed care does not mean that the care is tailored to a patient’s specific trauma. Nor does it mean that the team endeavors to heal or even address past trauma. Rather, in simple terms, trauma informed care recognizes that the patient has been through a traumatic ordeal and that the plan of care must take that trauma into account in order to properly care for the patient.
The patient’s reaction and compliance may be affected by their past trauma. Past trauma needs to be identified and acknowledged. Past trauma can affect all aspects of a patient’s life. Identifying and acknowledging the trauma and how the patients then needed coping mechanisms may now have become detrimental to their health is important for the overall well-being of the patient.
Through the identification of past trauma, the team can take the opportunity to ensure that the patient does not feel responsible for the life-altering trauma and understands that the trauma was not their fault. Identifying the trauma can lead to the revelation that there is a connection between past trauma and the patient’s current coping and functioning; this can change their overall perspective, thus changing their lives (4).

Self Quiz
Ask yourself...
- What is the goal of peer support in trauma informed care?
- Who is often involved in peer past trauma support groups?
Collaboration and Mutual Support
Collaboration and mutual support demand that the entire healthcare team see the patient as both an equal partner and the expert in their own personal experiences. The patient should be allowed to both identify and prioritize their goals, then the healthcare team can work with the patient to for the plan of care with these goals at the forefront.
Collaboration takes more time than the traditional healthcare “do as I say” method. It helps in the trust-building process and when done properly, it helps the patient to succeed as the goals have come from them. This is something that can also interrupt the re-traumatization cycle. It also increases the probability that the patient will be compliant with the plan of care and any after-care appointments and follow-ups (1,4).


Self Quiz
Ask yourself...
- How is the patient seen through the collaboration lens?
- What destructive cycle can collaboration and peer support interrupt?
Cultural and Historical Sensitivity
The entirety of the patient’s identity is surrounded in cultural and historical sensitivity. Not only does this encompass aspects such as race, gender, age, etc. but it also includes life experiences and relationships right down to the base familial associations.
It also includes beliefs, core values and experiences in open spaces. When seeking healthcare, no matter the reason, all of these aspects of the patient’s identity are brought to the facility and laid in front of the healthcare team (1).
Empowerment of Voice and Choice
As discussed earlier in the peer support principle, empowerment is paramount in trauma informed care. Empowerment of voice and choice is perhaps the chief cornerstone and is present in all the other principles of trauma informed care. Trauma informed care empowers patients by making them feel heard and that their voice is important for their overall well-being.
Though the healthcare team may not always agree, trauma informed care also enforces the concept that the patient’s choices are to be respected. Through the trauma informed care journey, the patient develops their ability to navigate the healthcare setting and becomes familiar with the language of the medical community.
This further empowers the patient as they can understand the plan of care and become an active participant in that plan, as they were involved in both the planning and implementation. Further, trauma informed care creates a safe and supportive environment where the patient is allowed to grow and mature in their knowledge and understanding of themselves and their health (1).

Self Quiz
Ask yourself...
- In what ways does trauma informed care empower patients?
Effects of Traumatic Stress
Traumatic stress has shown to increase chronic illness, mental health issues and early death. When left alone, traumatic stress can morph into enduring stress. Some forms of enduring stress include:
- Chronic stress – this happens when trauma is continuous over a long period of time.
- Toxic stress – this is found in children who experience long-standing and/or frequent trauma such as emotional and physical abuse, neglect, and exposure to violence.
Traumatic stress can have altering effects on multiple brain processes. This includes the neurologic, immunologic, endocrinologic, metabolic, inflammatory, and autonomic processes. Depending on which phase of brain development the patient is in when the trauma occurs, it determines the long-lasting effects.
Repeated trauma can cause a hormonal cascade which can result in the allostatic load phenomenon. This phenomenon distorts the normal stress mediating processes.
Traumatic stress often leads to self-destructive behavior. Drug and alcohol use/addiction, violence and risky behavior often follows those who have had traumatic stress events. There is a fear among traumatic stress victims that they may be judged for their choices and lifestyle (1).


Self Quiz
Ask yourself...
- Can you name a type of enduring stress?
- What is the phenomenon that distorts the normal stress mediating processes of the brain?
- What can traumatic stress lead to?

Self Quiz
Ask yourself...
- What are two things that trauma informed care is not?
- What can be accomplished through identifying trauma?
- What does the healthcare team need to do in connection with the patient’s trauma?
Adverse Childhood Experiences
Nearly 40% of people have been exposed to adverse childhood experiences. Of course, adverse childhood experiences harm the overall health and well-being of the child; they also have a potential great impact on the physical and mental health of the person as they transition into adulthood.
Adverse childhood experiences and childhood trauma have shown to leave the patient with an increased risk for developing leading causes of death and disability. These conditions include heart disease, stroke, cancer, and depression with suicidal ideation. Also, childhood trauma can lead to symptoms of chronic headache and pain as an adult (2).
As most childhood trauma is perpetrated by someone known and close to the child such as a parent, relative or close family friend, the child is often left with an altered expectation of interpersonal relationships. This may lead to severe insecurity and distrust where negative personal perception especially in relation to others may develop.
People who develop physical symptoms of past childhood trauma are often unable to adjust and move on post-trauma. On the other hand, those who are able to form positive relationships tend to have fewer physical symptoms and are more liable to have adjusted to childhood trauma. Childhood trauma can have long-reaching effects on the ability of the adult to form positive relationships and have normal responses to stressful situations (2).

Self Quiz
Ask yourself...
- What percentage of people have had adverse childhood experiences?
- What type of conditions are victims of adverse childhood experiences in danger of developing?
- Can you discuss what adverse childhood experiences can lead to?
Trauma Informed Approach
The trauma informed approach was born out of the research on adverse childhood experiences. The research showed that the more adverse childhood experiences that took place in the patient’s life, the more it affected their mental and physical health; even leading to early death.
Trauma informed approach begins at the organizational level, where the culture must adapt to prevent re-traumatization. There are a few aspects that need to be incorporated into the organization’s training to accomplish the changes needed.
The organization must put on trauma glasses and view the healthcare team through the trauma lens. There is a direct correlation between trauma and mental health; this needs to be acknowledged, accepted, and viewed as part of the care plan.
In order for the trauma informed approach to be effective, the organization must realize that trauma expands beyond Post Traumatic Stress Disorder (PTSD). Rather, trauma incorporates a multitude of differing issues that can include mental, emotional, physical, and other multiple trauma sources.
The healthcare professional who is assessing the patient should be trained in recognizing the signs of trauma and, if disclosed, be able to respond appropriately in the moment. Training needs to be done with all front-line staff who the patient may confide in.
The healthcare team should be aware of resources that are available in the community and be able to refer the patient to those organizations. Being as trauma specific as possible so that the patient can connect in a meaningful way with this new support system. The organization should partner with these support groups to ensure easy transitioning from the institution to the community.
The team should also prioritize the principles of trauma informed care. For instance, to promote trustworthiness and transparency, the team could limit the number of healthcare providers who will ask the patient to repeat the story of their traumatic experience. Turning to more collaborative communication and relationships between the team and the patient; allowing the patient to be actively involved in their plan of care also promotes the trauma informed care principles.
Promoting safety within the organization for both the patient and the healthcare team also helps to change the culture to one of a trauma informed approach. Trauma survivors could be approached and asked to help design, implement, and evaluate the trauma informed approach to be used. What better way to get to know your community resources than to actively partner with them to help meet the needs of the organization’s patients.
It needs to be recognized that not all patients who show similar symptoms to those of past trauma have gone through a traumatic event. There is no cookie-cutter plan of care, and all patients must be properly assessed to determine what their plan of care will be (6).

Self Quiz
Ask yourself...
- How was the trauma informed approach born?
- What must change to prevent patient re-traumatization?
- What are two ways that an organization can change to a trauma informed care culture?
- Should all past trauma survivors be cared for in the same manner?
Impact of Trauma Informed Care
The impact of trauma-informed care on the patient and on the healthcare system as a whole cannot be overstated. By understanding that a great deal of the patients who seek out healthcare services have undergone a traumatic event and tailoring the assessment with that in mind, an organization can minimize the occurrence of re-traumatization.
When we understand how trauma has affected our patients’ lives, how they perceive the healthcare system and what their previous experiences within that system have been like, steps can be taken to ensure better outcomes within this population (1).

Self Quiz
Ask yourself...
- What are healthcare institutions trying to prevent through trauma informed care?
- How can healthcare facilities ensure better outcomes within the past trauma patient?
- What are two ways that nurses can help minimize re-traumatization?
Nursing Implications
In order for trauma informed care to be properly accepted and put into use in the healthcare setting, the culture must be transformed to be a trauma-informed culture. Nursing is with the patient around the clock and nurses have the ability to touch patient’s lives in the most impactful way.
Here are a few considerations that pertain to the healthcare team but when used by nurses have the ability to transform the patient’s environment into a true trauma informed care setting.
Introductions
Even if the nurse believes that the patient knows who they are, it is important for the nurse to reintroduce themselves with every interaction. The patient generally has many different members of the healthcare team entering their space throughout their stay. Team members tend to meld into one anonymous face to the patient; the nurse by introducing and reintroducing themselves and their role to the patient will not only foster an understanding of who does what, but they will empower the patient to be engaged and involved in their plan of care.
Body Language
Body language is important when caring for any patient. When caring for a patient who has experienced trauma, this becomes even more impactful. Open body language sets the stage for trust.
Trauma survivors may often feel a sense of being trapped or confined which may lead to an overall sense of powerlessness. Unintentional threatening body language magnifies these feelings and could bring on a re-traumatization episode. By contrast, non-threatening body language decreases the trauma center and leaves the patient calm and non-triggered.
Trauma informed care body language includes being at the patient’s level; commit to sit or raising the bed so that both patient and nurse are at the same level. Knowing the environment and deescalating trauma by the nurse positioning themselves properly in relation to the patient and the door; allowing for both to access so that the patient does not feel confined.
Anticipatory Guidance
Past trauma may have been unpredictable or an outright surprise. Verbally telling a patient what will be expected during their stay will reassure them even if the coming procedure or test may cause pain. Sharing who will be part of their care during their stay will also set them at ease. Knowing and understanding the expectation further empowers the patient to be involved in their plan of care.
The expectation, when known, decreases those feelings of surprise which could bring them back to that time of trauma.


Self Quiz
Ask yourself...
- Why should the nurse introduce themselves by name and role?
- How can the nurse’s body language affect the past trauma patient?
- What is incorporated in anticipatory guidance?
Permission
Touch can have an incredible impact on the patient who has experienced past trauma. Unwanted or inappropriate touch quite likely may have been a part of their traumatic experience. It can activate those traumatic memories and activate the re-traumatization cycle. Touch is also inevitable when it comes to the nurse/patient relationship.
What the nurse can control is when the touch occurs. Touch should always be preceded by the nurse explaining what they are going to do and asking for the patient’s permission to touch them. Asking permission puts the ball in the patient’s court and empowers them as they are now given a choice and are in control of their body and space.
Permission to touch the patient should never be assumed; permission should be asked every time the nurse needs to touch the patient.
Protect
In many cases, patients who have experienced trauma experienced it at the hand of someone that they know. Many times, the patient will not be alone in their room; family and friends are often present. Patient advocacy has always been the primary role of the nurse.
As part of being an advocate for the patient, the nurse should protect the privacy and safety of the patient. Asking those present in the room to leave prior to discussing the patient’s plan of care is a way to protect both privacy and safety. The patient may not feel safe asking visitors to leave their room especially if they may have been involved in the prior traumatic episode.
By asking visitors to leave, the nurse gives control back to the patient. In private, the nurse can ask who the patient is comfortable with remaining in the room. Once again, the patient is empowered.
Clear and Consistent
Realistic expectations are understood when given in clear and consistent language. This will also foster trust especially if the entire healthcare team is on the same page and vocalizing the same message, consistency. Using language that the patient understands is also paramount. Avoiding medical terminology and acronyms also builds trust as the patient knows that the nurse has made it their priority that the patient understands their plan of care.
Universal Precaution
Finally, trauma informed care needs to be a universal precaution regardless of if the patient’s past trauma history is known or not. By treating all patients as if they had experienced past trauma, those who have will be more apt to share the experience.
Also, nurses will be less likely to start the re-traumatization cycle by inadvertently triggering an episode. It will help treat the patient without relying on the patient to disclose something that they may not yet feel comfortable sharing. (3)

Self Quiz
Ask yourself...
- Why should the nurse ask permission to touch the patient?
- When should the nurse ask permission to touch the patient?
- What does universal precaution in terms of trauma informed care mean?
Resources
It is not easy to become a center where trauma informed care is practiced, it does not happen overnight. Trauma informed care is an intentional shift in culture at the facility and/or system level. Trauma informed training should be implemented, and the staff need to understand the “why” behind the training in order to buy-in.
Trauma informed care not only helps the patient to be empowered and prevent re-traumatization, but staff that have undergone past traumatic experiences can also be helped once a facility adopts this culture. This culture should be at the forefront of both staff and leadership minds. Staff meetings, unit huddles and any other opportunity where leadership actively communicates with staff should incorporate the messaging of trauma informed care.
Facilities should actively be hiring a trauma informed workforce. People from wide varieties of racial and cultural backgrounds should be on the interview panel. Questions pertaining to trauma informed care can and should be asked to pick up on skills and traits that will promote this culture.

A few environmental factors to create the trauma informed culture include keeping doors and common areas well-lit, keeping noise levels low and having warm, cozy colors as decoration in common areas.
Below are some websites that can be visited in order to help kickstart the culture of trauma informed care:
- https://TraumaInformedCare.chcs.org
- https://www.creatingpresence.net/
- https://www.chcs.org/resource/key-ingredients-for-successful-trauma-informed-care-implementation/
- https://store.samhsa.gov/product/SAMHSA-s-Concept-of-Trauma-and-Guidance-for-a-Trauma-Informed-Approach/SMA14-4884
(5)

Self Quiz
Ask yourself...
- What do staff need to understand to be participants in patient informed care?
- When should trauma informed care concepts be communicated to staff?
- How can facilities ensure that new hires are on board with trauma informed care?
- What are some environmental elements that facilities can apply to promote trauma informed care?
Conclusion
Though not a new concept, trauma informed care is essential to help patients who have had traumatic experiences navigate through the healthcare system. Trauma informed care empowers patients to take control of their care in collaboration with the healthcare team. Ultimately, the goal of trauma informed care is to prevent re-traumatization of the patient at the hands of the healthcare team.
The trauma background of any given patient is unknown when they arrive at the facility. It is the responsibility of the nurse to use trauma informed care to both assess the patient and create trust so that they will disclose the trauma. Once known, the team will be able to work together with the patient to prevent further trauma and have positive outcomes.
Conclusion
Though not a new concept, trauma informed care is essential to help patients who have had traumatic experiences navigate through the healthcare system. Trauma informed care empowers patients to take control of their care in collaboration with the healthcare team. Ultimately, the goal of trauma informed care is to prevent re-traumatization of the patient at the hands of the healthcare team.
The trauma background of any given patient is unknown when they arrive at the facility. It is the responsibility of the nurse to use trauma informed care to both assess the patient and create trust so that they will disclose the trauma. Once known, the team will be able to work together with the patient to prevent further trauma and have positive outcomes.
Nursing Care in Lewy Body Dementia
Introduction
Lewy body dementia is one of the more common causes of dementia. Currently it is the second most common dementia disorder following Alzheimer’s disease [2]. This condition is shown to affect more than 1.4 million people in the United States [1] [2]. Of dementia cases in older adults, Lewy body dementia is said to make up 5% of people with dementia [2]. Lewy body dementia is a disorder that progresses over time [1]. The progression of the disease differs between individuals and the severity of the symptoms [1].
On average an individual lives between five to eight years after diagnosis [1]. Currently there is not a cure for this disease [1]. This course will examine the causes of this disease, signs and symptoms patients might experience, diagnostic tests, types of management, and educational resources for family members. This course is designed to inform nurses about this common disease and to use this information in their daily practice to care for their patients.

Self Quiz
Ask yourself...
- What do you think is the most common form of dementia in the United States?
- How common is Lewy body dementia in other parts of the world?
- Is there currently a cure for Lewy body dementia?
- Why do you think Alzheimer’s disease is more common than Lewy body dementia?
Definition
Lewy body is an umbrella term that includes two separate diagnoses: Dementia with Lewy bodies and Parkinson’s disease dementia [5]. As these diseases progress, they develop together and are seen as one entity, not two separate conditions [4]. Lewy body dementia is a condition that involves neurocognitive disorders that include hallucinations, memory loss, behavior changes, and parkinsonism features [2]. This disease can also affect intellectual abilities and cause individuals to act out dreams during REM (rapid eye movement) sleep [2]. REM sleep behavior disorder sometimes may be experienced before any other symptoms are exhibited [2].
Lewy body dementia is known for a buildup of deposits of alpha- synuclein proteins called Lewy bodies [1]. Diagnosing this condition can be difficult because many neurological disorders have similar symptoms. Lewy body dementia and Parkinson disease dementia are very similar. For a diagnosis of Lewy body dementia, there must be a cognitive impairment with motor symptoms occuring in less than 12 months [3]. Parkinson’s disease dementia affects an individual’s movements; cognitive symptoms appear later (greater than one year) [5].
Lewy body dementia is known to affect older adults generally between the ages of 50 and 85 [2]. This disease is said to be underdiagnosed due to a large number of diagnoses occuring post-death during autopsies [4]. Several medications used to treat neurocognitive and behavioral symptoms in other conditions can worsen the symptoms of Lewy body dementia [4]. Therefore, an accurate diagnosis can impact an individual’s quality of life.

Self Quiz
Ask yourself...
- What are the two forms of Lewy body dementia?
- What are the differences between dementia with Lewy bodies and Parkinson’s disease dementia?
- Why is it difficult to diagnose Lewy body dementia?
Epidemiology
Lewy body dementia affects a significant number of individuals in the United States. This condition is found more often in men than women [4]. Age is thought to be the greatest risk factor for an individual developing this disease [4]. An individual who has a family history of Lewy body dementia and Parkinson’s disease is at a higher risk for developing this condition [3].
Lewy Body dementia is more widespread in European, Asian, and African ethnic groups [3]. In individuals with Parkinson’s disease, the incidence of Parkinson’s disease dementia is said to be around 25-30% [4]. The incidence of individuals with Parkinson disease developing this type of dementia after having Parkinson’s for more than 20 years increases to around 83% [4].


Self Quiz
Ask yourself...
- What is the greatest risk factor for developing Lewy body dementia?
- Are there certain ethnic groups that have a higher rate of Lewy body dementia?
- Which gender is Lewy body dementia prominent in?
Pathophysiology
There is a buildup of alpha- synuclein proteins that causes neurons to die in Lewy body dementia [2] [5]. As mentioned above in this course, this buildup of proteins is called Lewy bodies. The death of neurons that produce dopamine result in problems with movement, cognitive impairment, a decline in cognition, and sleep disturbances [4]. In Lewy body dementia there is a deficiency of acetylcholine [3]. There is also a decrease in acetylcholine with Alzheimer’s disease, but the deficiency is greater with Lewy body dementia [3]. The decrease in neurons that produce acetylcholine causes memory loss and learning impairment [4].
The mutation of synuclein alpha and synuclein beta genes can cause dementia with Lewy bodies [2]. Mutations in apolipoprotein E and GBA genes are potential risk factors for developing the disease [2]. There have been cases where a buildup of alpha-synuclein was found during an autopsy, but the individual did not show any clinical signs of Lewy Body dementia when alive [4]. The function of these proteins in this condition is still undetermined [5].

Self Quiz
Ask yourself...
- What are considered Lewy bodies?
- What other disease besides Lewy body dementia has a decrease in acetylcholine?
- What symptoms are a result of destruction of neurons that produce dopamine?
Etiology
The exact cause of Lewy body dementia is still unknown. While research is ongoing and new developments are occuring, the specific cause has not been determined. The accumulation of Lewy bodies cause cell death which causes symptoms, however, the reason for the buildup of Lewy bodies is still under research [5]. As mentioned earlier, there are specific gene mutations that have been shown to increase the likelihood of producing altered alpha- synuclein proteins, in turn causing them to clump together (forming the Lewy bodies) [2].
The mutation of the GBA gene interferes with the function of lysosomes, which can affect the breakdown of the alpha- synuclein proteins, causing the proteins to accumulate [2]. The e4 allele type of the APOE gene has been shown to increase the risk of developing Lewy body dementia [2]. These clumps of Lewy bodies form inside and outside of neurons in different areas of the brain, where they can alter the function of the cell and can cause the cell to die [2].
The neurons that develop the neurotransmitter dopamine are especially impacted by these clumps of Lewy bodies, which was addressed earlier in this course [2]. Further research is required to find out why these Lewy bodies develop in certain individuals. Currently, age, genetics, and environmental factors are some of the greatest risk factors [3].

Self Quiz
Ask yourself...
- What is the cause of Lewy body dementia?
- Why is age a risk factor for developing this disease?
- What does the buildup of Lewy bodies do to cells?
Clinical Signs and Symptoms
Lewy body dementia is a progressive disorder - the signs and symptoms worsen over time. The symptoms that are more common are sleep changes, impaired behavior, movement, and cognition [5]. Research shows that the location of Lewy body accumulation impacts the clinical signs and symptoms the individual experiences [3]. If Lewy bodies develop in the brainstem and cerebral cortex first, the condition is called dementia with Lewy bodies, and the onset of the dementia is early [3]. If Lewy bodies accumulate in the brain stem and then develop into the cerebral cortex as time passes, the onset of dementia appears later, and this condition is called Parkinson’s disease dementia [3].
Rapid Eye Movement Sleep Behavior Disorder
Rapid eye movement (REM) sleep behavior disorder is sometimes the first clinical sign of dementia with Lewy bodies [2]. Individuals with this disorder move and talk while dreaming in their sleep [2]. The movements can be violent and cause the individual to fall out of bed [5]. Individuals may kick, punch, and scream in REM sleep (the second half of their sleep) [4]. REM sleep behavior disorder is seen in 76% of patients with dementia with Lewy bodies [4].
This disorder can cause fractures and contusions in some individuals resulting from falling out of bed [4]. This can not only affect the individual, but also the sleep partner of the patient [4]. In some cases, separate sleeping arrangements are needed for the safety of the individual and their sleeping partner. A questionnaire by the patient and sleep partner is part of the diagnosis of REM sleep behavior disorder [14]. If the individual does sleep next to someone, this questionnaire can be helpful as most of the time the patient cannot recall the events while asleep [14]. Video polysomnography is required for a complete diagnosis of this disorder [14]. These events while asleep must be repeated to meet the diagnostic criteria [14].
Other Sleep Disorders
Other disorders of sleep include sleepiness in the daytime, restless leg syndrome, confusion when awakened, and obstructive sleep apnea [4].
Visual Hallucinations
Visual hallucinations are present in about 80% of individuals with Lewy body dementia [1]. Visual hallucinations are a core clinical symptom of dementia with Lewy bodies [4]. They are more common in women than in men [4]. Individuals are aware of these hallucinations and can tell others what they experienced [4]. Visual hallucinations are vivid to individuals and have been said to range from people walking around the house to seeing people that have died sitting next to them [6]. During the beginning stages of the disease, the hallucinations do not seem to affect the patient as much as when the disease progresses [6]. Patients are said to be afraid of these hallucinations in the later stages of the disease [6]. Nonvisual hallucinations are less common, however can occur in some patients [1]. These hallucinations include smelling or hearing something that is not in their surroundings [1].
Fluctuation in Cognition
Fluctuation in cognition is also a clinical sign that is associated with dementia with Lewy bodies [4]. This symptom includes changes in attention, concentration, and alertness [5]. These changes are random and can differ day-to-day [1]. Symptoms can include delirium, and mimic symptoms that are caused by metabolic diseases, which can further the difficulty with identifying the correct diagnosis [4]. To diagnose dementia with Lewy bodies, one of the episodes must be confirmed [4]. These fluctuations can be present in other forms of dementia in their later stages but when present in earlier stages, they point to dementia with Lewy bodies [4].
Memory loss that impacts activities of daily living can be found in later stages of Lewy body dementia [1]. Memory loss early on is more often a characteristic sign of Alzheimer’s dementia [1]. Confusion about the individual’s whereabouts, and inability to multitask can also occur in dementia with Lewy bodies [4].
Problems with Movement
Problems with movement are signs of Lewy body dementia. Bradykinesia (slow movements) and rigidity occur in about 85% of individuals with dementia with Lewy bodies [4]. Tremor at rest is less common in individuals with this condition [4]. Loss of coordination and difficultly swallowing can occur [1]. Problems with movement greatly increase the risk of falls for these individuals [4]. This can place strain on the individual’s caregivers [4].
Autonomic Dysfunction
Autonomic dysfunction can be present in dementia with Lewy bodies and Parkinson’s disease dementia. This symptom is seen in about 90% of patients with Lewy body dementia [4]. The symptoms that result from autonomic dysfunction can be constipation, urinary incontinence, orthostatic hypotension, erectile dysfunction, and dizziness [1] [4]. Orthostatic hypotension appears as early as five years prior to the diagnosis of Lewy body dementia [4]. Syncope and falls are usually the result of orthostatic hypotension [4]. Constipation can also occur earlier in the disease process [4].


Self Quiz
Ask yourself...
- What is REM sleep behavior disorder?
- Are visual hallucinations common in Lewy body dementia?
- What does cognitive fluctuation mean?
- What are symptoms of autonomic dysfunction seen in dementia with Lewy bodies?
Diagnostic Tests and Evaluations
Throughout this course, it has been mentioned that Lewy body dementia is significantly underdiagnosed. Individuals are usually diagnosed as the disease progresses due to the symptoms that overlap with other forms of dementia and other neurological and psychiatric disorders [3]. An autopsy of the brain after death is one of the only ways to have a conclusive diagnosis of Lewy body dementia [16]. There are certain diagnostic criteria and diagnostic tests that are used to diagnose an individual with Lewy body dementia.
Diagnosis by Symptoms
Lewy body dementia is probable when an individual experiences dementia and two main features of the disease. Lewy body dementia is a potential diagnosis if the individual experiences progressive dementia and one main feature of the disease [3]. As discussed in the clinical signs and symptoms section of this course, key features of Lewy body dementia are cognitive fluctuations, dementia that progresses, problems with movement (signs of parkinsonism), REM sleep behavior disorder, and visual hallucinations [3] [16].
Timing of symptoms is relevant for distinguishing between the two forms of Lewy body dementia [3]. Currently healthcare providers use the time span of one year to distinguish the two forms [3]. If dementia occurs within one year of the appearance of movement problems, then a diagnosis of dementia with Lewy bodies is used [3]. If an individual is diagnosed with Parkinson’s disease and starts experiencing symptoms of dementia more than one year after their Parkinson’s diagnosis, then Parkinson’s disease dementia is used [3]. Some indicative biomarkers in addition to clinical symptoms are used in diagnosis [4]. Some of these biomarkers can be found in cerebral spinal fluid (CSF) and are still under research [4].
Cognitive Tests
Cognitive testing can be used to show the cognitive impairment of patients with Lewy body dementia [3]. The Mini-Mental State Examination can be used as an initial screening test [4]. This exam tests cognitive function by focusing on concentration, orientation, and memory [15]. This test can be limited since symptoms of these patients can fluctuate day to day [3]. Another cognitive function test is the Montreal Cognitive Assessment (MoCA) [15]. Providers do not usually diagnose based on a single test; instead, they use the results to look for other signs and symptoms of Lewy body dementia [4].
Imaging Tests
There are certain imaging tests that can help with diagnosis and distinguishing between other dementia disorders. A single-photon emission computerized tomography (SPECT) scan can help support a diagnosis [16]. This is a nuclear scan that can sense radioactivity [16]. If the SPECT scan shows a reduced dopamine transporter uptake in the basal ganglia, this can be a sign of Lewy body dementia [16]. This will separate the diagnosis between Lewy body dementia and Alzheimer’s disease [4]. Performing this scan alone will not lead to a possible diagnosis of Lewy body dementia; however, in combination with other diagnostic tests, the scan can lead to a more certain diagnosis [4]. Results from these scans can appear normal initially, and the scan may need to be repeated [4].
An iodine- MIBG myocardial scintigraphy can be performed to support Lewy body dementia [16]. This would show decreased communication of cardiac nerves [16]. The results may be skewed by heart disease or certain drugs [4]. A CT or MRI may be used but these imaging tests can present mixed results [4]. With Alzheimer’s disease, significant atrophy is seen in the medial temporal lobes [4]. There is normally minimal atrophy in Lewy body dementia [4].
As mentioned earlier in the course, video polysomnography is needed for the diagnosis of REM sleep behavior disorder [14]. This sleep study without the loss of muscle tone can also point towards a diagnosis of Lewy body dementia as REM sleep behavior disorder has now moved to a key feature of this disease [14].

Self Quiz
Ask yourself...
- What types of imaging tests can be used in the diagnosis of Lewy body dementia?
- Why are cognitive tests used in diagnosis of this disease?
- What criteria are needed for a probable diagnosis of Lewy body dementia?
- Can the cost of diagnostic imaging lead to a reduction in diagnosing Lewy body dementia?
Case Studies
Case Study #1
A 74-year-old male presents to his primary care provider after his wife reports abnormal behavior over the past several months. His wife reports the patient kicks and screams during sleep. The patient reports seeing little people walking around the living room during the day. The wife states the patient some days will fall asleep throughout the day while completing activities. The patient states difficulty walking and muscle stiffness.
The wife states last week the patient was supposed to go to the local grocery store to buy milk. After two hours passed, the wife called her husband as she was worried about him. He states he got lost finding the grocery store and did not know where he was. The wife said she had to drive to find her husband and bring him home. The patient also reports dizziness when standing. After the nurse obtained an orthostatic blood pressure, the patient was positive for orthostatic hypotension.
- Which form of dementia is the patient most likely experiencing?
- What type of symptoms is the patient experiencing that would point to that diagnosis?
- What diagnostic tests or evaluations should the patient undergo?
- What types of supportive treatment should the healthcare provider include in the treatment plan for this patient?
Case Study #2
A 70-year-old female presents to the emergency department via EMS after falling at home. The patient’s daughter called 911 after finding her on the floor when going to visit her. Upon arrival at the emergency department the patient is oriented to self. The patient does not know where she is or what happened to precipitate the fall. The patient has a past medical history of hypertension, diabetes type II, and Parkinson’s disease.
The patient was diagnosed with Parkinson’s disease two years prior. The daughter states the patient has been forgetful lately and not acting like herself. The daughter reports that her mom’s behavior is different from day-to-day. An MRI and the National Institutes of Health Stroke Scale (NIHSS) are used to rule out a cerebrovascular accident. A complete blood count (CBC), a complete metabolic count (CMP), and urinalysis are obtained. The patient suffered a contusion to her right cheek and a right radius fracture. The patient states that she sees figures dancing in the room and smells popcorn. The patient appears to be frightened by the hallucinations. The patient’s daughter states for the last six months the patient has had difficulty swallowing and a reduced appetite.
- Which form of dementia is the patient most likely experiencing?
- What would the MRI of the patient most likely show?
- What clinical signs of dementia is the patient exhibiting?
Management
Currently there is not a cure for Lewy body dementia, only supportive treatment. The management of this disease involves a multifaceted approach, including therapies, pharmacological treatments, and family support.
Therapies
Specific therapies can help with symptom management and help improve the individual’s quality of life. Occupational therapy can help improve a patient’s ability to complete activities of daily living. Speech therapy can help with swallowing coordination and improve the clarity and volume of speech [5]. Physical therapy can aid patients with problems with movement [5]. Mental health counseling can help individuals and their families with managing behaviors and their emotions [5].
Medications
Pharmacotherapy can help with supportive treatment but can also worsen symptoms if certain medications are taken. Below are some examples of medications that are used by patients with Lewy body dementia.
- Cholinesterase Inhibitors are used to help cholinergic activity to improve cognitive function [6].
- Rivastigmine was one of the first of these drugs to be tested [6]. Patients were noted to have improved on their cognitive exams [6]. It is also shown to reduce hallucinations and lessen anxiety [6]. This class of drugs has been said to improve the quality of life for some patients [4].
- Donepezil and Galantamine are also used to reduce dementia symptoms of hallucinations [6]. These drugs were initially targeted for patients with Alzheimer dementia, however, they are effective for individuals with Lewy body dementia as well [3]. A study was done stating even if there is not a sign of cognitive improvement, this should not be the criteria to stop the medication as this medication has been proven to protect the individual from further impairment of cognition [4].
- Atypical Antipsychotics are prescribed to patients that are not seeing a reduction of symptoms while on cholinesterase inhibitors [3]. These types of drugs are seen as controversial due to the many adverse effects that have been seen in patients [4]. Drugs such as haloperidol and olanzapine should be avoided in patients with Lewy body dementia as they can cause neuroleptic malignant syndrome (a life-threatening condition) [5]. Quetiapine, clozapine, pimavanserin, and aripiprazole are atypical antipsychotic drugs that can be used to improve agitation and help prevent cognitive fluctuations [5].
- Carbidopa-Levodopa can be used in patients to manage problems with movement [3]. This medication can cause side effects and can result in hallucinations, delusions, and increase confusion [3]. Providers should begin with low doses of this medication [3].
- Clonazepam is a benzodiazepine that can lessen the REM sleep behavior disorder that patients with dementia with Lewy bodies can experience [5]. Between 33-65% of patients with REM sleep behavior disorder can experience an injury while sleeping [5]. This medication has been proven to decrease injuries that occur during sleep [5]. Clonazepam can adversely affect individuals with gait disorders or sleep apnea [5].
- Melatonin is a hormone that can be used for patients that are affected by REM sleep behavior disorder (5). Studies have shown that the use of melatonin lessened the frequency and the severity of symptoms associated with REM sleep behavior disorder [5]. Melatonin can have side effects such as headaches in the morning, sleepiness during the day, and hallucinations [5].
- Memantine is used to treat dementia symptoms [5]. This medication is an NMDA receptor antagonist that stops effects of glutamate in the brain [5]. Memantine has been shown to improve symptoms of patients early in the disease [5].


Self Quiz
Ask yourself...
- What type of therapies are used in management of Lewy body dementia?
- What class of drugs are used to help improve cognitive function?
- What are some medications that should be avoided in patients with Lewy body dementia?
- Why is melatonin used in patients with Lewy body dementia?
Nursing Care
As mentioned before, there is not a cure for Lewy body dementia. Caring for patients with Lewy body dementia includes supportive treatment. Nurses can play a significant role in caring for these patients and providing the family with support. Home health nurses can help with frequent assessment of the patient and their environment [3]. Environmental changes may be needed to protect the patient from falls and other accidents. Home health nurses can assess the type of assistance the patient would benefit from.
Nurses can aid the family by providing education to assist in how to care for the patient. Family members and caregivers must be aware of the changes in behavior, fluctuations in cognition, and hallucinations that the individual might experience [3]. Nurses must also provide education to the caregiver of the patient on the side effects of certain medications, as they can affect an individual with this disease [7].

Self Quiz
Ask yourself...
- Are there any modifications that nurses must apply to care for patients with Lewy body dementia?
- Whose role is it to educate patients and their family members?
Family Support
Lewy body dementia is growing in recognition; however, many people might not be aware of this condition and the disease process. Family members need support from health care professionals to better care for their loved ones. Support can come in the form of education and preparing the family for the symptoms the individual may experience. The cognitive function of patients with this disease can be very limited [3]. Family members must be educated on monitoring the individual closely to promote safety [3]. These individuals are at a high risk for falling and developing aspiration pneumonia (due to swallowing difficulties) [3]. Family members should be educated in preparing for an emergency.
Individuals with Lewy body dementia may need care and the family needs to know how to inform health care providers of their specific needs. It is important to educate family members that their roles in their past relationship with the patient will likely change due to the disease process. To prevent caregiver burnout, family members must be aware of their limitations and know when they need help [7]. Modifying the patient’s home may be needed for patient safety [3]. Each patient may have specific needs and family members should know what modifications may be necessary [3].

Self Quiz
Ask yourself...
- What type of support do you feel is important to give family members of loved ones with this disease?
- What should nurses include in education for fall risk safety for family members while the individual is at home?
- Can nurses help to prevent caregiver burnout?
Prognosis
The prognosis of Lewy body dementia can be viewed as poor. As mentioned briefly earlier in this course, this disease is progressive and after diagnosis, the life expectancy is five to eight years [3]. The range of expectancy has also been attributed to delay in diagnosis, which can further delay supportive treatment to improve quality of life for the individual [3]. Patients can die from complications from the disease. Complications can include cardiac complications, falls, adverse effects from medications, pneumonia, and suicide [3].
Compared to Alzheimer’s dementia, the risk of hospitalization or death due to respiratory infections is higher in patients with Lewy body dementia [8]. The median age at death is said to be similar between patients with Alzheimer’s dementia and Lewy body dementia [8]. The life expectancy from diagnosis to death is shorter in patients with Lewy body dementia [8]. The patient’s environment has been shown to play a role in the increased risk of mortality [8]. Patients in nursing homes have been shown to have a higher risk of mortality [8]. Caregivers can decrease the risk of complications by educating themselves on this disease and keeping their loved ones safe.

Self Quiz
Ask yourself...
- What are some complications of Lewy body dementia?
- How can the patient’s environment increase the risk of mortality with this disease?
- Why do you think there is delay in diagnosis with Lewy body dementia?
Resources for Family Support
Lewy body dementia is a diagnosis that can affect all aspects of an individual’s life and their family members lives. As nurses we must provide support for family members so they can better care for their loved ones and improve their quality of life. As recognition of this condition grows, family support resources are increasing. The Lewy Body Dementia Association is a nonprofit organization that raises awareness and provides support for families with individuals that suffer from Lewy body dementia [10]. Support groups can be found on their website to help families across the country in their local area [10].
The Lewy Body Dementia Association was started by caregivers of individuals with this condition. They also focus on education and research into the disease. This association is a resource for family members [10].
Another resource for family members is The Lewy Body Dementia Resource Center. This is a nonprofit charitable organization that gives assistance and support to those who care for someone with Lewy body dementia [9]. This organization was founded by caregivers of individuals with Lewy body dementia. They have a support phone line that is available seven days a week to answer questions [9]. They also promote research and early diagnosis of this disease [9].

Self Quiz
Ask yourself...
- How can support of family members improve the quality of life of a patient with Lewy body dementia?
- What are some examples of resources for caregivers of individuals with Lewy body dementia?
- Can providing resources to the community help with early diagnosis of this disease?
Research Programs
Lewy body dementia is the second most common form of dementia in the United States [4]. This illness is thought to be underdiagnosed and commonly mistaken for other neurological disorders [3]. Research on Lewy body dementia can decrease the time it takes to diagnose a patient, and can help with management of the condition.
The National Institute of Neurological Disorders and Stroke provides support for a variety of research endeavors for Lewy body dementia [11]. In 2021 The National Institute of Health spent $93 million dollars on Lewy Body dementia research [11]. One program is the Biomarkers for Lew body dementias program. This program aims to increase clinical data collection from patients with this condition, find biomarkers to expand further research, and allow access to the science community to help with further studies [11]. Another program is the Parkinson’s Disease Biomarkers Program. This program’s purpose is to collectively research with healthcare professionals, patients and family members, and technology professionals to increase biomarker research [11].
Biomarker research has been increasing in Lewy body dementia. A biomarker is a feature that can specifically indicate a certain disease [12]. For quite some time there were not any identified biomarkers for Lewy body dementia. There are certain biomarkers that aid in distinguishing Alzheimer’s disease from Lewy Body dementia [13]. These biomarkers can be assessed through imaging or in cerebral spinal fluid [13]. Currently these biomarkers are only helpful if another disease is doubtful [13].
New biomarkers are needed to separate Alzheimer’s dementia from Lewy Body dementia and other neurological disorders [13]. Biomarkers that can help with early diagnosis would be beneficial for early treatment [13].

Self Quiz
Ask yourself...
- Why is researching biomarkers important for early diagnosis of Lewy body dementia?
- Is there more research conducted on Alzheimer dementia than on Lewy body dementia?
- What are some organizations that promote research for this disease?
Conclusion
Lewy Body dementia affects 1.4 million Americans [2]. The disease is underdiagnosed and often diagnosed incorrectly. Incorrect diagnoses can lead to worsening of symptoms and the administration of drugs that can lead to adverse effects.
Educating healthcare providers and the community about Lewy body dementia can improve quality of life for individuals with the disease. As nurses, we must be informed about this disease to better educate our patients and their caregivers, and to know how to advocate for our patients.
Understanding Lupus Nephritis
Introduction
A lupus diagnosis and the complications that arise can be devastating for patients. Nurses are often looked to for support and answers, so it is important to educate ourselves on these serious conditions. Lupus nephritis (LN) is considered one of the most severe organ manifestations of the autoimmune disease systemic lupus erythematosus (SLE). Essential knowledge on lupus nephritis includes the defining features, epidemiology, pathophysiology of normal kidney function and lupus nephritis, clinical presentation, and treatments.
Lupus Nephritis
Lupus nephritis (LN) is an organ manifestation of the autoimmune disease systemic lupus erythematosus (SLE). The cause of lupus erythematosus is not known. Researchers suggest a genetic predisposition, but a genetic link has not been identified (2). This is a difficult reality, as patients and healthcare providers usually hope for a why. We will discuss the definition, prevalence, pathophysiology, manifestations, clinical diagnosis guidelines, and treatment regimens for LN.
Definition
Lupus nephritis (LN) is considered a condition and a manifestation. LN is one of the most severe organ manifestations of the autoimmune disease systemic lupus erythematosus (SLE). LN is a form of glomerulonephritis, which is inflammation of the glomeruli (the tiny filters within the kidneys). This inflammation causes significant imbalances within the body due to impaired kidney function.
Overview of Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by a loss of immune tolerance of endogenous nuclear material, which leads to systemic autoimmunity that may cause damage to various tissues and organs (1). Essentially, the damage to DNA structures causes the body’s immune system to be incidentally programmed to attack its own tissue. There are two types of lupus: systemic lupus erythematosus and “discoid” lupus erythematosus. SLE is systemic, meaning it can affect almost any organ system or tissue and presents in different manifestations impacting the skin, joints, kidneys, and brain (2). “Discoid” lupus erythematosus only affects the skin tissue. Our focus will be on systemic lupus erythematosus (SLE) as we gain a deeper understanding of lupus nephritis.
The causes of SLE are unknown but many attribute it to genetic, environmental, and hormonal factors. SLE is hard to diagnose because the symptoms are often mistaken for those of other conditions. There is no cure for SLE, but symptoms can be managed. SLE presentation and prognosis are highly variable, with symptoms ranging from minimal to life-threatening. Patients with lupus may experience periods of exacerbation of symptoms, sometimes called 'flares', as well as periods of remission. SLE is associated with substantial morbidity and mortality, particularly caused by renal and cardiovascular disease and infections. LN is considered one of the most severe manifestations of SLE.
SLE can be compared to a guard dog intended to protect your home. The guard dog (immune system) protects you from unwanted intruders (infection), but also bites friends, family, and the mailman (your own organ tissue)!

Self Quiz
Ask yourself...
- Have you cared for a patient with autoimmune disorders impacting the skin or joints?
- Are you familiar with other autoimmune conditions?
- How are systemic and focal conditions different?
- Can you list the two types of lupus?
Epidemiology and Statistical Evidence of Lupus Nephritis
Systemic lupus erythematosus has an estimated prevalence of about 10–150 per 100,000 persons (2). However, a large number of people could be undiagnosed due to being asymptomatic or the symptoms mistaken for other diseases. An average of 40% of SLE patients develop lupus nephritis (LN). Those diagnosed with SLE at a younger age are at a higher risk of developing LN and other complications (1).
SLE in general is more prevalent in women, especially women of reproductive age, than in men; the ratio is 9:1 (1). Therefore, 90% of SLE cases are women. However, men who have been diagnosed with SLE more commonly develop LN than women with SLE. Numerous studies have also found that the prevalence of LN in patients with SLE is higher in African American, Hispanic, and Asian populations (1). The impact of SLE disproportionately affects children and adults living in poorer geographic areas (8).
Within 10 years of the initial SLE diagnosis, 5–20% of patients with LN develop end-stage kidney disease and the multiple comorbidities associated with immunosuppressive treatment (1). Mortality in LN is quite variable ranging in between 15% and 25% (6). It is important to remember that the treatments are also very risky because it is difficult to balance the risks and benefits of suppressing the immune system. LN is a topic of significant research, so nurses can have a meaningful impact in raising awareness and encouraging hope for more advanced treatment development.

Self Quiz
Ask yourself...
- Can you name the population at greatest risk for developing SLE?
- Do you think all ethnicities are impacted equally?
- Do you think men and women are impacted equally when developing SLE and LN?
- Have you ever cared for a patient with systemic lupus erythematosus? If so, what were specific problems they faced?
Normal Kidney Function
The kidneys have several life-sustaining functions. The kidney serves to maintain fluid and chemical homeostasis and to contribute to hemodynamic stability (3). The renal tubules of the kidneys have unique and vital roles. Daily urine output is about 1–2 L, and over 98% of the glomerular filtrate is reabsorbed by the renal tubules (3).
There is a delicate balance and interdependency between the kidneys and other organs. For example, the kidneys produce hormones that help regulate blood pressure and control calcium metabolism, the kidneys also release a hormone that stimulates red blood cell production. A simple and fun mnemonic formula to help you remember the vital functions: A WET BED.
A WET BED: Functions of the Kidneys
A - controlling ACID-base balance
W - controlling WATER balance
E - maintaining ELECTROLYTE balance
T - removing TOXINS and waste products from the body
B - controlling BLOOD PRESSURE
E - producing the hormone ERYTHROPOIETIN
D - activating vitamin D
Controlling acid-base balance
- Our bodies always have a state of delicate equilibrium among the acids and bases, which has a parameter known as pH.
- The kidneys excrete or retain acids and bases when there is an excess or lack of them.
- The normal pH of the blood is 7.35 to 7.45.
Controlling water balance
The kidneys regulate the volume of urine produced and adapt to one’s hydration level to maintain water balance.
Maintaining electrolyte balance
The kidneys filter specific electrolytes from the blood, return them back into circulation, and excrete excess electrolytes into the urine. Kidneys maintain electrolyte balances like sodium and phosphate.
Removing toxins and waste products from the body
The kidneys remove water-soluble waste products and toxins and excrete them in urine.
Controlling blood pressure
The kidneys produce an enzyme called renin, which converts the angiotensinogen produced in the liver into angiotensin I, that is later converted in the lungs into angiotensin II. Angiotensin II constricts the blood vessels and increases blood pressure. Another way the kidneys help reduce elevated blood pressure is they produce more urine to reduce the volume of liquid circulating in the body to compensate.
Producing the hormone erythropoietin
The kidneys produce a hormone called erythropoietin, which aids in the creation of more red blood cells (erythrocytes), which are vital for the transport of oxygen throughout all the tissues and organs.
Activating vitamin D
The kidneys transform calcifediol into calcitriol, the active form of vitamin D.

Self Quiz
Ask yourself...
- What are ways to help remember the major functions of the kidneys?
- How do the kidneys regulate and maintain electrolyte balance?
- Can you list examples of how electrolyte imbalances affect various organ functions? (example: cardiovascular system)
- What are some ways the kidneys help to regulate blood pressure?
Anatomy and Physiology of the Kidneys
It is important to review the anatomy and physiology of the kidneys. The urinary system as a whole is composed of two kidneys, a pair of ureters, a bladder, and a urethra. The kidneys are located at the back of the abdominal wall and at the beginning of the urinary system. The size of each kidney is dependent on age, sex, and height, but the average length is approximately 10–12 cm, and the right kidney may be slightly smaller than the left kidney (3). The kidneys are made up of nephrons, which are microscopic structures composed of a renal corpuscle and a renal tubule.
The average human kidney is composed of approximately one million individual functioning nephrons, each containing a single glomerulus or filtering unit (3). The function of filtration is accomplished by three major components of nephron activity: (1) glomerular filtration, (2) tubular reabsorption, and (3) tubular secretion. These components respond to factors including renal blood flow, neuroendocrine effects, and the fluid and nutrient supply to the body.
Vascular Structure of the Kidneys
The kidneys are perfused with 1.2 liters of blood per minute, which represents about 25% of the cardiac output (3). From the abdominal aorta, the main renal artery carries blood into the kidney and then branches to segmental arteries, then to interlobar arteries, then branches to arcuate arteries, followed by branching to interlobular arteries, and finally onto afferent arterioles (3). Vascular resistance in the kidney is low when compared to other vascular beds within the body.
Figure 2. Vascular Structure of the Kidneys (3)

Self Quiz
Ask yourself...
- Can you explain the major functions of the kidneys?
- How would you describe the vascular structure of the kidneys?
- Are you familiar with focused physical assessment techniques for assessing peripheral edema?
- Have you ever cared for a patient with impaired renal function?
Pathophysiology of Lupus Nephritis

Have you ever played dominos? If aligned properly, the domino effect will rapidly cause a consecutive reaction. The immune response can be compared to this domino effect. One cellular action will cause the response and activation of many other cells. A perceived foreign body activates certain immune responses. In most cases, this maintains life. In some cases, it is harmful to vital tissue.
An autoimmune response to the renal system involves the T- and B-cell interactions stimulating interstitial plasma cell generation in the kidney; interstitial tissue leads to restricted autoantibody-producing plasma cells (6). This cascade of inflammatory response is facilitated by the production of interferon-α (IFN-α), which augments autoreactive B-cell activation and its reciprocal interaction in T-cell activation. This prolonged local injury and inflammation attracts neutrophils that try to help alleviate this inflammation, but the sustained local injury leads to neutrophil apoptosis (cell death), which further causes local injury. This injury further augments the inflammatory response by enhancing the intrarenal autoimmunity and inflammation, leading to kidney tissue injury (6).
Lupus nephritis is considered a type-3 hypersensitivity reaction. A hypersensitivity reaction is an inappropriate or overreactive immune response to an antigen. Symptoms typically appear when an individual has had a previous exposure to the antigen. Hypersensitivity reactions can be classified into four types (9).
- Type I - IgE mediated immediate reaction
- Type II - Antibody-mediated cytotoxic reaction (IgG or IgM antibodies)
- Type III - Immune complex-mediated reaction
- Type IV - Cell-mediated, delayed hypersensitivity reaction
In type III hypersensitivity reactions, antigen-antibody aggregates called "immune complexes” are formed. When someone has lupus, a number of DNA are damaged and have cell death, which exposes parts of the nucleus in the cell, and parts of the nucleus are recognized by the immune system as “nuclear antigens.” Remember, the immune system attacks antigens. The antigen-antibody complexes are transported by the blood and are deposited in various tissues, such as the kidneys.
When the complexes are deposited, it initiates the recruitment of inflammatory cells (monocytes and neutrophils) that release lysosomal enzymes and free radicals at the site of immune complexes, causing damage to that tissue (9). Examples of tissues that it may deposit in include skin, joints, blood vessels, or glomeruli. In the case of LN, the site of damage is the glomeruli of the kidneys, and it can have a disastrous impact.

Self Quiz
Ask yourself...
- Can you describe the differences between the types of hypersensitivity reactions?
- How would you describe the composition of the antigen-antibody complexes?
- Can you name types of inflammatory cells?
- Can you think of reasons the glomeruli of the kidneys may be a deposit site for free radicals and antigen-antibody complexes?
Clinical Presentation
The clinical manifestations of LN can be unpredictable and very different among patients. Patients may present with no symptoms at all, while other patients may have significant proteinuria progressing to acute renal failure. Understanding the disease and its progress is vital for nurses to provide optimal care and education to the patient. Remember, these patients may have signs and symptoms from their lupus already, so isolating renal impairment is essential.
Signs and Symptoms
Signs and symptoms of systemic lupus erythematosus depend on the body systems that are affected by the disease. Systemic symptoms include fatigue, malaise, weight loss, anorexia, and fever. The patient may report musculoskeletal symptoms, including joint and muscle pain, joint swelling and tenderness, hand deformities, and skin lesions such as the characteristic “butterfly rash” or maculopapular rash (small, colored area with raised red pimples). Other symptoms stem from the central nervous system (visual problems, memory loss, mild confusion, headache, depression).
It is important for the nurse to establish a history of symptoms related to the hematological system (venous or arterial clotting, bleeding tendencies), cardiopulmonary system (chest pain, shortness of breath, lung congestion), or gastrointestinal system (vomiting, difficulty swallowing, diarrhea, and bloody stools). To differential LN, it is important to focus on specific function impairment and manifestations arising from the kidneys (1).
Nephritic symptoms related to hypertension and poor kidney function:
- Peripheral edema
- Headache and dizziness
- Nausea and vomiting
Nephrotic symptoms related to proteinuria:
- Peripheral or periorbital edema
- Coagulopathy
Patients may report the following:
- Foamy urine
- Blood in the urine
- Dark urine
- Changes in the frequency of urination
- Weight gain and swelling, including the legs and hands
Classifications of Lupus Nephritis
There are six classifications of lupus nephritis:
- Class I: Minimal mesangial
- Prevalence 10-25% of people with lupus (SLE)
- 5% of lupus nephritis cases
- Clinical findings: Kidney biopsy shows build-up of antigen-antibody complex deposits; urinalysis is normal
- Class II: Mesangial proliferative
- Prevalence: 20% of lupus nephritis cases
- Clinical findings: Mesangial hypercellularity of any degree with mesangial immune deposits
- Class III: Focal LN
- Prevalence: 25% of lupus nephritis cases
- Clinical Findings: Active lesions exist in less than half of the glomeruli; hematuria and proteinuria
- Class IV: Diffuse proliferative
- Prevalence: 40% of lupus nephritis cases
- Very severe subtype
- Clinical findings: More than 50% of the glomeruli are affected with active lesions
- Immune complex deposits exist under the endothelial when viewed with an electron microscope
- Hematuria and proteinuria
- Hypertension, elevated serum creatinine, and raises anti-dsDNA (an antibody tested to diagnose lupus)
- Kidney failure is common
- Class V: Membranous
- Prevalence: 10% of lupus nephritis cases
- Clinical findings:
- Hematuria and proteinuria
- Significant systemic edema
- The glomerular capillary wall is thicker in segments
- High risk for renal vein thromboses, pulmonary embolism, or other thrombotic complications; active lesions are present
- Class VI: Advanced sclerotic LN
- Global sclerosis – typically more than 90% of the glomeruli are damaged and have active lesions
- Clinical findings: Progressively worsening kidney function

Self Quiz
Ask yourself...
- What are some differences in clinical manifestations between renal impairment and renal failure?
- Can you name the different classes of LN?
- How are clinical findings of Class I and Class IV different?
- Can you describe the glomerular function impairment in Class IV LN?
Diagnosis
LN is often the presenting manifestation resulting in the diagnosis of SLE (1). SLE is diagnosed clinically and serologically with the presence of certain autoantibodies. Evaluating kidney function in patients diagnosed with SLE is important as timely detection and management of renal impairment has been shown to greatly improve renal outcomes. The clinical presentation and laboratory findings for LN may differ, ranging from normal urinalysis and normal renal function test results to severe proteinuria, nephrotic syndrome, or acute nephritic syndrome, which can result in acute kidney failure (1). Monitoring for the development of lupus nephritis is done by a urinalysis, creatinine, urine albumin-to-creatine ratio, glomerular filtration rate (GFR), and a kidney biopsy.
Laboratory tests for SLE disease activity include the following:
- Antibodies to double-stranded DNA (dsDNA)
- Complement (C3, C4, and CH50)
- Erythrocyte sedimentation rate (ESR)
- C-reactive protein (CRP)
Laboratory tests to evaluate kidney function in SLE patients:
- Urinalysis
- Check for protein, red blood cells (RBCs), and cellular casts
- Serum creatinine assessment
- Blood urea nitrogen (BUN) testing
- Spot urine test for creatinine and protein concentration
- 24-hour urine test for creatinine clearance and protein excretion
Urinalysis
A high level of protein or red blood cells in the urine signifies kidney damage. The Systemic Lupus International Collaborating Clinics (SLICC) define renal involvement in lupus as a 24-hour urinary protein excretion of 0.5 g daily or the presence of red blood cell casts in urinary sediment (1). Urinary protein excretion in a 12-hour or 24-hour urine collection provides the best estimate of proteinuria. The most common abnormalities in urinary sediment in patients with LN are leukocyturia, hematuria, and granular casts (1).
Blood Tests
Creatinine is a waste product from the normal breakdown of muscles in your body. Kidneys remove creatinine from the blood. An elevated creatinine reveals damage to the kidneys because it is not functioning as it should. Glomerular filtration rate (GFR) also is an important test to determine how well the kidneys are functioning.
Kidney Biopsy
The next step in diagnosing LN would be a kidney biopsy. Kidney biopsy is currently the gold standard for confirming a diagnosis of LN and characterizing the LN subtype on the basis of histological patterns (1). A kidney biopsy is usually performed as a percutaneous needle biopsy with minimization of risk factors for bleeding complications. The piece of tissue removed is examined under a microscope by a pathologist.
A kidney biopsy can (1):
- Confirm a diagnosis of lupus nephritis
- Help in determining how far the disease has progressed
- Guide treatment

Self Quiz
Ask yourself...
- Can you describe what the glomerular filtration rate (GFR) is important for?
- Can you name components of a urinalysis?
- Have you cared for a patient with hematuria?
- Can you think of reasons a patient may be apprehensive about having a kidney biopsy?
Treatment
Treatment of LN is highly individualized. There is not a specific FDA-approved drug specifically given for the treatment of LN. Treatment cannot be a “one-size fits all” approach, but a plan to target renal impairment and avoid causing further damage. The goal of immunosuppressive therapy is the resolution of inflammatory and immunologic activity. Unfortunately, aggressive treatments can result in additional harm to patients. As the therapy of LN consists of potentially toxic drugs, it may be harmful to begin treatment without a definitive diagnosis (4).
Treatment of LN usually involves immunosuppressive therapy and glucocorticoids. The goals of LN treatment are to achieve rapid remission of active disease, prevent renal flares, prevent progression of chronic kidney disease (CKD), minimize treatment-associated toxicity, and preserve fertility (1). Immunosuppressive therapy is used to treat active focal (class III) or diffuse (class IV) LN or lupus membranous nephropathy (class V); but not usually used to treat minimal mesangial (class I), mesangial proliferative (class II), or advanced sclerosing (class VI) LN.
The treatment of focal or diffuse LN has two main components: initial therapy with anti-inflammatory and immunosuppressive agents to slow kidney injury, followed by long-term subsequent immunosuppressive therapy to control the chronic autoimmune processes of SLE and encourage the repair of damaged nephrons (1).
Treatment Goals
- Reduce inflammation in the kidneys
- Decrease immune system activity by blocking immune cells from attacking the kidneys directly and making antibodies that attack the kidneys
- Treatment of systems (hypertension, fluid retention)
- Support kidney function
Medications
Medications for the treatment of LN include (2):
- Corticosteroid
- Prednisone
- Immunosuppressant
- Cyclophosphamide
- Mycophenolate mofetil
- Hydroxychloroquine (Quinoline drug used to treat or prevent malaria; used for autoimmune response)
Blood pressure control:
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)
- Diuretics
- Beta blockers
- Calcium channel blockers
| Risk Target and Goals | Interventions |
| Lupus nephritis-related mortality | Chloroquine or hydroxychloroquine |
| Control of blood pressure and hyperlipidemia | |
| SLE and LN activity to avoid ESRD | Immunosuppression no less and no more than necessary |
| Hyperfiltration and proteinuria to avoid end-stage renal disease (ESRD) | Renin-angiotensin-aldosterone system inhibition |
| Avoid drug toxicity | Infections: Reduce or eliminate corticosteroids, PJP prophylaxis, vaccination, personal infection control |
| Malignancy: Avoid cumulative cyclophosphamide of over 30 grams | |
| Fractures: Reduce or eliminate corticosteroids, vitamin D supplementation, bone density monitoring | |
| Symptoms | Improvement or stabilization of the serum |
| Improvement of the urinary sediment | |
| Nephrotic syndrome: loop of Henle diuretics |
Treatment Guidelines
Key points of American College of Rheumatology guidelines for managing lupus nephritis (1):
- Patients with clinical evidence of active and previously untreated lupus nephritis should have a kidney biopsy to classify the disease according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) criteria.
- Patients with lupus nephritis should receive background therapy with hydroxychloroquine, unless contraindicated.
- Glucocorticoids plus either cyclophosphamide intravenously or mycophenolate mofetil orally should be administered to patients with class III or IV LN.
- Patients with class I/II nephritis do not require immunosuppressive therapy.
- Angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers should be administered if proteinuria reaches or exceeds 0.5 g/day (1).
- Blood pressure should be monitored and maintained at or below 130/80 mm Hg.
- Patients with class V lupus nephritis are generally treated with prednisone for one to three months, followed by tapering for one to two years if a response occurs.
For those who progress to kidney failure, treatment options include dialysis and kidney transplant.

Self Quiz
Ask yourself...
- Can you think of examples of treatments for blood pressure control other than medications?
- Can you think of treatments for LN and the risks of these treatments?
Complications
Complications of LN can be categorized into comorbidities from the actual condition and treatment-associated adverse outcomes. As mentioned, immunosuppressive therapy and glucocorticoids have harmful risks of their own. Comorbidities can include complications of the renal system and cardiovascular system. Treatment-associated complications can include infections, osteoporosis, cardiovascular disease, and reproductive impairment (1).
SLE and treatments, including glucocorticoids and calcineurin inhibitors, can cause hypertension, hyperlipidemia, and nephrotic syndrome (1). Many patients with LN have progressive CKD with associated comorbidities, such as anemia, osteoporosis, and other bone and mineral diseases. These factors contribute to vascular risks of progressive CKD and can lead to cardiovascular disease.
The prevalence of osteopenia and osteoporosis is high in patients with LN taking immunosuppressive therapy. In patients with severe nephrotic syndrome, a loss of plasma proteins, including clotting inhibitors, transferrin, immunoglobulins, and hormone-carrying proteins (such as vitamin D-binding protein), can lead to protein malnutrition, anemia, hormonal and vitamin deficiencies, hyperlipidemia, and increased risk for venous or arterial thrombosis (1). High-dose cyclophosphamide therapy correlates with premature gonadal failure in some cases, which is a complication of male and female reproductive organs (1).
Immunosuppressive agents increase the risk of infection, which can be further increased by disease activity, leukopenia, and CKD-related factors, such as nephrotic syndrome. Patients receiving immunosuppressive treatment can be at risk for poor outcomes with pneumocystis jirovecii pneumonia, herpes, hepatitis B, tuberculosis, influenza, and pneumococcal infection (1).
End-stage renal disease (ESRD) is a major complication of LN. Some 5–20% of patients with LN develop ESRD (1). By definition, all patients with LN have chronic kidney disease (CKD), but not all patients with CKD progress to ESRD. Essentially, ESRD occurs when the kidneys are no longer able to function to maintain life and either dialysis or a kidney transplant is needed.
Figure 4. Complications of Lupus Nephritis

Self Quiz
Ask yourself...
- Can you describe how renal impairment impacts the cardiovascular system?
- Can you name signs and symptoms of renal impairment?
- Have you cared for a patient experiencing adverse reactions to medications for immunosuppression?
- Are you familiar with risk factors for long-term corticosteroid use?
Screening and Prevention of Lupus Nephritis
Screening for LN onset and relapses is important for prompt treatment to improve outcomes. There are many new biomarkers under exploration for predicting and assessing LN (1). Patients with SLE should be screened periodically, even during periods of remission, every six to 12 months, or more frequently when clinically indicated (1). During regular check-ups, screening for LN onset or flares in patients with SLE should include evaluation of volume status, blood pressure measurement, urinalysis, and measurement of serum parameters. Elevation of serum creatinine level, the appearance of dysmorphic erythrocytes, cellular casts and new-onset proteinuria may indicate onset of LN (1). Nurses should encourage patients to regularly attend their appointments.
Patient Education
Patient education must be individualized to each unique patient. Lupus or LN may be a new diagnosis, or the patient may have been diagnosed previously. Teaching topics should include education on the disease process, the purpose of treatment regimens, and the importance of compliance.
Education on medication regimens is essential. Include the purpose, dosage, and possible side effects of all medications. Teach the patient when to seek medical attention. Provide tips such as wearing a medical alert bracelet or lanyard noting the condition and medications so appropriate action can be taken in an emergency. Provide resources on smoking cessation for patients who use tobacco. Teach the female patient the importance of planning pregnancies with medical supervision because pregnancy is likely to cause an exacerbation of the disease and the disease may cause negative pregnancy outcomes.
Discuss all precipitating factors that need to be minimized or avoided, including fatigue, vaccination, infections, stress, surgery, certain drugs, and exposure to ultraviolet light. Teach the patient to avoid strenuous exercise, but instead set goals of steady pace and balance. Describe pain management strategies and the importance of adequate nutrition. The patients may have concerns about skin care products and cosmetics. Teach the patient that these products should be hypoallergenic and approved by a provider prior to use. Encourage the patient to contact appropriate support groups available in the area.
Diet
Education on diet and nutrition for patients with LN can be very helpful in managing this condition. A diet regimen can be challenging because many people with this condition may also experience weight loss or gain, inflammation, osteoporosis, high blood pressure, and atherosclerosis. Recognizing specific nutritional concerns for each condition is important. A registered dietitian would be a meaningful resource for those with LN.
A kidney-healthy diet consists of low salt, low fat, and low cholesterol, with an emphasis on fruits and vegetables. Eating the right foods can help patients manage kidney impairment, maintain a healthy weight, and lower their blood pressure. Steroid medications can cause significant fluctuation in weight and energy.
The provider may advise restrictions on dietary protein intake. According to nephrology research, consuming more than 1.5 g of protein per kilogram per day can overwork the kidney filters, causing hyperfiltration (5). Many proteins are composed of amino acids that are converted to acids that are harmful to the kidney in large amounts; a diet high in animal proteins also contains sulfuric and phosphate acids that promote kidney damage (5). Potassium intake is also an important aspect of diet for those with LN. Potassium is secreted by the kidneys and may rise when kidney function declines; abnormal potassium levels can impact muscle function and increase the risk of hypertension, coronary artery disease, or stroke (5). A balanced diet with special considerations is a key teaching factor. It may be helpful to seek out resources from registered dieticians when needed.
Overview of Teaching Topics
Topics for education:
- Disease process
- Treatment plan
- Diagnostic studies and lab results
- Medications
- Purpose
- Dosage
- Side effects
- Contraindications
- Infection control
- Diet
- Tobacco cessation resources
- Reproductive complications
- Techniques to minimize ultraviolet exposure
Resources
The American College of Rheumatology (ACR) and Lupus Foundation of America (LFA) are excellent resources for patient with lupus that provide education and resources to improve overall well-being. The Lupus Foundation of America has a team of physicians, scientists, health educators, and individuals with lupus who work together to create resources, support groups, awareness initiatives, and programs. Patients can go to the “Ask our Health Educator” portal and get answers to questions they may have.
Conclusion
Nurses need to have a good understanding of lupus nephritis to provide patients with appropriate support and advice about how to maintain wellbeing and lead meaningful active lives. Knowledge on disease pathophysiology, manifestations, treatments, and complications is valuable for this serious condition. Patients often rely on nurses to support and empower them on this pathway.

Self Quiz
Ask yourself...
- How would you describe lupus to a non-medical person?
- Can you describe the difference between normal immune response and autoimmune response?
- Can you name clinical signs and symptoms specific to lupus?
- What is the most reliable diagnostic tool for LN?
- What are some ways the nurse can advocate for a patient having a kidney biopsy?
- How would you empower a patient with a new diagnosis of LN in knowledge of medications and their treatment regimen?
Alcohol Use Disorder: Long Term Effects and Complications
Introduction
Alcohol use disorder (AUD) is one of the most widespread psychiatric disorders and is estimated to affect approximately 29 million individuals aged 12 and older in the United States (9). According to the 2021 National Survey on Drug Use and Health, 28.6 million adults ages 18 and older have AUD and roughly 900,000 adolescents ages 12 to 17 have AUD (7).
Alcohol is the most prevalent misused substance in America with alcohol-related issues causing more than 85,000 deaths annually in the United States and three million deaths a year globally (9, 12). Globally, about 240 million people are affected by AUD, especially in Europe and America (9).
AUD is linked to motor vehicle accidents, dementia, depression, homicide, and suicide (9). The condition can also lead to social complications, such as legal problems, relationship issues, and economic stressors (due to the cost of alcohol, required medical care, missed time at work, and job loss). Individuals with AUD can end up isolating themselves from the individuals who are trying to help them most, including family members and friends.
Moderate alcohol use for most adults—no more than two drinks a day for men and one for women—is relatively harmless. An alcoholic drink includes 12 fluid ounces of regular beer, 10 fluid ounces of malt liquor, five fluid ounces of wine, or 1.5 fluid ounces of 80-proof distilled spirits (3, 4). Heavy alcohol drinking is defined as having four or more drinks per day or eight or more drinks per week for women and five or more drinks per day or 15 or more drinks per week for men (4).
Small amounts of alcohol, in particular red wine, can have beneficial cardiovascular effects, but heavy drinking can lead to serious health issues, such as cancer, cardiovascular disease, liver disease, osteoporosis, and infections (3, 4). Men are more likely than women to develop AUD, but women’s health suffers more than men’s, even at lower levels of consumption (3). Individuals with lower levels of education and of lower income experience AUD more than their counterparts (9).
Drinking can become problematic for those people who have psychological traits of impulsiveness, low self-esteem, and the constant need for attention and approval. Individuals who lack personal insight about drinking can develop AUD. For example, those who use alcohol to emotionally self-medicate (i.e., unwind from a stressful day) can develop alcohol-related issues (3).
Unfortunately, many individuals with AUD deny having a problem with alcohol and therefore do not seek treatment until faced with medical issues or legal complications. Regardless of how severe a patient’s drinking problem may seem, evidence-based treatment that includes medications, behavioral therapies, and support groups can help individuals achieve and maintain recovery.


Self Quiz
Ask yourself...
- How extensive is AUD in the United States and globally?
- How many fluid ounces constitute an alcoholic drink for beer, malt liquor, wine, and distilled spirits?
- To which health risks does heavy drinking contribute?
- Which psychological traits can increase the risk of AUD?
Pathophysiology
Alcohol ethanol or ethyl alcohol is a chemical substance found in alcoholic beverages, such as beer, hard cider, malt liquor, wines, and distilled spirits (liquor). Alcohol is the byproduct of yeast fermentation of sugars and starches. Alcohol is also found in some medicines, mouthwashes, and household products, including vanilla extract and other flavorings (4).
Alcohol use disorder is defined by the National Institute on Alcohol Abuse and Alcoholism as a medical condition characterized by an impaired ability to stop or control alcohol use despite adverse social, occupational, or health consequences (7). The term alcohol use disorder includes the terms alcohol abuse, alcohol dependence, alcohol addiction, and alcoholism. The condition affects brain function and is classified as mild, moderate, or severe (7).
Several evidence-based theories explain the development of AUD in individuals. These theories are (9):
- Positive-effect regulation: This occurs when people consume alcohol to seek positive rewards like pleasure or euphoria. Alcohol may be consumed to enhance social experiences and positive emotions.
- Negative-effect regulation: Individuals may drink alcohol in response to negative or distressing situations as a means to self-medicate and alleviate feelings of anxiety, depression, or low self-worth.
- Pharmacological vulnerability: This theory explains the differences in how individuals respond to the acute and chronic effects of alcohol. Some individuals are more prone to the rewarding effects of alcohol, or they have a reduced ability to metabolize alcohol, thus increasing their chances of developing AUD.
- Deviance proneness: Individuals with a history of deviant behavior or impaired socialization during childhood are more prone to AUD because these individuals use alcohol to self-medicate to alleviate symptoms of anxiety, depression, or low self-worth.
Cytosolic alcohol dehydrogenase (ADH) is the enzyme that metabolizes alcohol in the liver, and this metabolism process produces acetaldehyde as a byproduct. Acetate is then formed by the metabolism of acetaldehyde by the enzyme aldehyde dehydrogenase (ALDH).
Acetate then enters the body’s various metabolic pathways. Additionally, ADH is present in the gastrointestinal tract as well as the liver, which leads to the initial metabolism of alcohol during its ingestion. This is referred to as first-pass metabolism. The enzyme CYP2E1 of the cytochrome P450 system is upregulated in chronic alcohol users, which leads to an increased rate of alcohol metabolism (9).
Several factors affect the metabolism of alcohol (9):
- Women have a higher initial blood alcohol concentration following consumption because they have a slower first-pass metabolism due to lower levels of ADH; however, women eliminate alcohol consumption faster than males.
- Alcohol elimination declines with age, which is why older adults may experience a lower tolerance to alcohol than when younger, or why they may experience more severe hangover symptoms.
- In pregnancy, the fetal liver has an incomplete expression of enzymes CYP2E1 and ADH, leading to slower alcohol metabolism. This means that the fetus is exposed to the mother’s consumption of alcohol for a prolonged period, increasing the risk of fetal alcohol spectrum disorders.
- Native Americans have been found to metabolize alcohol faster due to the expression of beta-3 Class 1 ADH isoforms than individuals who express only the beta-1 Class 1 ADH isoform.
- When an individual fasts, alcohol metabolism is slowed due to decreased levels of ADH during a fasting state. Alternatively, food intake increases blood flow to the liver and allows fructose and other sugars to speed the metabolism of alcohol.
- The highest rates of alcohol elimination occur in the late evening versus during the daytime hours.
- Medications that have ADH inhibition or H2 receptor-blocking properties decrease the rate of alcohol elimination. This reduces first-pass metabolism in the stomach and increases blood alcohol levels.
- Heavy drinking increases the expression of the CYP2E1 enzyme, which increases alcohol elimination; however, this is eventually slowed in individuals with advanced liver disease.
Alcohol use can permeate every organ and tissue in the body, resulting in systemic dysfunction. The Complications and Long-Term Effects section explores how alcohol use impacts the body.

Self Quiz
Ask yourself...
- Which terms are included in AUD?
- What are the differences between positive-effect regulation and negative-effect regulation theories?
- How does the body’s metabolism process of alcohol work?
- How do pregnancy, fasting, and medications impact the metabolism of alcohol?
Risk Factors
Several factors are believed to contribute to the development of alcohol use disorder, including the home environment, peer interactions, genetic disposition, cognitive functioning, and other mental health disorders, such as schizophrenia, depression, and personality disorders (9).
Risk factors for AUD include (3, 7, 9):
- Genetics and family history. Hereditary factors can influence AUD by as much as 60%. Parents’ drinking habits are directly correlated to the child’s development of AUD. Certain genes have been found to increase an individual’s susceptibility to AUD, including:
- GABRG2 and GABRA2, COMT Val 158Met, DRD2 Taq1A, and KIAA0040.
- Drinking at an early age. Those individuals, especially females, who begin consuming alcohol before age 15 are three times more likely to have AUD. Research indicates that younger people who wait to start consuming alcohol until age 21 are less likely to have AUD.
- Mental health disorders. Psychiatric conditions, such as depression, post-traumatic stress disorder (PTSD), and attention deficit hyperactivity disorder (ADHD) are linked to an increased risk of AUD. Childhood trauma is also strongly correlated with AUD. The presence of both AUDs and psychiatric disorders leads to a worsened prognosis for both disorders.
- Social issues. Poverty and lack of education are significant risk factors for AUD.
Additionally, the risk for developing AUD can depend on how much, how often, and how quickly the individual consumes alcohol. Binge drinking and heavy drinking over time can lead to AUD (7).

Self Quiz
Ask yourself...
- Which mental health disorders can contribute to AUD?
- Which types of drinking can lead to AUD over time?
- Which genes have been found to increase an individual’s susceptibility to AUD?
- Which social factors are significant risk factors for AUD?
Signs and Symptoms
Nurses need to perform a thorough assessment of those individuals suspected of having AUD. The Cut Down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire is the widely accepted gold-standard assessment tool and is comprised of these questions (2):
1. Have you ever felt that you need to cut down on your drinking?
2. Have people annoyed you by criticizing your drinking?
3. Have you ever felt bad or guilty about your drinking?
4. Have you ever had a drink first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The scoring for the CAGE includes 0 for “no” answers and 1 for “yes” answers with a total score of two or greater being clinically significant for AUD. However, healthcare providers are encouraged to regard a total score of one or greater as the potential for substance abuse disorder that requires further patient exploration (2).
The Alcohol Use Disorders Identification Test (AUDIT) is a 10-item screening tool created by the World Health Organization (WHO) to assess alcohol-related conditions. There is a patient test that individuals may administer to themselves, and a test designed for healthcare providers to administer to patients. Both tests ask the same questions and use a similar scoring method. The provided answers are 0 for “never”, 1 for “monthly or less”, 2 for “2-4 times a month”, 3 for “2-3 times a week”, and 4 for “4 or more times a week”. A score of 8 or more indicates harmful alcohol use (8, 12).
The AUDIT questions are (8, 12):
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking? (The answer options for this question are 0 for “1 or 2”, 1 for “3 or 4”, 2 for “5 or 6”, 3 for “7 to 9”, and 4 for “10 or more”.)
- How often do you have six or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected to you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because of your drinking?
- Have you or someone else been injured because of your drinking? (The answer options for this question are “no”, “yes, but not in the last year”, and “yes, during the last year”.)
- Has a relative, friend, doctor, or other healthcare worker been concerned about your drinking or suggested that you cut down? (The answer options for this question are “no”, “yes, but not in the last year”, and “yes, during the last year”.)
Nurses and other healthcare professionals can ask patients the additional questions below about their alcohol use to determine the risk and presence of AUD.
In the past year, have you (7):
- Ended up drinking more or longer than you intended?
- Tried to cut back on drinking, but have been unable to do so?
- Experienced symptoms of illness (hangover) related to your drinking?
- Craved a drink so much that it distracted you from your current activity?
- Found that your drinking has interfered with maintaining your responsibilities and obligations regarding home, family, work, or education?
- Continued to drink despite knowing that it caused problems with family and friends?
- Foregone pleasurable activities (hobbies, etc.) that you enjoy to drink?
- Increased your risk for injury (driving, swimming, using machinery, walking in a dangerous area, engaging in unsafe sexual behavior) due to drinking?
- Continued to drink despite the social, health, or economic problems it causes?
- Experienced feelings of anxiety, depression, or memory blackout due to your drinking?
- Needed to drink more alcohol than in the past to get the same effects?
- Experienced alcohol withdrawal symptoms, such as tremors, restlessness, irritability, nausea and vomiting, sweating, malaise, racing heartbeat, hallucinations, or seizures?
The patient’s positive response to two to three of these criteria indicates mild symptoms, four to five indicates moderate symptoms, and six or more indicates severe symptoms (7).
Patients with AUD often report frequent falls, blackout spells, motor instability and tremors, visual disturbances, hangover symptoms (headache, nausea and vomiting, dry mouth, photophobia), hypertension, heart palpitations, seizures, confusion, mood swings, and sleep disturbances. Social symptoms include school or job instability or loss, relationship separation or divorce, estrangement from family and friends, and homelessness (9). Nurses should also assess patients suspected of AUD for post-traumatic stress disorder, bipolar disorder, panic disorder, anxiety disorder, dysthymic disorder, major depressive disorder, and insomnia (9).
During the assessment of a patient with AUD, nurses may note ataxia, fine motor skill disturbances, mental status changes, mood changes, slurred speech, tachycardia, hypotension, nystagmus, asterixis, ruddy palms, jaundiced coloring, and ascites. Signs of liver disease include hepatomegaly, splenomegaly, cirrhosis, spider angiomata, and liver atrophy (9).

Self Quiz
Ask yourself...
- What are the four CAGE alcohol use screening questions?
- When using the AUDIT screening tool, which score indicates harmful alcohol use?
- Which social symptoms are individuals with AUD likely to experience?
- Which physical symptoms may the nurse observe in patients with AUD?

Prevention
The stage in life when a person is exposed to alcohol is an important predictor for alcohol misuse. Adolescence is a time when many people begin experimenting with drinking, and research indicates that drinking before the brain is fully formed (age 26) can negatively impact brain development and compromise cognitive function. The main goal of preventing AUD is to impede, or at least delay, the onset of drinking in the youth population (6).
Adolescents and young adults seek independence and favor transitioning to adult roles. Most of this population drives automobiles and spends more time with friends than family members, thus making them easily influenced by their peer group. This also means that this population is at high risk for alcohol-related injuries, with 5,000 18- to 24-year-olds dying each year due to alcohol-related incidents (6). This group engages in binge drinking, which can lead to blackouts, alcohol poisoning, sexual assault, sexually transmitted infections, and poor academic performance.
Other factors that influence drinking behaviors that should be considered in prevention interventions are genetics, health status, cultural background, educational background, socioeconomic status, community attitudes toward alcohol use, and exposure to social media. Preventative measures should also be geared toward pregnant women and older adults since these populations have special considerations, such as the risk for fetal alcohol spectrum disorders in pregnant women and the inability to effectively metabolize alcohol in older adults that can lead to injuries (6).
Regular screenings for alcohol misuse are a key prevention strategy, and research shows that most patients do not object to being screened for alcohol use and thus are open to receiving advice about alcohol use (6). Evidence also shows that alcohol-related incidents can be prevented by both individual and environmental interventions. For example, college students who are taught to monitor their drinking and how to effectively refuse alcohol when offered can prevent AUD. College communities that restrict happy hours, enforce the minimum drinking age, and prevent alcohol price promotions can decrease the risk of AUD (6).

Self Quiz
Ask yourself...
- Which factors are predictors of alcohol misuse?
- Which factors put adolescents and young adults at risk for alcohol-related injuries?
- How do most patients react to screenings and advice regarding alcohol use?
- Which measures can college students and college communities implement to prevent AUD?
Diagnostics and Treatment
The preliminary source used to diagnose alcohol use disorder is the Diagnostic and Statistical Manual of Mental Disorders (DSM).
According to the DSM, the criteria for alcohol use disorder include (11):
A maladaptive pattern of substance use leading to clinically significant impairment or distress, as manifested by two or more of the following, occurring at any time in the same 12-month period:
- Alcohol is often taken in larger amounts or over a longer period than was intended.
- There is a persistent desire or unsuccessful efforts to cut down or control alcohol use.
- A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects.
- Craving, or a strong desire or urge to use alcohol.
- Recurrent alcohol use failing to fulfill major role obligations at work, school, or home.
- Continued alcohol use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of alcohol.
- Important social, occupational, or recreational activities are given up or reduced because of alcohol use.
- Recurrent alcohol use in situations in which it is physically hazardous.
- Alcohol use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by alcohol.
- Tolerance, as defined by either of the following:
- A need for markedly increased amounts of alcohol to achieve intoxication or desired effect.
- A markedly diminished effect with continued use of the same amount of alcohol.
- Withdrawal, as manifested by either of the following:
- The characteristic withdrawal syndrome of alcohol
- Alcohol (or a closely related substance, such as a benzodiazepine) is taken to relieve or avoid withdrawal symptoms.
Laboratory testing in patients with AUD can indicate blood disorders; vitamin, mineral, and electrolyte imbalances; cancers; cardiovascular disease; liver disease; and pancreatitis. Alcohol-related organ damage can be determined by biomarkers, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), phosphatidylethanol (PEth), fatty acid ethyl ester (FAEE), total serum sialic acid (TSA), mean corpuscular volume (MCV), cholesteryl ester transfer protein (CETP), carbohydrate-deficient transferrin (CDT), N-Acetyl-β-Hexosaminidase (Beta-Hex), macrophage migration inhibitory factor (MIF), and D-dopachrome tautomerase (DDT). Biomarkers like alcohol and ethyl glucuronide levels can determine recent alcohol consumption. CDT and PEth levels can help monitor abstinence (1, 9).
Treatment approaches for AUD involve pharmacological and nonpharmacological interventions.
The Federal Drug Administration (FDA) has approved three pharmacological treatments for AUD (9, 10):
- Naltrexone (Vivitrol) is a mu-opioid antagonist that blocks the effects of opioids and is widely used in treating addiction to narcotics. In alcoholism, the medication blocks the effects of naturally occurring opioids, such as endorphins. Therefore, the drinker does not experience any psychological benefits or “high” from the alcohol. Naltrexone also reduces the craving for alcohol leading to improved abstention rates, reduced number of drinking days, and reduced risk of relapse. The potential side effects—nausea, headaches, and fatigue—also work as an alcohol deterrent.
- Acamprosate is a glutamate agonist that promotes a balance of inhibitory and excitatory neurotransmitters in the brain that are disrupted by alcohol abuse. The discontinued brand name is Campral, but the generic equivalent is still available. The medication can cause weight gain and severe depression.
- Disulfiram (Antabuse) is an alcohol antagonist drug that inhibits ALDH, resulting in the accumulation of acetaldehyde in the body. The purpose of disulfiram is to promote fear in the drinker because drinking alcohol in conjunction with taking the medication can result in serious adverse reactions, including flushing, headache, dyspnea, diaphoresis, dizziness, shock, and even death.
- Gabapentin (Neurontin) and topiramate (Topamax) are not yet FDA-approved for AUD, but they are currently being used to treat the disease. Gabapentin, an anticonvulsant used to treat neuropathic pain, corrects dysregulation caused by alcohol use and cessation; it also decreases alcohol cravings. Topiramate, also an anticonvulsant, decreases alcohol cravings.
The most effective non-pharmacological approach to AUD is psychological therapy, including (9):
- Motivational interviewing. This technique is most effective in those patients who are ambivalent about changing their behaviors and quitting alcohol. The approach is client-centered and helps patients recognize and address their issues. It helps them become motivated to make long-lasting, healthy changes.
- Motivational enhancement therapy (MET). MET is a manual-based intervention that includes motivational interviewing and focuses on strengthening the motivation of the patient to change their alcohol use behaviors.
- Cognitive behavioral therapy (CBT). CBT helps individuals explore the connections among their thoughts, emotions, and behaviors. This therapy can increase motivation to stop drinking, identify triggers that prompt alcohol use, and teach effective coping mechanisms to deal with alcohol cravings and alcohol-seeking behaviors. In one study, 58% of patients receiving cognitive-behavioral treatment fared better than those who did not receive this therapy (3).
Other programs that support patients with AUD are residential facilities (in-patient rehabilitation), community programs like Alcoholics Anonymous (AA) or other 12-step programs, and faith-based programs that align with the principles of certain religions.


Self Quiz
Ask yourself...
- How does the DSM define tolerance?
- Which biomarkers can indicate AUD?
- Which three medications has the FDA approved for AUD treatment?
- What are the differences among motivational interviewing, motivational enhancement therapy, and cognitive behavioral therapy?
Complications and Long-Term Effects
AUD impacts each body system and can cause complications that have long-term negative effects. For example, alcohol impairs the brain’s ability to regulate balance, memory, speech, and judgment, which can lead to injuries. With long-term, heavy drinking the neurons reduce in size, the brain’s mass shrinks, and the brain’s inner cavity grows larger.
Alcohol interferes with glutamate action, which inhibits the creation of new memories and leads to blackouts. Alcohol also influences the neurotransmitters dopamine and serotonin, which can cause mood dysregulation, impaired concentration, and decreased motivation. Even though alcohol can cause euphoria at first, it is a depressant that can lead to suicidal ideation, behaviors, and death.
Additionally, excessive alcohol consumption can weaken the immune system, which can lead to increased rates of infection, such as pneumonia (4).
Additional complications of AUD by the organ system are (4, 5, 7):
- Cardiac/Circulatory: Cardiomyopathy, arrythmias, stroke, hypertension, ischemic heart disease, heart failure
- Endocrine: Acute and chronic pancreatitis, pancreatic cancer, diabetes
- Gastrointestinal: Esophageal cancer, oral cavity cancer, larynx cancer, pharynx cancer, gut leakiness, gastritis, ulcers, microbial dysbiosis, colorectal cancer, inflammatory bowel disease (IBD)
- Hepatic: Steatosis, steatohepatitis, fibrosis, cirrhosis, alcohol-associated hepatitis, liver cancer
- Immune/Lymphatic: Infections, such as tuberculosis
- Integumentary: Melanoma
- Neurologic: Ischemic stroke, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, multiple sclerosis (MS)
- Reproductive: Breast cancer, sexually transmitted infections
- Respiratory: Acute respiratory distress syndrome, alcohol-associated lung disease, pneumonia
- Skeletal: Muscle myopathy, muscle wasting, impaired bone fracture repair, reduced bone density (osteopenia, osteoporosis)
- Urinary: Kidney disease, prostate cancer
Alcohol is a toxic substance that contains carcinogens, such as nitrosamines, asbestos fibers, phenols, and hydrocarbons, which are the cause of 3.5% of cancer deaths in the United States (4).
Some cancers associated with alcohol consumption are (4):
- Head and neck cancer. Drinkers are at increased risk for oral cavity, pharynx, and larynx cancers. The risk increases among those individuals who both drink and use tobacco.
- Esophageal cancer. Esophageal squamous cell carcinoma risk is high in moderate to heavy drinkers.
- Liver cancer. The risk for hepatocellular carcinoma and intrahepatic cholangiocarcinoma doubles in individuals who are heavy drinkers.
- Breast cancer. Light drinkers have a slightly increased risk of breast cancer, whereas moderate to heavy drinkers increase their risk substantially. Tobacco use in conjunction with alcohol use increases the risk of breast cancer.
There are multiple ways in which alcohol can increase the risk of cancer, such as (4):
- Metabolism of ethanol to acetaldehyde, which is a toxic carcinogen, acetaldehyde damages DNA and proteins.
- Oxidation can damage DNA, proteins, and lipids.
- Inability to digest and assimilate vital nutrients that can protect against cancers, such as vitamin A, vitamin B complex, vitamin C, vitamin D, vitamin E, and carotenoids.
- Increasing levels of estrogen can lead to breast cancer.
The prognosis for patients with AUD is very challenging with less than 20% to 30% achieving long-term abstinence and with most acquiring serious to permanent health conditions that are irreversible (9).

Self Quiz
Ask yourself...
- How does alcohol negatively impact the brain?
- How does alcohol affect the gastrointestinal and integumentary systems?
- Why does oxidation increase the risk of cancer?
- What is the prognosis of patients with AUD?
Patient Education
Nurses play a critical role in educating patients with AUD, and they should do so in a non-judgmental and non-confrontational manner.
Educational topics nurses should address with patients include (9, 10):
- Healthy diet. Most patients with AUD suffer from malnourishment, particularly regarding folate. Foods rich in folate include legumes, eggs, leafy greens, citrus fruits, nuts and seeds, and fortified grains. Patients should be taught the importance of maintaining a healthy diet that is rich in protein, complex carbohydrates, fresh fruits and vegetables, healthy fats, and vitamins and minerals.
- Reducing risky behaviors. Individuals with AUD should be taught that alcohol consumption lowers inhibitions, which can lead to risk-taking behaviors, such as driving, swimming, operating heavy machinery, and engaging in unprotected sexual practices.
- Importance of support groups. Patients with AUD are more likely to make progress and stay motivated if they are supported by family, friends, and community resources like Alcoholics Anonymous (AA). Family members should also be encouraged to attend support groups like Al-Anon.
- Maintaining a temptation-free environment. Patients should be encouraged to remove all alcohol from their homes and offices. When traveling, they can request that hotels remove alcohol from the guest room minibar.
- Importance of maintaining health screenings. AUD can lead to other serious health conditions, such as cardiovascular disease, osteoporosis, and various cancers. Patients should be taught the importance of receiving regular medical check-ups and health screenings to maintain optimal health.
Nurses should also ensure that patients can access educational and support resources in their native language, as well as be provided with information about resources available to low-income individuals, if applicable.

Self Quiz
Ask yourself...
- Which foods high in folate should the nurse recommend for patients with AUD?
- Why do individuals with AUD engage in risky behaviors?
- Which serious health conditions are patients with AUD at risk of acquiring?
- How can alcoholics maintain a temptation-free environment at home and when traveling?
Resources
There are many resources available to assist patients with AUD.
- Alcoholics Anonymous: AA is a global fellowship community that helps individuals resolve their issues with alcohol. https://www.aa.org/
- Al-Anon Family Groups: Like AA, Al-Anon is a fellowship community that helps friends and family members of alcoholics gain the support they need. https://al-anon.org/
- Alateen: Alateen is a component of Al-Anon, a fellowship group for adolescents who have been affected by someone else’s drinking. https://al-anon.org/newcomers/teen-corner-alateen/
- Centers for Disease Control and Prevention: The CDC provides resources, such as publications, online tools, and healthcare information related to individual states. https://www.cdc.gov/alcohol/resource-center/index.html
- FindTreatment.gov: This is a national website for locating treatment facilities for mental and substance use disorders. https://findtreatment.gov/locator
- National Association for Children of Addiction (NACoA): This organization provides programs, such as The Clergy Education and Training Project, Celebrating Families, and Children’s Program Kit to support the children of addicted parents. https://nacoa.org/
- Rethinking Drinking: The National Institute on Alcohol Abuse and Alcoholism (NIAAA) website provides evidence-based information for patients and healthcare providers, as well as listings for treatment centers. https://www.rethinkingdrinking.niaaa.nih.gov/

Self Quiz
Ask yourself...
- Which organization assists friends and family members of individuals with AUD?
- Which organization supports teenagers who are affected by someone else’s drinking?
- Which programs by NACoA support the children of addicted parents?
- Which websites provide listings for treatment centers?
Case Study
B.P. is a 32-year-old Caucasian man who presents to the emergency department after a motor vehicle accident during which B.P. lost control of his car and drove it into a roadside ditch. There were no other cars on the road at the time, making the accident a one-person collision. B.P. was brought to the hospital by his girlfriend and presented with superficial lacerations and bruising to his face from deployment of the driver’s side airbag and more serious lacerations to his left forearm that were injured by the breaking glass of the driver’s side window. He states that he thinks he may have hit his head on the automobile’s steering wheel before airbag deployment, but then says, “I’m not completely sure about that little detail.”
B.P.’s vital signs are blood pressure 158/84 mmHg, pulse 78 beats per minute, respirations 16 breaths per minute, and oxygen saturation 95%. His lung fields are clear to auscultation bilaterally, and his cardiovascular exam reveals a regular rate and rhythm without murmurs. Bowel sounds are confirmed in four quadrants via auscultation. Radial, pedal, and dorsal pedal pulses are normal bilaterally. Pupils are sluggish to react to penlight; the patient’s eyes have a glassy appearance. B.P. is oriented to place, but not day or time.
He can state his name and birthdate without hesitation, but he pauses before stating his complete address and incorrectly states his zip code. When speaking, B.P.’s words are slurred, but his hearing bilaterally is intact. During the musculoskeletal and neurological assessments, B.P. is unable to follow all the instructions the nurse provides and at one point he whines, “Can we stop now? This is super hard and I’m so sleepy. I need to go to bed now.” The patient’s left forearm is red and edematous with two lacerations that are both roughly 1.5 inches in length.
There are evident shards of glass present in both lacerations and bleeding has slowed significantly since the patient arrived at the hospital. B.P.’s face has several minor red contusions and a couple of superficial lacerations. The patient denies any reports of pain, headache, nausea, or dizziness, and says that he only feels fatigued.
B.P.’s health history includes chronic hay fever for which he takes over-the-counter antihistamines. He denies any further health issues. The nurse is unable to obtain a detailed social history or family history due to B.P.’s noncompliance.
After B.P.’s face and left arm are cleaned, and the left arm sutured and bandaged, he is taken for a chest x-ray, which is normal, and a computerized tomography (CT) scan of the head, which shows no intracranial hemorrhage, mass, or stroke. Blood test results reveal folate deficiency, and ALT, AST, MCV, GGT, and CETP levels consistent with heavy alcohol use. Intravenous folate and normal saline are administered during the next several hours, and the patient is consistently monitored for pain and alcohol withdrawal symptoms. As B.P. becomes sober, the nurse initiates the AUDIT questionnaire; B.P. scores a 32.
When the nurse explains B.P.’s AUDIT score to him, he states, “Yeah, I’m not surprised. I’ve been drinking since I was 13, and my girlfriend keeps telling me that my drinking is getting worse. I just like to have a few to chill after a hard day at work; my job is super stressful. Maybe she’s right. And after tonight, I should probably look into it. These hospital and car bills aren’t going to be cheap.”
B.P. is discharged with a referral to a psychiatrist for further AUD evaluation and potential treatment. He is also given a couple of brochures about alcohol abuse and in-patient rehabilitation programs. B.P. is encouraged to follow up with his primary care physician for continued care of his injured arm.

Self Quiz
Ask yourself...
- Which social factors about B.P.’s situation put him at risk for AUD?
- Which assessment findings indicate B.P. may have AUD?
- Is B.P.’s AUDIT score concerning? If so, why?
- Which factors indicate it was effective for the nurse to wait to administer the AUDIT to B.P.?
Conclusion
AUD puts individuals at risk for serious health complications, legal problems, and impaired interpersonal relationships. Many alcoholics do not receive appropriate medical care for AUD due to a lack of screening by healthcare providers. Therefore, nurses must use their assessment skills to evaluate patients for AUD and provide them with proper education about the disease, including its long-term effects and complications.
Healthcare screenings for cardiovascular disease and mental health disorders, as well as referrals to community support programs such as Alcoholics Anonymous, help patients take responsibility for their health and recovery.
SSRI Use in Anxiety Disorders
Introduction
Selective Serotonin Reuptake Inhibitors (SSRIs) are a medication class typically used to treat depression. SSRIs are considered first-line treatment for depression and other psychological disorders, including (2):
- Major Depressive Disorder
- Generalized Anxiety Disorder
- Bulimia Nervosa
- Bipolar Depression
- Obsessive-Compulsive Disorder
- Panic Disorder
- Premenstrual Dysphoric Disorder
- Treatment-Resistant Depression
- Pot-traumatic Distress Disorder
- Social Anxiety Disorder
Additional disorders may be treated off-label, including (2):
- Binge Eating Disorder
- Body Dysmorphia Disorder
- Fibromyalgia
- Premature Ejaculation
- Paraphilias
- Autism
- Raynaud’s Phenomenon
- Vasomotor symptoms associated with menopause.
SRRIs remain relatively safe, tolerable, and efficient in treating patients (2).
Fluoxetine
The Fluoxetine drug class includes brand names of Prozac, Rapiflux, Sarafem, and Selfemra
(11).
Indications
Fluoxetine is an FDA-approved medication used to treat major depressive, obsessive-compulsive, binge eating, premenstrual dysphoric, and panic disorders. Fluoxetine is also prescribed for bulimia and bipolar depression.
Off-label uses include social anxiety disorder, adult post-traumatic stress disorder (PTSD), borderline personality disorder, Raynaud phenomenon, and selective mutism (21).
Mechanisms of Action and Pharmacokinetics
Fluoxetine blocks the reuptake of serotonin into the presynaptic serotonin neurons. This is accomplished by blocking the reuptake transporter proteins located in the presynaptic terminal. There have been some indications of mild activity at the 5HT2A and 5HT2C receptors. Conversely, there is minimal activity on noradrenergic reuptake (21).
Fluoxetine produces an activating effect that takes two to four weeks to see antidepressant effects. Fluoxetine actives norfluoxetine, a metabolite produced when cytochrome p450 enzyme acts on it. This indicates that fluoxetine has a high risk for drug-drug interactions that metabolize at the CYP2D6 isoenzyme (21).
Fluoxetine has a half-life of two to four days. Fluoxetine’s active metabolite, norfluoxetine, has a longer half-life of seven to nine days (21).
Contraindications and Precautions
Fluoxetine is contraindicated in patients with (21):
- Hypersensitivity to fluoxetine or its components
- Use of monoamine oxidase inhibitors (MAOIs) to treat psychiatric disorders
- Prescribers should discontinue MAOIs for > 2 weeks before initiation of fluoxetine
- Prescription of:
- Linezolid
- Pimozide
- Thioridazine
- Tamoxifen
- History of seizures
- Elderly patients
- Suicidal thoughts or ideation
- Especially in pediatric patients or those aged 18 to 24 years old
- Monitor closely for the first two months of initiating therapy
- Especially in pediatric patients or those aged 18 to 24 years old
- During pregnancy
- Use in early pregnancy increases the risk of septal heart defects
- After 20 weeks, it is linked with a possible risk for pulmonary hypertension in newborns
- This may increase the risk of gestational hypertension and preeclampsia
- Breastfeeding
- Trace amounts found in breast milk
Precautions should be taken when prescribing Fluoxetine in patients with liver cirrhosis, as this diagnosis reduces clearance of fluoxetine and its active metabolite. This increases the elimination or half-life. Lower or less frequent doses should be considered for these patients (21).
Adverse Effects and Black Box Warnings
Fluoxetine’s most common side effects include (21):
- Insomnia
- Nausea
- Diarrhea
- Anorexia
- Dry mouth
- Headache
- Drowsiness
- Anxiety
- Nervousness
- Yawning
- Decreased libido
- Decrease arousal or lubrication
- Bruising
- Hyperhidrosis
- Weight gain or loss
- Decreased orgasm
- Muscle weakness
- Tremors
- Pharyngitis
Less common adverse effects include (21):
- Bleeding
- Seizures
- Suicidal ideation and behaviors
- Especially in teenagers
Most adverse effects will appear upon initiation of treatment and should fade with time. It is advised to wait for side effects to subside before changing treatment, as most side effects are dose- and time-dependent.
If side effects continue after several weeks, dose adjustment then changing medication is recommended (21).
If insomnia is present, patients should take medication in the early morning (21).
A Black Box Warning is present for Fluoxetine, as this medication increases suicidal thoughts and behaviors. Individuals prescribed this medication should be closely monitored, especially teenagers as they are more prone to experiencing these severe adverse effects (5).
Drug Interactions
Fluoxetine interacts with several medications including (22):
- Monoamine Oxidase inhibitors
- Medications that increase serotonin levels
- Tricyclic antidepressants (TCAs)
- Lithium
- Triptan migraine medications
- Certain opioids
- Other SSRIs
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- St John’s Wort
- Medications that raise the risk of bleeding
- Warfarin
- Apixaban
- Rivaroxaban
- Clopidogrel
- Prasugrel
- Pimozide and thioridazine
- Medications metabolized by certain liver enzymes
- Flecainide
- Propafenone
- TCAs
- Benzodiazepines
- Antipsychotics
- Seizures

Self Quiz
Ask yourself...
- What are the different medication names for Fluoxetine’s class of SSRIs?
- What is the primary use of Fluoxetine?

Sertraline
The Sertraline drug class includes the brand name: Zoloft (13).
Indications
Sertraline is FDA-approved to treat the following (20):
- Major depressive disorder
- Obsessive-compulsive disorder (OCD)
- Panic disorder
- Post-traumatic stress disorder (PTSD)
- Premenstrual Dysphoric Disorder (PMDD)
- Social Anxiety Disorder (SAD)
Sertraline may be prescribed off-label for the following disorders (20):
- Binge eating disorder
- Body dysmorphic disorder
- Bulimia Nervosa (BN)
- Generalized Anxiety Disorder (GAD)
- Premature ejaculation
Mechanisms of Action and Pharmacokinetics
Sertraline inhibits serotonin reuptake on the presynaptic terminals. This leads to an accumulation of serotonin, leading to better mood regulation, personality stability, and wakefulness or arousal (20).
Sertraline has a half-life of 24 to 32 hours. Sertraline is metabolized by the liver (7).
Contraindications and Precautions
Sertraline is contraindicated in (20):
- Patients with hypersensitivity to this drug or any of its components
- Coadministration with:
- Thioridazine
- Pimozide
- Monoamine oxidase inhibitors
- Sertraline should not start <2 weeks of discontinuing any monoamine oxidase inhibitors, as this may lead to toxicity with serotonin syndrome.
- Serotonergic medication
- Disulfiram
- may cause an alcohol-disulfiram reaction.
Adverse Effects and Black Box Warnings
Sertraline has multiple adverse effects, including (20):
- Syncope
- Lightheadedness
- Diarrhea
- Nausea
- Sweating
- Dizziness
- Xerostomia
- Confusion
- Hallucinations
- Tremor
- Somnolence
- Impotence
- Disorders of ejaculation
- Fatigue
- Rhinitis
- Female sexual disorder
More serious adverse effects include (20):
- Prolonged QT interval
- Inhibiting platelet aggregation, leading to bleeding risk
- Serotonin syndrome, including symptoms of:
- Myoclonus
- Muscle rigidity
- Diaphoresis
- Tremor
- Hyperreflexia
- Agitated delirium
- hyperthermia
Sertraline has a Black Box Warning related to increased risk of suicidal thoughts and ideation, particularly in pediatric patients and adult patients aged 18 to 24 (20).
Drug Interactions
Due to Sertraline’s interaction with many CYP enzymes, several drug-drug interactions are possible (7). These include:
- Bepridil
- Bromopride
- Cisapride
- Dronedarone
- Eliglustat
- Furazolidone
- Iproniazid
- Isocarboxazid
- Levoketoconazole
- Linezolid
- Mesoridazine
- Methylene Blue
- Metoclopramide
- Ozanimod
- Phenelzine
- Pimozide
- Piperaquine
- Procarbazine
- Rasagiline
- Safinamide
- Saquinavir
- Selegiline
- Sparfloxacin
- Terfenadine
- Thioridazine
- Toloxatone
- Tranylcypromine
- Ziprasidone

Self Quiz
Ask yourself...
- What are the most common side effects of Sertraline?
- What contraindications are present for Sertraline?
Paroxetine
The Paroxetine drug class includes brand names of Brisdelle, Paxil, and Pexeca (19).
Indications
Paroxetine is FDA-approved to treat the following disorders (19):
- Major depressive disorder (MDD)
- Obsessive-compulsive disorder (OCD)
- Social anxiety disorder (SAD)
- Panic disorder
- Post-traumatic stress disorder (PTSD)
- Generalized anxiety disorder (GAD)
- Premenstrual dysphoric disorder (PMDD)
- Vasomotor symptoms associated with menopause
Paroxetine is prescribed off-label for the following disorders (19):
- Obsessive-compulsive disorder (in children and adolescents)
- Social anxiety disorder
- Dysthymia
- Body dysmorphic disorder
- Postpartum depression
- Premature ejaculation
- Malignancy related to pruritus
- unresponsive to standard treatment
Mechanisms of Action and Pharmacokinetics
Paroxetine blocks the serotonin reuptake transports (SERT), increasing the concentration of synaptic serotonin. The diminished serotonin concentration induces the upregulation of serotonergic receptors. Paroxetine induces the downregulation of upregulated serotonin receptors to balance the receptor concentration.
Paroxetine has also shown some affinity for muscarinic, adrenergic (alpha and beta), dopaminergic (D2), serotonergic (5-HT2), and histaminergic (H1) receptors – which assist with antidepressant effects (19).
Paroxetine is metabolized via hepatics CYPP450 2D6 and has a half-life of approximately 21 hours (19).
Contraindications and Precautions
Paroxetine is contraindicated in the following situations (19):
- Concurrent use:
- Monoamine oxidase inhibitors (MAOIs)
- Causes serotonin syndrome
- Thioridazine
- Causes cardiac arrhythmias
- Pimozide
- Causes serotonin syndrome
- Monoamine oxidase inhibitors (MAOIs)
Precautions should be taken when prescribing paroxetine with (19):
- Tricyclic antidepressant (TCA) use
- Inhibits TCA metabolism
- Tamoxifen use
- Inactivates tamoxifen
- Any drugs affecting hepatic metabolism
- Pregnant or breastfeeding patients
- Increased risk of cardiovascular malformations in the first trimester
Adverse Effects and Black Box Warnings
Paroxetine has dose-dependent side effects. The most common side effects include (19):
- Alopecia
- Apathy
- Chest pain
- Constipation
- Diarrhea
- Dizziness
- Drowsiness
- Dry mouth
- Eczema
- Edema
- Headache
- Hypomania
- Hyponatremia
- Loss of appetite
- Mania
- nausea
- Palpitations
- Photosensitivity
- Sexual side effects
- Sleep disturbance
- Sweating
- Tachycardia
- Tremor
- Vasodilation
- Weight gain
Discontinuation syndrome is more severe with Paroxetine versus other SSRIs. Withdrawal may occur, and symptoms include (19):
- Dizziness
- Lethargy
- Nausea
- Vomiting
- Headache
- Fever
- Chills
- Vivid dreams
- Electric shock-like sensations
- Dyskinesia
- Anxiety
- Crying
- Irritability
- Depersonalization
A Black Box Warning is present for Paroxetine related to increased suicidal thoughts and behaviors. Anyone under the age of 24 has a higher risk for these serious adverse effects and should be carefully considered and monitored when prescribed (9).
Drug Interactions
Drug-drug interactions for Paroxetine include (19):
- Monoamine oxidase inhibitors (MAOIs)
- Thioridazine
- Pimozide
- Tricyclic Antidepressant (TCA)
- Tamoxifen use
- Any drugs affecting hepatic metabolism

Self Quiz
Ask yourself...
- What are the different medication names for Paroxetine’s class of SSRIs?
- What are the most common side effects of Paroxetine?
Fluvoxamine
The Fluvoxamine drug class includes the brand names Luvox and Luvox CR (16).
Indications
Fluvoxamine is FDA-approved to treat the following disorders (1):
- Obsessive-compulsive disorder (OCD)
- Depression
- Social anxiety disorder
Fluvoxamine has recently been prescribed off-label for the treatment of COVID-19. However, the COVID-19 Treatment Guidelines Panel recommends against it. There is insufficient evidence for the use of fluvoxamine in combination with inhaled budesonide for non-hospitalized patients.
Conversely, patients with are receiving fluvoxamine for underlying treatments should continue therapy if diagnosed with COVID-19 (16).
Mechanisms of Action and Pharmacokinetics
Fluvoxamine blocks the reuptake of serotonin at the sodium-dependents serotonin reuptake transports (SERT). This enhances the action of serotonin on the 5HT1A auto receptors, leading to an increase in the concentration of synaptic serotonin (16).
Fluvoxamine is metabolized by the liver and has a half-life of 12 to 15 hours (4).
Contraindications and Precautions
Fluvoxamine is contraindicated in the following situations (16):
- Concurrent use of:
- Ethanol
- Tobacco
- Comorbidities, as this medication may impact the illness or medications to treat:
- Bipolar disorder
- Bleeding problems
- Depression
- Glaucoma
- Heart-attack
- Heart disease
- Heart rhythm problems
- Hyponatremia
- Mania
- Mental health problems
- Seizures
- Liver disease
Precautions should be taken when prescribing Fluvoxamine to (16):
- Patients who drink or have a history of alcoholism
- Patients with vocations that include operating heavy machinery
- May cause drowsiness
- Patients with bleeding problems
- This medication may increase the risk for bleeding
- Patients who are expecting or trying to conceive
- There is some link between this medication and infertility
Adverse Effects and Black Box Warnings
Fluvoxamine has the following side effects include (16):
- More common side effects include:
- Sexual side effects
- Decreased libido, erectile dysfunction
- Less common side effects include:
- Changes to behavior or mood
- Decreased orgasm
- Difficulty breathing
- Difficulty urinating
- Twitching
- Rare
- Agitation
- Blurred vision
- Chills
- Clumsiness
- Confusion
- Diarrhea
- Fever
- Inability to move eyes
- Decreased or increased ability to move the body
- Menstrual changes
- Nosebleeds
- Overactive reflexes
- Poor coordination
- Red or irritated eyes
- Skin issues include:
- Redness
- Tenderness
- Itching
- Burning
- Peeling
- Restlessness
- Seizures
- Shivering
- Skin rash
- Sore throat
- Sweating
- Excitement that cannot be controlled
- Trembling or shakiness
- Unusual bruising
- Unusual or sudden body or facial movements
- Unusual secretion of milk in females
- weakness
A Black Box Warning is present for Fluvoxamine related to increased suicidal thoughts and behaviors (6).
Drug Interactions
Drug-drug interactions for Fluvoxamine include (16):
- Alosetron
- Pimozide
- Ramelteon
- Thioridazine
- Tizanidine
- Increase side effects
- Monoamine oxidase inhibitors (MAOIs)
- Should not take until MAOIs are discontinued for > 2 weeks
- Linezolid
- Methylene blue injection
- Phenelzine
- Selegiline
- Tranylcypromine
- Buspirone
- Fentanyl
- Lithium
- Tryptophan
- St John’s Wort
- Amphetamines
- Some pain medications
- Blood thinners

Self Quiz
Ask yourself...
- What is the primary use of Fluvoxamine?
- What contraindications are present for Fluvoxamine?
Citalopram
The Citalopram drug class includes the brand name of Celexa (11).
Indications
Citalopram is FDA-approved to treat the following disorders (11):
- Depression in adults
Citalopram is prescribed off-label to treat:
- Obsessive-compulsive disorder (OCD)
- Panic disorder
- Generalized anxiety disorder (GAD)
- Social anxiety disorder (SAD)
- Premenstrual dysmorphic disorder (PMDD)
- Binge eating disorder
- Posttraumatic stress disorder (PTSD)
- Premature ejaculation
- Poststroke depression
Mechanisms of Action and Pharmacokinetics
Citalopram escalates the brain-derived neurotrophic factor (BDNF) in the hippocampus and preferential cortex, which can be decreased in patients with depression (18).
Citalopram is metabolized by the liver and has a half-life of 24 to 48 hours. This may take longer due to hepatic impairment (18).
Contraindications and Precautions
Citalopram is contraindicated in (18):
- Concomitant use of:
- monoamine oxidase inhibitors (MAOIs)
- Serotonin syndrome, including symptoms of:
- Myoclonus
- Muscle rigidity
- Diaphoresis
- Tremor
- Hyperreflexia
- Agitated delirium
- Hyperthermia
- Urokinase
- Pimozide
- Methylene blue
- Linezolid
- Dapoxetine
- Hypersensitivity to the drug or excipient
- Increases thioridazine concentrations
- Lead to dangerous arrhythmias
- Increases thioridazine concentrations
Adverse Effects and Black Box Warnings
Adverse effects of Citalopram include (18):
- Most common
- Drowsiness
- Insomnia
- Dizziness
- Headache (dose-dependent)
- Diaphoresis
- Nausea
- Vomiting
- Xerostomia
- Constipation
- Diarrhea
- Ejaculation disorder (dose-dependent)
- Less common
- Myocardial infarction
- Prolonged QT interval
- Torsades de pointes
- Hemorrhage
- Cerebrovascular accident
- Suicidal ideation
- Manic
- Serotonin syndrome
Citalopram has a Black Box Warning related to increased risk of suicidal thoughts and ideation, particularly in pediatric patients and adult patients aged 18 to 24 (18).
Drug Interactions
Citalopram interactions significantly with the following medications, and should be monitored closely (18):
- Bupropion
- Omeprazole
- Esomeprazole
- Cimetidine
- Fexinidazole
- Voriconazole
- Fluoxetine
- Fluvoxamine
- Fluconazole


Self Quiz
Ask yourself...
- What are the different medication names for Citalopram’s class of SSRIs?
- What contraindications are present for Citalopram?
Escitalopram
The Escitalopram drug class includes the brand name Lexapro (14).
Indications
Escitalopram is FDA-approved to treat the following disorders (8):
- Depressive disorder in adults and adolescents, ages 12-17
- Generalized anxiety disorder for adults and children, ages7+
Escitalopram is prescribed off-label for (8):
- Social anxiety disorder
- Obsessive-compulsive disorder
- Panic disorder
- Posttraumatic disorder
- Premenstrual dysphoric disorder
- Vasomotor symptoms associated with menopause
Mechanisms of Action and Pharmacokinetics
Escitalopram binds to the sodium-dependent serotonin transporter protein (SERT) in the presynaptic neuron. This inactivates the SERT and causes an elevation in the synaptic serotonin levels (8).
Escitalopram is metabolized by the liver and has a half-life of 27 to 33 hours (8).
Contraindications and Precautions
Escitalopram is contraindicated in the following situations (8):
- Hypersensitivity to the medication or any of its components
- Concurrent use of:
- MAOIs
- pimozide
- QT prolongation
- Or family history
Precautions should be taken when prescribing Escitalopram, including assessment and reduction of risk for serotonin syndrome. Medications that should be avoided to prevent this include (8):
- Other antidepressants
- Triptans
- Fentanyl
- Lithium
- Tramadol
- Tryptophan
- Buspirone
- Amphetamines
- St John’s Wort
- Methylene blue
- Linezolid
- MAOIs
If possible, obtaining genetic testing for patients before prescribing could show an individual’s enzyme activity. If the patient is classified as a poor metabolized of the enzyme, CYP2C19, they are at a greater risk for drug reactions and may want to reduce the dose or consider alternative treatment (8)
Adverse Effects and Black Box Warnings
Escitalopram has the following side effects include (8):
- Insomnia
- Sexual dysfunction
- Reduce libido
- Anorgasmia
- Male ejaculatory delay
- Nausea
- Increased sweating
- Fatigue
- Somnolence
- QT prolongation
- Serotonin syndrome
- Rare but serious
A Black Box Warning is present for Escitalopram for increased risk of suicidal ideation. Patients should be closely monitored for behavioral changes or destructive behaviors, particularly in pediatric and adolescent patients (8).
Drug Interactions
Drug-drug interactions for Escitalopram include (8):
- Rasagiline
- Serotonin syndrome risk
- Antiplatelet agents
- Increased risk for bleeding
- NSAIDS
- Upper GI bleeding risk
- Aspirin or dual antiplatelet therapy
- Risk of bleeding
- Other agents that prolong QTc intervals
- Amiodarone
- Especially in patients with chronic kidney disease
- Amiodarone

Self Quiz
Ask yourself...
- What is the primary use of Escitalopram?
- What are the most common side effects of Escitalopram?
Vilazodone
The Vilazodone drug class includes the brand name Viibryd (3).
Indications
Vilazodone is FDA-approved to treat the following disorders (3):
- Depression
Mechanisms of Action and Pharmacokinetics
Vilazodone binds to the sodium-dependent serotonin transporter protein (SERT) in the presynaptic neuron. This inactivates the SERT and causes an elevation in the synaptic serotonin levels (17).
Vilazodone is metabolized by the liver and has a half-life of 25 hours (17).
Contraindications and Precautions
Vilazodone is contraindicated in the following situations (23):
- Concurrent use of:
- MAOIs
- Methylene blue
- Must be discontinued > 14 days before starting Vilazodone
Precautions should be taken when prescribing Vilazodone for patients at risk for (23):
- Suicidal thoughts
- Bleeding risk
- Fractures
- Ocular issues
- Serotonin syndrome
- Sexual dysfunction
- SIADH and hyponatremia
- Mania/hypomania
- Seizure disorder
This medication also poses a risk of discontinuation syndrome and should only be stopped under the direction of a provider throughout monitored time (23).
Adverse Effects and Black Box Warnings
Vilazodone has the following side effects include (3):
- Allergic reaction
- Bleeding
- Irritability
- Confusion
- Irregular heartbeat
- Muscle stiffness
- Sweating high fever
- Seizures
- Chills
- vomiting
- diarrhea
- fatigue
- dizziness
- headache
- sudden eye pain
- changes in vision
- suicidal ideation
- change in sex drive or performance
- dry mouth
- trouble sleeping
A Black Box Warning is present for Vilazodone
Drug Interactions
Drug-drug interactions for Vilazodone include (3):
- Linezolid
- MAOIs
- Methylene blue
- Amphetamines
- Aspirin
- Buspirone
- Diet medications
- Dexfenfluramine
- Fenfluramine
- Phentermine
- Sibutramine
- Headache medications
- Almotriptan
- Eletriptan
- Frovatriptan
- Naratriptan
- Rizatriptan
- Sumatriptan
- Zolmitriptan
- Blood clots medications
- Warfarin
- Enoxaparin
- Daletparin
- Infection treatment
- Clarithromycin
- Itraconazole
- Voriconazole
- Ketoconazole
- Rifampin
- Seizure medications
- Carbamazepine
- Phenytoin
- Digoxin
- Fentanyl
- Lithium
- NSAIDS
- Other antidepressants
- St John’s Wort
- Tramadol

Self Quiz
Ask yourself...
- What are the most common side effects of Vilazodone?
- What contraindications are present for Vilazodone?
Conclusion
Selective Serotonin Reuptake Inhibitors (SSRIs) are a medication class typically used to treat depression. SSRIs are considered the first-line treatment for depression and other psychological disorders. However, many SSRIs can be used to treat various anxiety disorders.
Some of the SSRIs are FDA-approved for the treatment of anxiety, while others the provider may prescribe off-label if circumstances permit. While SRRIs remain relatively safe, tolerable, and efficient in treating patients. providers should pay special attention to serious or life-threatening side effects of SSRIs including suicidal ideation, discontinuation syndrome, serotonin syndrome, prolonged QTc interval, and increased risk for bleeding (2).
Anticoagulant Therapy
Introduction
Anticoagulants are a class of medications that prevent and treat blood clots, or venous thromboembolism (VTE).
The Centers for Disease Control and Prevention (CDC) estimates that around 900,000 people in the United States are affected by some form of venous thrombosis every year. Furthermore, the CDC estimates around 60,000 to 100,000 Americans die from some form of venous thromboembolism each year.
They further state that sudden death is the first symptom that occurs in about 25% of people who have a pulmonary embolism [4]. Thus, healthcare providers must be knowledgeable of the signs and symptoms of venous thromboembolism and the available anticoagulants for prevention and treatment.
Understanding the different pharmacokinetics of anticoagulant medication is essential during drug selection. This course outlines anticoagulant pharmacology and addresses pharmacokinetics, including mechanism of action, side effects, usage, and contraindications.
Definitions
Anticoagulants - medications used to prevent and treat blood clot formation, commonly referred to as “blood thinners” [5].
Venous Thromboembolism - a condition where a blood clot forms in a vein, including deep vein thrombosis and pulmonary embolism, and appears during periods of hemostasis [5].

Self Quiz
Ask yourself...
- What are anticoagulants?
- What is a venous thromboembolism?
Medications Overview
Anticoagulant medications are used for the prevention and treatment of venous thromboembolism in both inpatient and outpatient settings.
The major anticoagulant medication classes include:
- Unfractionated Heparin
- Low Molecular Weight Heparin
- Vitamin K Dependent Antagonists
- Direct Oral Anticoagulants
- Direct Thrombin Inhibitors
- Direct Factor Xa Inhibitors [14]
Unlike anticoagulants, antiplatelets act on platelet formation. Although commonly mistaken as an anticoagulant, they are not a part of this class [14].
Depending on the type of anticoagulant, they have other indications for use, such as:
- Atrial Fibrillation stroke prevention
- Left Ventricular Thrombus
- Left Ventricular Aneurysm
- Prosthetic Heart Valve
- Venous Thromboembolism treatment
- VTE prevention in people with cancer
- Pulmonary Embolism
- Pregnancy
- Heparin-Induced Thrombocytopenia

Self Quiz
Ask yourself...
- What settings are anticoagulants used in?
- What medical conditions can anticoagulants be used for treatment and prevention?
- What are the different classes of anticoagulant medications?
Pharmacokinetics
Unfractionated Heparin
Unfractionated heparin, also known as heparin, is a medication used to prevent excess blood coagulation. It’s used to prevent and treat thrombotic events like deep vein thrombosis (DVT) and pulmonary embolism (PE).
Heparin is also used in patients with atrial fibrillation to prevent blood clot formation and during medical procedures, like dialysis, continuous renal replacement therapy, extracorporeal circulation, and heart catheterizations and surgeries. It’s also used for various off-label indications and usually in inpatient settings, such as acute coronary syndrome, percutaneous coronary intervention (PCI), or as a bridge medication when converting to oral anticoagulants [15].
Heparin works by binding to an antithrombin that inactivates thrombin and thus, blocks the clotting cascade. More specifically, by inactivating thrombin factor IIa and factor Xa, fibrinogen is not converted to fibrin, and in turn, prevents clot formation [15].
Heparin is available via intravenous (IV) and subcutaneous (SQ) forms. Intravenous heparin is infused via continuous infusion until therapeutic levels are achieved, while the SQ form is administered intermittently for VTE prophylaxis. Starting dosages of IV heparin usually begin with a bolus of 80 units per kilogram (kg), and then a continuous infusion rate of 18 units/kg/hour. However, this is dependent on the underlying medical condition being treated and is titrated to achieve therapeutic levels.
In hospital settings, heparin flushes are available to lock and maintain IV-line patency. Additionally, subcutaneous dosages of heparin are weight-based and dependent on the medical condition [15].
Common side effects of heparin include:
- Bleeding
- Thrombocytopenia
- Injection site reactions
- Hyperkalemia
[15]
As bleeding is a common side effect, nurses and healthcare providers must monitor patients for evidence of bleeding. Some signs or symptoms of bleeding can include petechiae, bruising, nosebleeds, hematuria, and bright red or dark, tarry stools. Moreover, heparin-induced thrombocytopenia (HIT) usually occurs within five days of initiating heparin therapy, causing serious adverse effects, like myocardial infarction, stroke, DVT, PE, and even death [15].
Before ordering this medication, healthcare providers should be aware of this anticoagulant’s contraindications. Heparin is contraindicated in individuals who have a platelet count of less than 100,000/mm or they have an active, uncontrolled bleed except if they have disseminated intravascular coagulation (DIC). It should also be avoided in patients with a history of HIT or who cannot have routine blood monitoring of heparin therapeutic levels.
Monitoring the patient’s hemoglobin, hematocrit, and vital signs is also essential to detecting a possible hemorrhage [15].
As heparin affects clotting time, healthcare providers must order therapeutic monitoring for activated partial thromboplastin time (aPTT) and activated clotting time (ACT). Prior to patients being initiated on a heparin infusion, a baseline aPTT level is drawn and then subsequently monitored every 6 hours until a therapeutic aPTT level is achieved. After there are two or more aPTT therapeutic results, then an aPTT is drawn every 24 hours.
The therapeutic range of heparin is typically 1.5 to 2 times the normal range, and titration algorithms are dependent on the healthcare facility’s protocols. ACT monitoring is less specific than aPTT and is typically reserved for PCI or cardiac bypass [15].

Self Quiz
Ask yourself...
- What is the pharmacokinetics of unfractionated heparin?
- What are common side effects and contraindications for unfractionated heparin?
- Which lab value(s) require routine monitoring when administering this medication?

Low Molecular Weight Heparin
Another medication used to prevent excess blood coagulation is low molecular weight heparin (LMWH). This medication is used to prevent venous thromboembolic disease in hospitalized patients and is also used to treat DVT and PE. Low molecular weight heparin is also approved for use and treatment of medical conditions such as ST-elevation myocardial infarction (STEMI), unstable angina, and extracorporeal clot prevention.
Common names of LMWH include enoxaparin and dalteparin [13].
Low molecular weight heparin’s mechanism of action works on the body’s clotting cascade by activating antithrombin III. Once antithrombin III is activated, it binds to factor Xa, which inhibits thrombin activation. Therefore, clot formation is prevented since fibrinogen is not converted to fibrin. This medication’s mechanism of action slightly differs from heparin, as LMWH inhibits factor Xa only, while heparin acts on factor IIa and factor Xa [13].
Low molecular weight heparin is available via subcutaneous injection. Dosages of this medication are typically calculated by body weight and are dependent on whether it’s being used for prevention or treatment. LMWH is typically administered once or twice daily and is safe to administer during pregnancy.
Some adverse effects of LMWH include:
- Bleeding
- Heparin-induced Thrombocytopenia
- Injection site reactions
- Osteoporosis [13].
Some other side effects are spontaneous fracture, hyperaldosteronism, and hypersensitivity reactions. Before prescribing this medication, healthcare providers must be knowledgeable of LMWH contraindications. It’s contraindicated in patients with hemorrhagic disorders, peptic ulcer disease, cerebral hemorrhage, trauma, and recent eye or nervous system surgeries.
Additionally, healthcare providers should caution when prescribing LMWH to patients with chronic kidney disease, as it increases the risk of accumulation in their system. Lower doses may be required for these individuals. A benefit of low molecular weight heparin when compared to unfractionated heparin is that the patient’s aPTT blood levels do not need to be monitored [13].

Self Quiz
Ask yourself...
- What is the pharmacokinetics of low molecular weight heparin?
- What are the common side effects and contraindications of low molecular weight heparin?
Vitamin K Dependent Antagonists
Vitamin K antagonists (VKAs) are a class of anticoagulant medications used in the prevention and treatment of blood clots. Warfarin is a commonly prescribed VKA and other less common VKAs are acenocoumarol, fluindione, and phenprocoumon [7].
Warfarin is approved for the treatment and prevention of pulmonary embolism, venous thrombosis, and thromboembolic complications of heart valve replacement and atrial fibrillation. It’s also used to reduce mortality risk after a myocardial infarction or stroke. Prevention of transient ischemic attack and stroke are additional off-label indications [11].
Vitamin K antagonists act by inhibiting the liver’s vitamin K epoxide reductase complex 1 (VKORC1). This reduces the amount of vitamin K reserves and therefore reduces the production of the body’s clotting factors that require vitamin K. Some clotting factors that are reduced include factors II, VII, IX, and X [7].
Vitamin K antagonists are available in oral form and are typically taken once daily in the afternoon or evening. Warfarin is rapidly absorbed, and onset of action is about 24 to 72 hours. The medication’s therapeutic effects aren’t normally seen until five to seven days after taking the medication. The typical starting dosage is 5 mg but might be less (2.5 mg) in patients who are elderly or who have liver disease [11].
Some common side effects of this class of medications include:
- Bleeding or bruising
- Nausea
- Vomiting
- Abdominal pain
- Altered sense of taste [11].
As VKAs alter the body’s ability to clot, it can cause more serious adverse effects, such as:
- Significant bleeding or hemorrhage
- Purple toe syndrome
- Warfarin-induced skin necrosis
- Calciphylaxis [11].
Warfarin and other VKAs have several contraindications such as gastrointestinal bleeding, cerebral aneurysm, dissecting aortic aneurysm, or other hemorrhagic conditions. Additionally, if the patient has had a recent nervous system, eye, or trauma surgery, then this medication should not be prescribed. Threatened abortion, malignant hypertension, and regional or lumbar anesthesia blocks are contraindications as well [11].
When prescribing VKAs, healthcare providers must order therapeutic monitoring and assess the patient’s prothrombin (PT) or international normalized ratio (INR). INR is usually the preferred method where a baseline level is initially drawn, and then subsequent levels are drawn within a week. The therapeutic range is dependent on the underlying medication condition, where the goal can range from an INR of 2 to 3 for most patients or from 2.5 to 3 for patients with a mechanical mitral valve.
Once the patient has reached their therapeutic goal, levels are typically drawn every four weeks unless there have been changes in their medications. Additionally, patients who take VKAs should have their hemoglobin and hematocrit levels and liver and kidney function checked at least every 6 months [11].

Self Quiz
Ask yourself...
- What is the pharmacokinetics of vitamin K dependent antagonists?
- What are common side effects and contraindications of vitamin K dependent antagonists?
- Which lab value(s) require routine monitoring when administering vitamin K antagonists?
Direct Oral Anticoagulants
Direct oral anticoagulants (DOACs) are a class of anticoagulant medications mainly used to prevent thrombosis formation. There are two main classes of DOACs, which include direction thrombin inhibitors and direct factor Xa inhibitors [6].
Direct Thrombin Inhibitors
Direct thrombin inhibitors (DTIs) are used to treat and prevent thrombosis in patients with heparin-induced thrombocytopenia, acute coronary syndrome, or during percutaneous coronary intervention. Common names of direct thrombin inhibitors include argatroban, bivalirudin, fondaparinux, and dabigatran [9]. Additional off label uses may include anticoagulation during dialysis or renal replacement therapy, and it can be used as an alternative anticoagulant for patients who are resistant to heparin [10].
Direct thrombin inhibitors block coagulation activities that are involved in fibrin clot formation. There are two types of direct thrombin inhibitors, which are univalent DTIs that act by binding to the active site of thrombin, and bivalent DTIs that bind to two sites, the active thrombin site and exosite I.
As the thrombin pathways are blocked and cleavage of fibrinogen to fibrin is prevented, this ultimately inhibits clot formation and coagulation [9]. Some DTIs, like argatroban, are metabolized primarily via the cytochrome P450 enzyme [10].
This class of anticoagulant medications is typically available in intravenous form. However, it is also available in oral form, but dabigatran is the only available oral form. Starting dosages for DTIs depend on the medical condition being treated [9].
Some side effects of DTIs may include:
- Bleeding
- Gastrointestinal symptoms and bleeding
- Hypotension
- Dyspnea
- Fever
- Sepsis
[10]
When DTIs are used during PCI, some potential side effects are back pain, nausea, chest pain, and headache. Contraindications include increased bleeding, a history of medication hypersensitivity, lumbar puncture, and spinal anesthesia [10].
As most patients who are treated with DTIs have HIT, aPTT monitoring is common and the goal is 1.5 to 3 times the patient’s baseline. For patients who undergo PCI, ACT is used, and levels are obtained according to the hospital’s protocol or policy [10].
Direct Factor Xa Inhibitors
Direct factor Xa inhibitors are used to prevent and treat several blood clotting disorders. Examples of direct factor Xa inhibitors include apixaban, rivaroxaban, and edoxaban and they are only available in oral form [9].
These medications are often used to reduce the risk of stroke in patients with atrial fibrillation or other cardiac arrhythmias. Direct Xa inhibitors are also used to prevent and treat conditions like DVT or PE [1]. Rivaroxaban is approved for secondary prevention and adjunct therapy after acute coronary syndrome and peripheral artery disease [12].
Direct factor Xa inhibitors directly bind to factor Xa, preventing it from cleaving prothrombin to thrombin. This results in blocking the propagation phase of the coagulation cascade [9]. It is metabolized via the liver and by the CYP3A4/5 and CYP2J2 mechanisms [12].
Starting dosages of direct factor Xa inhibitors depend on the underlying medical condition. Rivaroxaban usually is started at 20mg daily for atrial fibrillation stroke prevention or 10mg daily for VTE prevention [12].
Some side effects of direct factor Xa inhibitors may include:
- Dizziness
- Hemorrhage
- Abdominal Pain
- Pruritis
- Nausea
[1] [12]
Direct factor Xa inhibitors have several drug interactions and thus, the healthcare provider must review any potential interactions before initiating this medication. Common drugs that have potential interactions are aspirin, ketoconazole, phenytoin, rifampin, and carbamazepine [1].
They are also contraindicated in patients with hepatic and renal impairment, antiphospholipid syndrome, and increased bleeding risk. Most have black box warnings of increased risk of thrombotic adverse events and spinal or epidural hematoma. They should also be avoided in patients who are pregnant or breastfeeding [12].
Although this class of medications does not require routine blood monitoring, the healthcare provider should consider obtaining baseline aPTT, PT, and kidney and liver function.
If the patient is scheduled for surgery with a moderately high risk of bleeding, direct factor Xa inhibitors should be held for at least 48 hours before the procedure [1].

Self Quiz
Ask yourself...
- What is the pharmacokinetics of direct thrombin inhibitors?
- What are common side effects and contraindications of direct thrombin inhibitors?
- What is the pharmacokinetics of direct factor Xa inhibitors?
- What are the common side effects and contraindications of direct factor Xa inhibitors?
Considerations for Prescribers
This section reviews potential considerations when prescribing anticoagulants.
When prescribing anticoagulant medications, healthcare providers must consider and review several factors. First, the route and dosage are typically determined by the setting, inpatient versus outpatient, if it’s being used for prevention or treatment, and the underlying medical condition.
Additionally, healthcare providers should strive to follow current guidelines, approved uses, and hospital protocols when initiating or adjusting these medications. Healthcare providers must review the patient’s medical history, baseline lab values, contraindications, and recommended therapeutic medication range as discussed above.
Low molecular weight heparin is often considered safe during pregnancy [13]. Certain anticoagulants, especially warfarin, should be avoided in patients who are elderly, prone to falls, or have reduced kidney function [11]. Oftentimes, anticoagulants are combined with antiplatelet medications to produce better therapeutic results in certain medical conditions [8].
Healthcare providers should also review the potential common and adverse side effects of anticoagulants with patients. Additionally, as bleeding is a serious adverse effect, they should review potential signs of bleeding, such as dizziness, uncontrolled bleeding to the skin, blood in the stool or urine, and other symptoms [15]. Patients should be instructed to seek immediate medical treatment if they experience any of these symptoms, fall at home, or have another traumatic injury.
In addition, they should be instructed on how to control bleeding when cuts occur to the skin [11]. A common side effect of LMWH is pain and bruising around the injection site. Therefore, patients should understand the importance of rotating injection sites and if side effects become a concern, then an oral alternative should be considered [13].
As periodic blood monitoring is required for many anticoagulants, such as INR, PT, or aPPT, the healthcare provider must review the importance of completing these labs and coming to their scheduled visits [15, 11]. If the patient is non-compliant with laboratory monitoring, then the healthcare provider should consider other alternatives, such are direct oral anticoagulants that do not require routine bloodwork or DTIs that have a wider therapeutic range [6] [9].
In addition, the healthcare provider must be knowledgeable about the potential drug interactions for each medication and dietary concern. For example, patients on vitamin K antagonists should reduce their intake of foods high in vitamin K since it counteracts the therapeutic effects of the medication. They should avoid foods rich in vitamin K, such as spinach, kale, and broccoli [7]. Co-administration of other anticoagulants, aspirin, and non-steroidal anti-inflammatory medications also increases bleeding risk [11].
Most anticoagulant medications can potentially cause toxicity and patients should seek emergency medical treatment. Therefore, healthcare providers must be aware of the signs and symptoms of toxicity and the reversal agent, or antidote, for each medication.
Protamine sulfate is the reversal agent for heparin and low molecular weight heparin. Protamine sulfate is administered via IV push and if administered too rapidly can lead to pulmonary edema, vasoconstriction, and pulmonary hypertension [15]. Vitamin K is the antidote for vitamin K antagonists [11]. Andexanet alfa is the reversal agent for direct factor Xa inhibitors and idarucizumab is approved for the reversal of dabigatran specifically [12].


Self Quiz
Ask yourself...
- What factors should healthcare providers consider when prescribing anticoagulants?
- What are the reversal agents for each anticoagulant class?
- Which anticoagulant medication is often prescribed during pregnancy?
- What health conditions and lab values are important when selecting anticoagulants?
Upcoming Research
This section reviews upcoming research and medications for anticoagulant treatment.
There have been many new anticoagulant medications introduced over the past several years and research continues to evolve for this type of medication. More recently, a newer class of anticoagulant medications called anti-factor XI and Xia inhibitors, affect the factor XI pathway for clot formation. However, it’s still being researched and there are ongoing clinical trials [3].
Moreover, in November 2023, the American Heart Association released promising information about abelacimab, a monoclonal antibody that acts as a factor XI inhibitor, which has been shown to reduce stroke risk in patients with atrial fibrillation. The same report stated that bleeding risk was reduced by more than 60% while taking this medication [2].
Potential factor VII and VIII inhibitors are also being researched [16].

Self Quiz
Ask yourself...
- What new research is there about anticoagulants?
- Which new class of medications has shown promise in reducing bleeding risk in patients with atrial fibrillation?
Conclusion
Anticoagulants are typically indicated for the prevention and treatment of blood clotting conditions, including deep vein thrombosis and pulmonary embolism. However, they have several additional approved and off-label uses. Healthcare providers should understand the pharmacokinetics, potential side effects, and contraindications when selecting an anticoagulant. They should also follow current clinical guidelines and their facility’s protocols for a more evidence-based approach.

Self Quiz
Ask yourself...
- What new research is there about anticoagulants?
- Which new class of medications has shown promise in reducing bleeding risk in patients with atrial fibrillation?
SNRIs for Depression
Introduction
Depression can significantly interfere with daily activities and diminish quality of life among patients who experience it. Major depressive disorder (MDD) is one of the leading causes of the burden for worldwide diseases (5). At the present stage, the first-line treatment of MDD is selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). Research has found that SNRIs showed faster antidepressant effects than SSRIs (5).
The serotonin norepinephrine reuptake inhibitors (SNRIs) are a family of antidepressants that inhibit the reuptake of both serotonin and norepinephrine; however, it is important to recognize the differences among each specific drug in this class. In this course, we will examine pharmacological properties, clinical indications, mechanism of action, metabolism and excretion, dosing schedules, side effects, and warnings of each SNRI currently approved by the United States Food and Drug Administration (FDA) for the treatment of depression.
Forms and Symptoms of Depression
Depression can have crippling effects. Research suggests that genetic, biological, environmental, and psychological factors play a role in depression (7). Depression can be present with other comorbidities, such as mental disorders, diabetes, cancer, heart disease, and chronic pain. Depression can make these conditions worse, and vice versa. Certain medications have been shown to increase depressive symptoms.
Common forms of depression are: (7)
- Major depression - which includes symptoms of depression most of the time for at least two weeks that regularly interfere with work, sleep, eating, and quality of life.
- Persistent depressive disorder (dysthymia), which often includes less severe symptoms of depression that last much longer, typically for at least two years.
- Perinatal depression - occurs when a woman experiences major depression during pregnancy or after delivery (postpartum depression).
- Seasonal affective disorder – impacted by change in seasons, typically starting in late fall and early winter and improving during spring and summer.
- Depression with symptoms of psychosis - a severe form of depression in which the patient experiences psychosis symptoms, such as delusions or hallucinations
Common symptoms of depression include:
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness
- Irritability, frustration‚ or restlessness
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies or activities once enjoyed
- Fatigue or lack of energy
- Aches, musculoskeletal pain, or headaches without injury or obvious causation, and do not improve with medication.
- Gastrointestinal issues
- Difficulty concentrating, remembering, or making decisions
- Poor sleeping patterns
- Changes in appetite
- Suicide attempts or thoughts of death or suicide


Self Quiz
Ask yourself...
- Are you aware of certain biological, environmental, or psychological factors that play a role in depression?
- How would you describe the difference between occasional sadness and depression?
- Can you name common symptoms of depression?
- Which drug class has shown faster antidepressant effects in studies, SSRIs or SNRIs?
Pharmacokinetics of SNRIs
The major mechanism of action of SNRIs is the inhibition of presynaptic neuronal uptake of 5-HT (serotonin) and norepinephrine after release from the synaptic cleft (3). Blocking the reuptake prolongs the presence of monoamines in the synaptic cleft within the central nervous system (CNS). This causes an increase in postsynaptic receptor stimulation and additional post synaptic neuronal transmission (3).
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT), is a neurotransmitter that has an important role in regulating various activities, including behavior, mood, memory, and gastrointestinal homeostasis (1). It is often referred to as the “feel good hormone” and delivers messages between brain cells, contributing to well-being, mood, appetite, social behavior, as well as helping to regulate the body’s sleep-wake cycle.
Serotonin is synthesized in the raphe nuclei of the brainstem and the enterochromaffin cells of the intestinal mucosa (1). Serotonin is a primary treatment target for major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), and anxiety disorders.
Norepinephrine
Norepinephrine, also known as noradrenaline, is a neurotransmitter in the brain that plays an essential role in the regulation of arousal, attention, cognitive function, and stress reactions. It also functions as a hormone peripherally as part of the sympathetic nervous system in the “fight or flight” response.
During states of stress or anxiety, norepinephrine and epinephrine are released and bind to adrenergic receptors throughout the body which exert effects such as dilating pupils and bronchioles, increasing heart rate and constricting blood vessels, increasing renin secretion from the kidneys, and inhibiting peristalsis.
It works closely with dopamine and serotonin systems and is thought to help mobilize the brain for action, increasing alertness, focus, and the retrieval of memory (4).
The increased availability of serotonin and norepinephrine in the nerve synapse means that information can be transmitted easier from one nerve to another.
There are currently five SNRIs approved by the FDA for use in the U.S.:
- Venlafaxine (Effexor XR)
- Duloxetine (Cymbalta, Irenka)
- Desvenlafaxine (Pristiq, Khedezla)
- Levomilnacipran (Fetzima)
- Milnacipran (Savella)
- Milnacipran (Savella TM) is not approved by the FDA to treat depression, but rather only fibromyalgia. For this reason, this course will not review this SNRI.
As mentioned, all SNRIs regulate serotonin and norepinephrine, but there are subtle differences in how much absorption they prevent and the side effects of each type.

Self Quiz
Ask yourself...
- Do all SNRIs have the same uses?
- Can you define serotonin and norepinephrine in your own words?
- Are you familiar with these SNRIs? If so, have you known patients who have benefited from use?
- How would you describe the relationship between sleep cycles, nutrition, and mood?
Venlafaxine
Venlafaxine (Effexor ™) is approved by the FDA and was the first SNRI approved in the U.S. It is available in an extended-release formula and works by regulating levels of serotonin and norepinephrine.
Uses
- Major depressive disorder
- Generalized anxiety disorder
- Social anxiety disorder
- Panic disorder
Mechanism of Action
Venlafaxine increases serotonin, norepinephrine, and dopamine in the brain by blocking transport proteins and preventing their reuptake at the presynaptic terminal (10). This action leads to more neurotransmitters available at the synapse, which ultimately increases the stimulation of postsynaptic receptors.
Venlafaxine is a bicyclic phenylethylamine compound; it is a more potent inhibitor of serotonin reuptake than norepinephrine reuptake (8). Venlafaxine acts as a selective serotonin reuptake inhibitor at 75 mg, but when a higher dose is given, such as 225 mg/day, it has significant effects on the norepinephrine transporter as well (10). Venlafaxine does not have MAO-inhibitory properties.
Pharmacodynamics
The pharmacodynamics of venlafaxine is as follows: (9)
Absorption: 92-100% absorbed after oral administration.
Distribution: Extensive distribution into body tissues.
Metabolism and Excretion: Extensively metabolized in first pass through the liver. 5% of venlafaxine is excreted unchanged in urine; 30% of the active metabolite is excreted in urine.
Half-life: 3-5 hr.; ODV: 9-11 hr.
Contraindications
- Hypersensitivity
- Precaution advised for use in patients with:
- Cardiovascular disease
- Hepatic or renal impairment
- History of seizures or neurologic impairment
- History of mania
- History of drug abuse
- Angle-closure glaucoma
Obstetrics (OB): Use during pregnancy only if risks are closely examined. There is a potential for discontinuation syndrome or toxicity in the neonate when venlafaxine is taken during the 3rd trimester (9). Venlafaxine is a category C pregnancy drug and can potentially pass into breast milk (8).
Venlafaxine is contraindicated if it causes worsening suicidal ideation, depression, anxiety, and psychosis. Precaution is needed for patients with heart failure patients, hyperthyroidism, and those with recent myocardial infarctions, as it can raise blood pressure and increase heart rate. Venlafaxine raises the risk of seizures, and prescribers should avoid the drug in patients with a seizure disorder (8).
Adverse Reactions / Side Effects
Adverse reactions / side effects of venlafaxine include: (10)
CV: Chest pain, hypertension, palpitations, tachycardia.
Neuro: Abnormal dreams, anxiety, dizziness, headache, insomnia, nervousness, paresthesia.
Dermatology: ecchymoses, itching, photosensitivity, skin rash.
EENT: rhinitis, visual disturbances.
GI: abdominal pain, altered taste, anorexia, constipation, diarrhea, dry mouth, dyspepsia, nausea, vomiting, weight loss.
GU: Decreased libido, erectile dysfunction, urinary frequency, urinary retention.
Hematological: Bleeding.
Serotonin syndrome – a condition of building up high levels of serotonin in the body due to medication use. Although rare, serotonin syndrome is a very serious condition that has a high mortality rate (5). Signs of serotonin syndrome include tachycardia, sialorrhea (excessive saliva production), hyperactive bowel sounds, mydriasis (sustained dilated pupils), hyperthermia, and diaphoresis (6). This condition is caused by combining monoamine oxidase inhibitors, tricyclic antidepressants, triptans, additional serotonin receptor modulators, or over-the-counter drugs such as St. John's Wort (6).
If a clinician is suspecting serotonin syndrome, the Sternbach and Hunter criteria can be useful to help arrive at a definitive diagnosis.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors or serotonergic neurotransmitter systems, including tricyclic antidepressants, fentanyl, buspirone, tramadol, amphetamines, and triptans, may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
PO (Adults): Tablets: 75 mg/day in 2-3 divided doses; may increase by up to 75 mg per day every 4 days, up to 225 mg/day. Do not exceed 375 mg per day in 3 divided doses.
Extended-release capsules: 75 mg once daily (some patients may be started at 37.5 mg once daily) for 4-7 days; may increase by up to 75 mg/day at intervals for no less than 4 days (not to exceed 225 mg/day).
ALERT: US Boxed Warning
Antidepressants increased the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders (10). Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults older than 24 years old (10).
Venlafaxine is not approved for use in pediatric patients.

Self Quiz
Ask yourself...
- What are the major mechanisms of action for Venlafaxine?
- Can you explain the side effects and contraindications of Venlafaxine?
- Are SNRIs currently recommended for pediatric patients?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
Duloxetine
Duloxetine (Cymbalta ™) has the most FDA-approved uses of any SNRI, and, unlike Venlafaxine, is proven to be effective in the treatment of other conditions, such as neuropathy, fibromyalgia, and osteoarthritis (9).
Drug Class
Selective Serotonin/Norepinephrine Reuptake Inhibitors
Uses
Duloxetine is used for the following conditions: (9)
- Major depressive disorder
- Diabetic peripheral neuropathic pain
- Generalized anxiety disorder
- Chronic musculoskeletal pain (including chronic lower back pain and chronic pain from osteoarthritis)
- Fibromyalgia
Mechanism of Action
Duloxetine inhibits serotonin and norepinephrine reuptake, and also enhances dopamine levels within the prefrontal cortex. This increase in dopamine levels involve the inhibition of norepinephrine transporters, which are particularly attracted to dopamine, making it effective in transporting dopamine and norepinephrine (2). Essentially, inhibition of norepinephrine transporters can cause an increase in dopamine.
The secondary mechanisms of action, in increasing dopamine, is helpful in pain reduction. This occurs due to increased activity of noradrenergic and serotonergic neurons in the descending spinal pathway on the dorsal horn and suppression of excessive input from reaching the brain (2). The perception of pain can be reduced as these signals are interrupted.
Pharmacokinetics
Absorption: Well-absorbed following oral administration.
Distribution: Unknown.
Protein Binding: >90%.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP2D6 and CYP1A2 isoenzymes. Excretion: Fecal is 20%; renal is 70% as metabolites.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
| ROUTE | ONSET | PEAK | DURATION |
| PO | Unknown | 6 hr. | 12 hr. |
Contraindications:
Contraindications in the use of duloxetine include: (9)
- Hypersensitivity
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Severe renal impairment (CCr <30 mL/min).
- Hepatic impairment or substantial alcohol use (increased risk of hepatitis).
Use cautiously in:
- History of suicide attempt or ideation.
- History of mania (may activate mania/hypomania).
- History of seizure disorder.
- Diabetes (may worsen glycemic control).
- Angle-closure glaucoma.
OB/ Lactation: Use while pregnant or breastfeeding only if potential maternal benefit justifies potential risk to infant.
Geriatric Precaution: Appears on Beers list. May worsen or cause syndrome of inappropriate antidiuretic hormone (SIADH) secretion and/or hyponatremia in older adults; closely monitor sodium concentrations.
Adverse Reactions / Side Effects
CV: Hypertension, orthostatic hypotension.
EENT: Increased intraocular pressure, blurred vision.
Endocrine: SIADH.
Fluids and Electrolytes: Hyponatremia.
GI: Decreased appetite, constipation, dry mouth, nausea.
GU: Dysuria.
Neurological: Drowsiness, fatigue, insomnia.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitor before initiation of levomilnacipran (9). Concurrent use with MAO-inhibitor like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
- Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
PO (Adults): 40-60 mg/day (as 20 mg or 30 mg twice daily or as 60 mg once daily) as initial therapy, then 60 mg once daily as maintenance therapy (9).


Self Quiz
Ask yourself...
- What are the major mechanisms of action for Duloxetine?
- Can you explain the side effects and contraindications of Duloxetine?
- Which of these patient symptoms do you think should be a priority: mild drowsiness or tachycardia?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
Desvenlafaxine
Desvenlafaxine (Pristiq ™) received approval from the FDA in 2008. The drug is similar to Venlafaxine structurally and 10 times more potent in inhibiting serotonin reabsorbing than norepinephrine (6). It has been observed weakly blocking dopamine reuptake, similar to Venlafaxine and Duloxetine.
Uses
Desvenlafaxine is an antidepressant that is an FDA-approved drug to treat major depressive disorder in adults. For healthy women who have contraindications to estrogen, desvenlafaxine has an off-label use to treat hot flashes during menopause (6).
Mechanisms of Actions
Desvenlafaxine is the primary active metabolite of venlafaxine. Therefore, the two agents are structurally similar in that they both contain two chemical rings that are not next to each other. In vitro studies have shown that desvenlafaxine is ten times more selective for serotonin than for norepinephrine, making it similar to the drug duloxetine (6).
Pharmacokinetics
Absorption: 80% absorbed following oral administration.
Distribution: Widely distributed to tissues.
Metabolism and Excretion: 55% metabolized by the liver, 45% excreted unchanged in urine.
Half-life: 10 hr.
Time / Action Profile (Plasma Concentrations):
|
ROUTE |
ONSET |
PEAK |
DURATION |
|
PO |
Unknown |
7.5 hr. |
24 hr. |
Contraindications/Precautions
- Hypersensitivity to venlafaxine or desvenlafaxine.
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Should not be used concurrently with venlafaxine.
Use cautiously in:
- Untreated cerebrovascular or cardiovascular disease, including untreated hypertension (control BP before initiating therapy).
- Bipolar disorder (may activate mania/hypomania).
- Moderate or severe renal impairment.
- History of seizures or neurologic impairment.
- Moderate or severe hepatic impairment.
- Angle-closure glaucoma.
OB: Safety is not established in pregnancy. Desvenlafaxine is excreted into breast milk, with one study reporting that peak levels are 3.3 hours after a dose (6).
Adverse Reactions / Side Effects
There is a broad range of general adverse effects that patients have reported with desvenlafaxine. A study comparing desvenlafaxine to a placebo in treating major depressive disorder in adolescents noted the most common side effects to be abdominal pain, decreased appetite, headache, and nausea (6).
Less common side effects were diarrhea, dizziness, and cough.
Abruptly stopping the use of desvenlafaxine can cause irritability, nausea, and headaches (6). Gradually tapering the medication is recommended to avoid such side effects.
Drug-Drug Interactions
- The concurrent use of all SNRIs and MAO inhibitors may result in serious and potentially fatal reactions (it may increase the risk of serotonin syndrome) (9). A period of at least two weeks is recommended between stopping MAO inhibitors and initiating venlafaxine (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
Route and Dosage in Depression
PO (Adults): 50 mg once daily (range = 50-400 mg/day).
Renal Impairment:
PO (Adults): CCr 30-50 mL/min: 50 mg once daily; CCr <30 mL/min: 50 mg every other day or 25 mg once daily.
Hepatic Impairment:
PO (Adults): 50 mg once daily (not to exceed 100 mg/day).

Self Quiz
Ask yourself...
- What are adverse effects for desvenlafaxine?
- Can you explain why SNRI and MAO inhibitors are contraindicated to use together?
Levomilnacipran
Levomilnacipran (Fetzima ™) is the most recent medication approved for treating major depression.
Levomilnacipran is different from other SNRIs because it has greater inhibition of norepinephrine than serotonin; essentially, it has twice the potency of norepinephrine reuptake inhibition compared to serotonin (3). The more recent FDA approval and relatively lower number of prescriptions may be contributing factors to an overall low volume of research and evidence on this drug.
Levomilnacipran has a one-a-day extended-release form, which makes it easier for patients to stick to its regimen. The drug has no effect on dopamine and affects reuptake inhibition of serotonin and norepinephrine steadily at any dose (3).
Uses
- Major Depressive Disorder
Mechanisms of Actions
Levomilnacipran enhances the amount of serotonin (5-HT) and norepinephrine (NE) activity in the central nervous system by inhibition of reuptake at 5-HT and NE transporters (9). Levomilnacipran has shown to be a more potent inhibitor of NE versus 5-HT transporter (9).
Pharmacokinetics
Absorption: Well-absorbed (92%) following oral administration.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP3A4 isoenzyme. 58% eliminated unchanged in urine; 42% metabolized; metabolites are renally eliminated.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
|
ROUTE |
ONSET |
PEAK |
DURATION |
|
PO |
unknown |
6-8 hr. |
unknown |
Contraindications/Precautions
- Hypersensitivity to Levomilnacipran or milnacipran.
- Uncontrolled narrow-angle glaucoma.
- Use Cautiously in:
- Hypertension, cardiovascular or cerebrovascular disease (blood pressure should be controlled prior to treatment).
- Bipolar disorder (may activate mania/hypomania).
- Renal impairment.
OB: Use only if potential maternal benefit justifies potential fetal risk.
Geriatric: Consider age-related decrease in renal function, chronic disease state and concurrent drug therapy in older adults; increased risk of hyponatremia.
Adverse Reactions/Side Effects
CV: Hypertension, hypotension, palpitations, tachycardia.
Dermatology: Hyperhidrosis (excessive sweating), rash.
EENT: Mydriasis.
Fluids and Electrolytes: Hyponatremia (in association with syndrome of inappropriate antidiuretic hormone [SIADH]).
GI: Nausea, constipation, decreased appetite, vomiting.
GU: Sexual dysfunction, testicular pain, urinary hesitation, and retention.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitor before initiation of levomilnacipran (9). Concurrent use with MAO-inhibitor like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.

Self Quiz
Ask yourself...
- What are the major mechanisms of action for Levomilnacipran?
- Does an increased dosage of Levomilnacipran result in different neurotransmitter activity?
Review of SNRI Pharmacokinetics
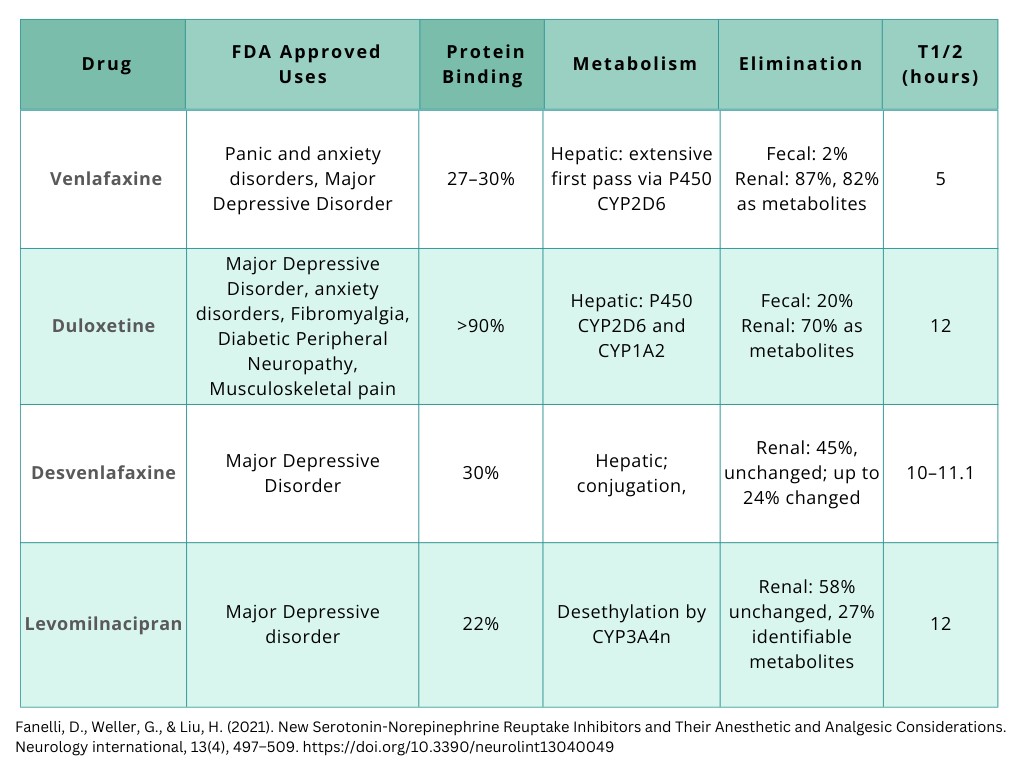
Figure 1. Pharmacokinetics of SNRIs [Created by course author].
Nursing Considerations
It is vital to recognize how each patient is unique and different in their journey of depression treatment. Nurses play a key role in assessing and providing advocacy for these patients.
The following are important nursing considerations:
- Thorough and focused mental status assessment
- Assess level of consciousness and orientation, appearance and general behavior, speech, motor activity, affect and mood, thought and perception, attitude and insight, and cognitive abilities.
- Assess status and mood changes.
- Assess suicidal tendencies, especially in early beginning of SNRI (9).
- Monitor BP before and periodically during therapy. Sustained hypertension may be dose-related; decrease dose or discontinue therapy if this occurs.
- Assess for serotonin syndrome.
- Provide patient education on medication use, dosage, and side effects. Provide patient education on support and resources available.
Nursing Diagnosis: Disturbed Thought Processes
Nursing Diagnosis: Ineffective Coping
Lab Assessments
Monitor CBC with differential and platelet count periodically during SNRI therapy. These medications can cause anemia, leukocytosis, leukopenia, thrombocytopenia, basophilia, and eosinophilia (9).
May cause an increase in serum alkaline phosphatase, bilirubin, AST, ALT, BUN, and creatinine, and serum cholesterol.
SNRIs can also cause electrolyte abnormalities (hyperglycemia or hypoglycemia, hyperkalemia or hypokalemia, hyperuricemia, hyperphosphatemia or hypophosphatemia, and hyponatremia).
Conclusion
SNRIs are an effective method of treatment for those suffering from depression. The effectiveness of SNRIs is dependent on a variety of factors like dosage and time spent in the body. Although the mechanisms of actions and makeup of SNRIs are consistent, each drug has differences on how much reuptake they prevent and the side effects of each type. Prescribers must be aware of these differences, the uses, mechanisms of action, side effects, and warnings.
Conclusion
SNRIs are an effective method of treatment for those suffering from depression. The effectiveness of SNRIs is dependent on a variety of factors like dosage and time spent in the body. Although the mechanisms of actions and makeup of SNRIs are consistent, each drug has differences on how much reuptake they prevent and the side effects of each type. Prescribers must be aware of these differences, the uses, mechanisms of action, side effects, and warnings.
Corticosteroid Therapy
Introduction
When many people hear of steroids, they may think of athletes. However, the steroids that some athletes misuse to enhance their strength and training abilities are not the same as those administered for allergic reactions and some inflammatory conditions. The steroids used by some athletes are called anabolic steroids. The steroids used for the treatment of disease are called corticosteroids.
Steroids are hormones naturally produced in the body (made from cholesterol) to regulate the function of various bodily systems [2]. Some steroid hormones are produced by the adrenal glands of the endocrine system (a set of two glands that rest atop the kidneys). The adrenal glands produce the hormone types: glucocorticoids, mineralocorticoids, and adrenal androgens [2]. Corticosteroid medication mimics glucocorticoids, more specifically a glucocorticoid called “cortisol.”
The Immune System
Corticosteroids are well-known for increasing a patient’s risk for infection. This is due to the medication’s effect on the immune system, particularly its anti-inflammatory properties. It is important to have a clear understanding of how the immune system works as well as the inflammatory process that takes place when a foreign pathogen enters the body.
How Does the Immune System Work?
In a typical immune system the immune system is triggered by an antigen – a marker in the body that alerts cells of the immune system that there’s a foreign invader present, like a pathogen or foreign object. Subsequently, a series of events follows. Like in a war, the body rounds up the first line of defense soldiers to fight the pathogen.
These include [11]:
- Proteins (called “the inflammasome”) to active inflammatory responses.
- Macrophages to engulf and digest pathogens.
- Neutrophils to help kill pathogens and rid of the leftover debris.
- Endothelial cells to help rid of the dead pathogens/debris.
The influx of these cells to the site of injury or infection is the beginning of inflammation.
What is Inflammation?
Inflammation is more than just “swelling.” Inflammation is a process in which a cascade of events occurs to help the immune system fight against pathogens and foreign bodies. The inflammatory process encompasses a variety of signs/symptoms including redness, warmth, swelling, and drainage [11].
For example, when a person sustains a traumatic injury to the skin causing an open wound, the accompanying redness (or erythema) is caused by a rush of red blood cells through the site [5]. The dilation of the blood vessels is what causes this “rush” of cells. The increased blood flow also causes the localized warmth that typically occurs. Blood vessel dilation is responsible for the associated swelling as well. The pus that forms is a combination of fluid, dead immune/pathogen cells, and debris from the fight, essentially, the “casualties at war” that must be removed from the body [5].
As with fever, inflammation aids in the fight against pathogens and therefore should not be inhibited unless it is likely to be damaging or fatal in and of itself. [11]. This inflammatory process is acute in nature, and typically goes away on its own or with the help of medication. While acute inflammation from a normal immune response is natural and beneficial, chronic inflammation is harmful and can lead to permanent damage of tissues and body systems.
Chronic inflammation can be seen in conditions including inflammatory bowel disease, cancer, heart disease, and autoimmune disorders like rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus [11]. Chronic inflammation is typically caused by an overactive immune system or autoimmune disease (when the immune system mistakenly attacks normal tissues). In cases like these, inhibiting the inflammatory process by suppressing the immune system is most beneficial. This is where corticosteroids come into play.

Self Quiz
Ask yourself...
- How often do you encounter patients with autoimmune disease or chronic inflammatory diseases?
- Have you ever cared for a patient who was on long-term corticosteroid therapy?
- Have you ever cared for a patient who developed an infection after corticosteroid use?
- How comfortable are you with the idea of immunosuppression?
Corticosteroids: The Basics
Corticosteroids are a group of steroidal therapy medications that are often the go-to for reducing inflammation in the body. Once referred to as “a miracle drug,” these medications are helpful in the treatment of acute and chronic inflammatory conditions and autoimmune diseases.
Corticosteroids are also useful in emergency situations, for example during asthma attacks or allergic reactions – when the inflammation is life-threatening. Corticosteroids are synthetically formulated to mimic the hormone cortisol.
What is Cortisol?
As mentioned earlier, the adrenal glands of the endocrine system produce three different types of steroid hormones, one of which is glucocorticoids. Cortisol is a major glucocorticoid hormone.
There are two major structures of the adrenal glands – the outer layer (cortex) and inner portion (medulla) – each producing its own hormones. The outer layer (cortex) produces cortisol, hence the name “corticosteroid” [6].
Cortisol levels in the body rise when a person is stressed [2]. This hormone helps to regulate metabolism, growth, reproduction, and the immune system [2]. Cortisol also aids in cardiovascular function by making blood vessels more sensitive to natural vasoconstrictors in the body (like adrenaline) [2].
How Do Corticosteroids Work?
Corticosteroids work by suppressing the immune system which in turn reduces inflammation. In patients with an overactive immune system or acute flareups from a triggering agent, the inflammation can cause troubling symptoms, become prolonged, and spread to other areas within the body. If left untreated, inflammation can lead to tissue scarring (or damage to affected structures) and loss of bodily functions [11].
The challenge with administering corticosteroids to suppress an overactive immune system is balancing the benefits of inflammatory symptom relief and the risks of infection due to the resulting underactivity of the immune system. In this case, clinicians are encouraged to start with the lowest therapeutic dose possible, carefully monitor its use in patients with infections, and ensure that the benefits exceed the risks [6].
Corticosteroids also stop the body from producing its own natural cortisol. In a sense, a course of corticosteroids gives the adrenal glands a break. In a short course of corticosteroids, once the medication is discontinued, the adrenal glands can start back releasing cortisol. However, the adrenal glands do not resume releasing cortisol right away and need time to start working again.
This is why corticosteroids (oral and parenteral forms) should not be stopped abruptly when taken for more than about two weeks [6]. If stopped abruptly, patients may develop adrenal insufficiency during this period, which can be life-threatening.


Self Quiz
Ask yourself...
- What do you think is the biggest concern of nurses and clinicians when prescribing/administering corticosteroids?
- Have you encountered patients with permanent bodily dysfunction caused by prolonged inflammation?
- How challenging is it to balance infection risk and therapeutic benefit when prescribing/administering corticosteroids?
- How do you determine what to begin with when initiating a new corticosteroid treatment? Or have you ever discussed dosing safety with a provider?
Corticosteroid Pharmacology
Common corticosteroid formulations include intravenous (IV), intramuscular (IM), intra-articular (injected directly into a joint), oral, inhaled, intranasal (nasal spray), topical, ophthalmic (eye drops), and otic (ear canal). The increased risk of infection is most associated with systemic formulations (oral and parenteral).
The following sections will cover the pharmacology of systemic corticosteroids, including indications, mechanisms of action, pharmacokinetics, precautions, adverse effects, drug interactions, and warnings [7].
Indications
Corticosteroids are useful in the treatment of many medical conditions. Some indications for corticosteroids among adults include the following diseases, conditions, and situations (maybe drug- or route-specific) [6][7].
Allergic Reactions
Corticosteroids are well-known for treating mild to severe allergic reactions. These include anaphylaxis, angioedema, and laryngeal edema (non-infectious). Others include hypersensitivity reactions to medications or food, severe seasonal allergies (like allergic rhinitis), contact dermatitis (or rash), and itching.
Autoimmune Diseases
Corticosteroids are used to treat autoimmune disorders including systemic lupus erythematosus, Crohn’s disease, rheumatoid arthritis, and plaque psoriasis. Others include autoimmune hepatitis, hemolytic anemia, celiac disease (gluten intolerance), sarcoidosis (marked by lumps of inflammatory cells throughout the body) and myasthenia gravis (marked by weakness in voluntary muscles).
Pulmonary Exacerbations
Corticosteroids are useful in the treatment of exacerbations from asthma and chronic obstructive pulmonary disease (COPD).
Adrenal Insufficiency
Corticosteroids may be used to treat both primary and secondary adrenocortical insufficiency. This includes Addison’s disease, congenital adrenal hyperplasia, and adrenogenital syndrome.
Inflammatory Bowel Disease
Inflammatory bowel disease, such as the aforementioned Crohn’s disease, and ulcerative colitis may be treated with corticosteroids.
Neuro/Musculoskeletal Disorders
Corticosteroids are used in the treatment of ankylosing spondylitis (chronic inflammation of spinal joints), bursitis, polymyalgia rheumatica (marked by muscle pain and stiffness), osteoarthritis, and psoriatic arthritis. Corticosteroids can also treat neurological conditions like multiple sclerosis and Bell’s palsy (facial paralysis, often temporary).
Inflammatory Eye Conditions
Corticosteroids are useful in the systemic treatment of inflammatory ophthalmic conditions including optic neuritis, allergic conjunctivitis, and other conditions that cause inflammation of various structures of the eye.
Organ Transplant Rejection Prophylaxis
Renal transplant guidelines recommend corticosteroids in combination with other medications to prevent kidney rejection after transplantation (called induction therapy or immunosuppressant therapy). Corticosteroids are also indicated for heart and liver transplant rejection prophylaxis.
Cancer
Corticosteroids may be used for the palliative treatment of cancers including multiple myeloma and chronic lymphocytic leukemia (CLL). They may also be used to treat Hodgkin lymphoma (in conjunction with cancer medications), aggressive non-Hodgkin lymphoma, peripheral T-cell lymphoma, and neoplastic-associated hypercalcemia.
COVID-19
Although still in the investigative stages, the World Health Organization (WHO) recommends systemic corticosteroids as an adjunctive treatment for severe COVID-19 [6][13].

Self Quiz
Ask yourself...
- Do you prescribe/administer corticosteroids most frequently for acute or chronic conditions?
- What is the most common indication for corticosteroid therapy you have witnessed in your practice?
- Have you ever prescribed/administered corticosteroids for prophylaxis treatment alone?
- Can you think of ways to minimize the severity of adverse effects of corticosteroid therapy among patients most at risk?
Mechanism of Action
As already mentioned, corticosteroids suppress the immune system. This is done by preventing protein synthesis in certain cells of the immune system (like macrophages and neutrophils), ultimately inhibiting their fighting potential [6][11]. More specifically, corticosteroids repress the activity of DNA within the immune cell – an activity that would otherwise serve as the beginning of protein synthesis.
Within immune cells, DNA sends messages to protein-making structures within the cell that prompts them to start making protein. However, the message must be “translated” or “decoded” (transcription) first before the structures can “understand” what to do [6][12]. Messenger RNA (mRNA) is the transcribed version of DNA. Corticosteroids work by making it difficult for DNA to be transcribed into mRNA.
Pharmacokinetics
The pharmacokinetics of corticosteroids differ based on drug type and route. The following is the pharmacokinetics of three common systemic corticosteroid drugs: prednisone, methylprednisolone, and dexamethasone [6].
Prednisone
Prednisone comes in oral forms, both immediate-release (IR) and delayed-release. Prednisone IR is quickly absorbed in the gastrointestinal (GI) tract with a peak effect after one to two hours. Delayed-release tablets are released four hours after ingestion and peak after about six hours. Prednisone is metabolized by the liver and excreted by the kidneys.
Methylprednisolone
Methylprednisolone is rapidly absorbed orally and peaks within one to two hours. After IV administration, effects can occur within one hour with nearly complete excretion in 12 hours. When given as an injection in the joint, methylprednisolone absorption can occur over several days. This medication is distributed into various organs and body structures including the kidneys, intestines, skin, liver, and muscle. Methylprednisolone is metabolized by the liver and excreted by the kidneys.
Dexamethasone
Oral dexamethasone peaks in one to two hours. Absorption times for parenteral forms depends on the dosage and indication. For example, in patients with cerebral edema, treatment response from an IV dose of dexamethasone followed by an IM dose occurs in 12 to 24 hours [1]. Dexamethasone is rapidly distributed into the skin, intestines, liver, muscle, and kidneys. As with prednisone and methylprednisolone, this medication is metabolized by the liver and excreted by the kidneys.


Self Quiz
Ask yourself...
- What is the most common corticosteroid you have prescribed/administered?
- Why do you think it is important to know the mechanism of action of corticosteroids?
- What is the main determining factor in prescribing/administering an oral corticosteroid as opposed to a parenteral form?
Contraindications and Precautions
Clinicians should not prescribe/administer prednisone to patients with an allergy to the drug or any components of its formulation. Precautions should be taken in patients with an allergy to other corticosteroids as cross-sensitivity can occur. Clinicians should also be aware that high-dose systemic corticosteroids place patients at risk for immunosuppression, especially when prescribed/administered with other immunosuppressant medications.
Although carrying a lower risk, moderate-dose and low-dose corticosteroid preparations should be monitored as well. Clinicians should avoid prescribing/administering these medications to patients with fungal or bacterial infections that are not well controlled with anti-infective medications.
Pregnancy and Breastfeeding Precautions
While corticosteroids are not contraindicated for pregnant or breastfeeding patients, clinicians have reason to be cautious when prescribing/administering these medications [6]. The following are precautions for pregnant and breastfeeding patients.
Pregnancy
If corticosteroids must be prescribed/administered during pregnancy, precaution should be taken as these medications cross the placenta and may be harmful to the fetus/infant (may cause adrenal insufficiency in the infant). Although the risks are small and inconsistent, oral or facial clefts (like cleft palate) may occur if systemic corticosteroids are prescribed/administered during the first trimester.
Breastfeeding
Corticosteroids are distributed into breastmilk. While no reported side effects have been reported in breast-fed infants, lower doses are recommended as high doses may cause problems with the infant’s growth and development. Corticosteroids can interfere with the infant’s ability to produce their own glucocorticoid hormones. With prednisone in particular, peak concentrations in breastmilk occur in about one hour after the dose is taken and it is recommended for patients to avoid breastfeeding during this time. However, the total daily dose of prednisone reaching the infant has been shown to be approximately 0.1% of the mother’s total daily dose [6].

Self Quiz
Ask yourself...
- How comfortable are you prescribing/administering corticosteroids to patients who already take immunosuppressive medications?
- Have you ever witnessed a patient have a severe allergic reaction to a corticosteroid? If so, what was the treatment/anecdote?
- Have you ever been in a situation in which you had no choice but to prescribe/administer corticosteroids to a patient who was pregnant?
Adverse Effects
Corticosteroids given systemically can affect multiple body systems, particularly with prolonged use. Short-term use in high doses typically does not cause adverse effects [6]. Adverse effects of corticosteroids can be as mild as a rash to as severe as psychosis or a ruptured heart wall.
The following are adverse effects categorized by body systems [6].
Neurological
Corticosteroids may increase intracranial and intraocular pressure which can lead to optic nerve damage and visual impairments. These medications can also lower seizure thresholds. Other neurological findings include headache, vertigo, and peripheral neuropathy.
Pulmonary
Corticosteroids can reactivate tuberculosis (TB) in patients who have a history of active TB.
Cardiovascular
Sodium retention, edema, and low potassium may occur in patients with high blood pressure or congestive heart failure (can also occur with renal failure/insufficiency). Left ventricular free-wall rupture can occur in patients with a recent myocardial infarction. Corticosteroids may also exacerbate arrhythmias and cause blood clots which may lead to a stroke.
Endocrine
Corticosteroids are known to decrease glucose tolerance and raise blood glucose levels. This can aggravate existing diabetes mellitus. Hypothalamic-pituitary-adrenal (HPA) suppression or signs/symptoms of Cushing’s syndrome (marked by high cortisol levels) can also occur with prolonged systemic use.
Gastrointestinal/Genitourinary
Corticosteroids can increase the risk of GI perforation and should therefore be monitored in patients with peptic ulcer disease, diverticulitis, or GI abscess. Additionally, esophageal ulcers and GI bleed may occur with use. Corticosteroids may cause weight loss, but can stimulate the appetite as well, leading to weight gain conversely. Menstrual irregularity may occur as well.
Musculoskeletal
Osteopenia (loss of bone density) and osteoporosis (more severe bone loss) can occur due to decreased bone formation, increased bone resorption, and inhibition of osteoblast function. Bone fractures (primarily in elderly patients) can occur as well. Acute generalized myopathy leading to quadriparesis (weakness in all extremities) may occur in patients with neuromuscular disease or those receiving neuroblocking medications. Muscle pain and waste can also occur due to protein depletion.
Skin
Corticosteroids may cause impaired wound healing due to protein depletion. Sweating, abnormal hair growth, and striae (stretch marks) can also occur with use. While corticosteroids may be useful in the treatment of rashes and itching, they can cause these symptoms as well.
Psychiatric
Psychiatric problems can occur with corticosteroid use, including euphoria, severe depression, anxiety, hallucinations, psychosis, personality changes, and withdrawn behavior. Patients may also experience insomnia, impaired cognition, mood swings, irritability, and restlessness.
Laboratory Changes
A common lab finding with corticosteroid use is leukocytosis (without an infectious or inflammatory cause). It is important for clinicians to differentiate between infectious and corticosteroid-related leukocytosis. Other lab findings may include low neutrophil count and abnormal electrolyte levels (high sodium, low calcium, and low potassium).

Self Quiz
Ask yourself...
- What are the most common adverse effects of corticosteroid therapy you have witnessed in your practice?
- Considering the severity of some adverse effects of corticosteroids, what is your strategy for safe dosing/administration?
- Has a patient under your care ever reported a serious adverse effect from a short-term course of corticosteroids?
- How often do you consult another provider in prescribing corticosteroid therapy for patients with multiple comorbidities? Or how often do witness providers doing so?
Black Box Warning
Black box warnings are issued by the U.S Food and Drug Administration (FDA) to warn the public about the serious adverse effects of some medications. The most recent black box warning for corticosteroids was issued in 2014. The FDA warned against administering corticosteroid injections into the epidural space of the spine for the treatment of neck/back pain and radiating pain in the extremities [4]. When administered in this way, serious (although rare) adverse effects can occur including vision loss, stroke, paralysis, and death.
Drug Interactions
The therapeutic effect of corticosteroids may be counteracted or enhanced when administered with certain medications. Clinicians should perform an accurate medication reconciliation when prescribing/administering corticosteroids to ensure effective and safe treatment.
The following are medications that interact with corticosteroids categorized by a decreased or increased therapeutic effect [1]. Corticosteroid doses should be adjusted accordingly.
Decreased Therapeutic Effect
Medications that can decrease the level of corticosteroids in the blood when administered together include:
- Barbiturates
- Carbamazepine
- Ephedrine
- Phenytoin
- Rifampin
- Cholestyramine (affects oral corticosteroids in particular)
Increased Therapeutic Effect
Medications that can increase the level of corticosteroids in the blood when administered together include:
- Ketoconazole
- Macrolide antibiotics

Self Quiz
Ask yourself...
- How often do you prescribe/administer corticosteroid injections (in any area of the body)?
- How comfortable are you prescribing/administering corticosteroids in conjunction with antibiotics?
- Have you ever had to increase a corticosteroid dose due to inadequate therapeutic levels (evidenced by persistent symptoms)?
- How often do you rely on pharmacists to check drug interactions for you?
Clinical Guidelines on Corticosteroid Use in the Critically Ill
Clinical practice guidelines for corticosteroid therapy were developed to assist clinicians and providers in ensuring optimal prescribing and administration practices. Currently, guidelines are in place for corticosteroid use in critical illness. The most recent 2024 guidelines were reestablished for the treatment of septic shock, acute respiratory distress syndrome, and community-acquired bacterial pneumonia, particularly in adult patients.
In the following sections, these guidelines are compared with prior 2017 recommendations [3]. Clinicians practicing in acute care settings can use the following guidelines when prescribing/administering corticosteroids to patients who are critically ill.
Sepsis and Septic Shock
Previously, the guidelines recommend against administering corticosteroids in adult patients with sepsis without shock. Instead, corticosteroids were recommended in patients with septic shock not responsive to fluid and moderate- to high-dose vasopressor therapy.
Current Recommendation: Administer corticosteroids to adult patients with septic shock. Do not administer high dose/short duration corticosteroids for adult patients with septic shock (no more than 400 mg per day of a hydrocortisone equivalent for no more than three days).
Acute Respiratory Distress Syndrome
Previously, the guidelines recommended corticosteroid use in patients with early moderate to severe acute respiratory distress syndrome (PaO2/FIO2 of less than 200 and within 14 days of onset).
Current Recommendation: Administer to adult hospitalized patients with acute respiratory distress syndrome.
Community-Acquired Bacterial Pneumonia
Previously, the guidelines recommended corticosteroid use for five to seven days at a daily dose of less than 400 mg IV hydrocortisone (or equivalent) in hospitalized patients with community-acquired pneumonia.
Current Recommendation: Administer to adult patients hospitalized with severe bacterial community-acquired pneumonia. There is no recommendation for adult patients hospitalized with less severe bacterial community-acquired pneumonia.

Clinical Guidelines on Corticosteroid Use for COVID-19
While data is limited and still developing, both the World Health Organization (WHO) and the National Institutes of Health (NIH) developed clinical practice guidelines for the use of corticosteroids as a treatment for COVID-19.
Clinicians practicing in acute care settings can use the following guidelines when prescribing/administering corticosteroids to patients with COVID-19.
Global Guidelines for Corticosteroid Use for COVID-19
As mentioned earlier, the WHO recommends corticosteroids for the treatment of severe COVID-19 [6]. The 2020 “Corticosteroids for COVID-19: Living Guidance” recommendations are as follows [13]:
- The WHO recommends systemic corticosteroids rather than no corticosteroids for the treatment of patients with severe and critical COVID-19.
- The WHO suggests not to use corticosteroids in the treatment of patients with non-severe COVID-19.
National Guidelines for Corticosteroid Use for COVID-19
The National Institutes of Health (NIH) developed the 2023 “Therapeutic Management of Hospitalized Adults with COVID-19” guidelines outlining various drug therapy recommendations for patients with COVID-19, including corticosteroids. Recommendations are as follows [9][10]:
- The panel recommends against the use of dexamethasone (or other systemic corticosteroids) for the treatment of COVID-19 in patients who do not require supplemental oxygen.
- The panel recommends against the use of dexamethasone (or other systemic corticosteroids) in nonhospitalized patients in the absence of another indication.
- The panel recommends patients with COVID-19 receiving dexamethasone (or another corticosteroid) for an underlying condition to continue this therapy as directed by their health care provider.

Self Quiz
Ask yourself...
- How does your facility/organization inform nursing staff of new clinical practice recommendations?
- How comfortable are you with the idea of using corticosteroids in the treatment of septic shock and COVID-19?
- How often do you review clinical practice guidelines on your own time?
- Are there any other practice recommendations your facility has shared with nursing staff about corticosteroid safety?
Patient Education
Therapeutic and safe corticosteroid use is dependent on safe prescribing and administration practices, as well as patient compliance. Optimal patient education is vital for successful treatment with corticosteroids.
The following are teaching points to share with patients about corticosteroid use [1][6][7].
- Adverse Effects: Patients should be informed of common adverse effects, particularly elevated blood sugar, mood changes, appetite changes, and weight gain.
- Vaccine Safety: Patients should be encouraged to check with their health provider before receiving vaccines (Corticosteroids should not be given with live vaccines)
- Infection Risk: Patients should be informed of the risk for developing an infection when corticosteroid doses are high or used to suppress the immune system, and to therefore avoid exposure to chickenpox or measles.
- Administration: Patients should be instructed to avoid abruptly stopping corticosteroids to prevent adrenal insufficiency and potentially death. They should also be made aware that stress on the body (for example, when having surgery) may require additional doses of corticosteroids.


Self Quiz
Ask yourself...
- What do you think is the most important teaching point for patients about corticosteroids?
- Have you ever had to adjust a patient’s corticosteroid dose due to a stressful event (like surgery)? Or have you witnessed a provider doing so?
- What is the protocol in your facility/organization for educating patients about corticosteroid therapy?
- Have you ever encountered a patient who abruptly stopped corticosteroid treatment? If so, did the patient develop any symptoms?
Future Implications
Corticosteroid use may be on the rise. As aforementioned, corticosteroids are useful in the treatment of autoimmune disease. In the U.S., researchers anticipate a rise in autoimmune diseases in the future.
In a 2020 study in Arthritis and Rheumatology, researchers noted that the prevalence of antinuclear antibodies (ANA) (markers of autoimmune disease) is increasing in the U.S. [8]. The study analyzed the antibodies of over 14,000 participants (age 12 and older) over the course of three time periods. Results revealed the following:
- From 1988 – 1991, 22 million people had antibodies.
- From 1999 – 2004, 27 million people had antibodies.
- From 2011 – 2012, 41 million people had antibodies.
The study also found that antibody prevalence is highest among males, non-Hispanic whites, adults over age 50, and adolescents. Young people age12 to 19 had the highest antibody prevalence. While it is unclear why this is the case among the youth, the study findings may suggest an increase in the use of corticosteroids in the future. Further, clinicians might anticipate changes in clinical guidelines for safe prescribing/administration practices.

Self Quiz
Ask yourself...
- How often do you encounter patients on long-term corticosteroid therapy for autoimmune disease?
- Have you noticed a rise in corticosteroid prescribing/use in your facility/organization?
- Why do you think antibody prevalence is on the rise in the adolescent population?
Conclusion
Corticosteroids are helpful in the treatment of many medical conditions and diseases. While the use of these medications is common, there are equally associated risks, many of which can be life-threatening. Safe prescribing/administration practices are imperative. Clinicians can ensure that treatments are safe and effective through the gathering of accurate health histories, careful evaluation of each patient’s situation, weighing of risks and benefits, and provision of effective education to patients.

Self Quiz
Ask yourself...
- In your opinion, what is the biggest public misconception about corticosteroid use?
- Do you feel that corticosteroids are over-prescribed/used?
- How can nurses enhance teaching for patients to ensure compliance with corticosteroid treatments?
- How can nurses advocate in the workplace for safer prescribing/administration of corticosteroids?
- What future clinical practice changes do you anticipate with regards to corticosteroid use in the U.S.?
Anti-arrhythmics
Introduction
Cardiac arrhythmias continue to present significant clinical challenges and remain a cause common of death and disability [5]. Arrhythmias encompass a wide array of heart rate and rhythm disturbances; they are classified into broad terms as bradyarrhythmia’s (heart rates below 60 beats per minute) and tachyarrhythmias (heart rates exceeding 100 beats per minute) [10].
The defining feature of cardiac arrhythmias is an irregular heartbeat, or a symptom of abnormal heart rhythm linked to irregular initiation of electrical impulses, or a combination of both factors.
The mechanisms underlying cardiac arrhythmias are complex. The management of these conditions often involves the administration of antiarrhythmic drugs. It is vital that prescribers are aware of the pharmacokinetics of this drug class.
Overview of Cardiac Arrhythmias
Arrhythmias impact an estimated 17 million individuals across the planet, they rank among the most prevalent forms of heart disease [8]. The clinical manifestations of arrhythmias vary from asymptomatic individuals to sudden cardiac death (SCD), contributing to 10–15% of all mortality cases [10].
The only pattern considered to be a normal heart rhythm is the normal sinus rhythm. In this state, an electrical impulse originates in the sinoatrial (SA) node and travels through the heart [1]. This delayed impulse occurs in the atrioventricular (AV) node, then it proceeds through the His-Purkinje network, which encompasses the bundle of HIS, the left and right bundle branches, and the Purkinje fibers [1]. These reactions essentially coordinate each beat of the heart.
Despite the notable limitations associated with existing antiarrhythmic medications, pharmacological intervention remains a fundamental aspect of managing cardiac arrhythmias [5]. Research suggests that arrhythmias occur in 1.5% to 5% of the general population, with atrial fibrillation being the most prevalent form [1, 10].
Arrhythmia refers to any deviation from the heart's normal rhythm, with the normal sinus rhythm being the baseline for a healthy heart rhythm [1]. The categorization of arrhythmias occurs through various criteria, with the most prevalent method being the heart rate of conduction. This includes bradyarrhythmia’s, where the heart beats slower than 60 beats per minute (bpm), and tachyarrhythmias, where the heart rate exceeds 100 bpm [1].
Atrial fibrillation (AF) is the most frequent occurring sustained cardiac arrhythmia and linked to heightened morbidity and mortality rates, in addition to increased healthcare costs [2][3].
Arrhythmias may originate from congenital anomalies (present from birth) or can develop due to irritation or damage to the myocardial tissue, causing disruptions or ‘short circuits’ in the heart's electrical system [13].
Basics of Antiarrhythmic Drugs (AADs)
Antiarrhythmic drugs (AADs) continue to be fundamental in the management of cardiac arrhythmias and classified based on the cardiac action potential [4]. The action of these drugs works toward the immediate cessation of atrial and ventricular arrhythmias (acute cardioversion) and for the long-term prevention of arrhythmia recurrence to maintain normal sinus rhythm [5].
A limited number of new AADs have reached the market despite the growing incidence of cardiac arrhythmias [5].
Two key objectives define the rationale for treating arrhythmias: (1) to mitigate significant clinical symptoms, including weakness, syncope, or the onset or worsening of congestive heart failure caused by an arrhythmia, and (2) to extend the patient's lifespan [8].
In the initial stages of antiarrhythmic drug (AAD) development, the primary focus was on controlling ventricular arrhythmias [6]. The direction of treatment changed following the adverse outcomes highlighted by the Cardiac Arrhythmia Suppression Trial (CAST) and the Survival with Oral D-Sotalol (SWORD) trial, which demonstrated that patients receiving AADs (encainide or flecainide) fared worse than those on placebo [6].
The primary criterion of antiarrhythmic drugs is safety. In the last decade antiarrhythmic drugs have been subject to intense reevaluation, prompted by the outcomes of large-scale human research studies that highlighted various risks and limitations associated with pharmacological treatments for arrhythmias [7].
In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, the trial demonstrated that although achieving rhythm control was associated with improved survival the adverse effects negated survival benefits [8].

Self Quiz
Ask yourself...
- How would you describe the range from asymptomatic cases to life-threatening conditions?
- How can healthcare professionals balance the immediate need for rhythm control with the long-term goal of minimizing adverse effects and mortality?
Definition
Antiarrhythmic agents, often referred to as cardiac dysrhythmia medications, constitute a category of pharmaceuticals designed to moderate the heart’s electrical impulse and mitigate rapid heart rhythms; these rhythms include atrial fibrillation (AF), supraventricular tachycardia (SVT), and ventricular tachycardia (VT) [11].
This class of drugs influence cardiac ionic channels or receptors, modifying the cardiac action potential, or its creation and transmission [11]. These alterations affect the activation spread or repolarization pattern, suppressing cardiac arrhythmias [12].
Common symptoms of arrhythmias include heart fluttering, abnormal or rapid heart rhythms, lightheadedness, fainting spells, chest pain, and breathlessness. Individuals may also experience heart palpitations, dizziness or feeling faint, chest pain or discomfort, weakness, and fatigue [12].

[10]

Self Quiz
Ask yourself...
- How do antiarrhythmic agents contribute to the suppression of arrhythmias and the alleviation of associated symptoms such as heart fluttering, lightheadedness, and chest pain?
- Do you have experience administering antiarrhythmic medications to patients?
Classification of Antiarrhythmic Medications
The categorization of arrhythmias is based on the location within the conduction pathway where they originate.
There are two main groups:
- Supraventricular - originating from the atria or the atrioventricular (AV) node.
- Ventricular - occur distal to the AV node.
[23]
Prior to 2018, the categorization of antiarrhythmic drugs was based on the Vaughan-Williams (VW) classification system, which organized these medications by their principal mechanism of action [4, 14]. The initial Vaughan-Williams classification had limitations. When introduced in the 1970s, the range of antiarrhythmic drugs focused on altering the function of Na+, K+, and Ca2+ channels, as well as targeting intracellular processes governed by adrenergic activity [15].
Today, there is a broader range of advanced antiarrhythmics, many possessing overlapping interactions with drugs in other classes. For example, amiodarone, categorized under Class III (Potassium channels blockers), also exhibits sodium and calcium-channel blocking properties (Class IV).

Self Quiz
Ask yourself...
- How do the limitations of the initial Vaughan-Williams classification system highlight the importance of continuous research and development in the field of cardiac pharmacology?
- Can you think of an example of a drug that contains overlapping drug actions?
Class 0: HCN Channel Blockers
Ivabradine
Ivabradine is designed to lower heart rate. It is used in the management of stable angina pectoris and chronic heart failure with heart rate ≥70 bpm across various clinical scenarios, including those with either preserved or compromised left ventricular (LV) function.
Ivabradine is effective by decreasing heart rate while preserving myocardial contractility and coronary vasomotor responsiveness, thereby reducing oxygen consumption, and extending diastolic duration [16] [17].

Self Quiz
Ask yourself...
- How does Ivabradine's mechanism of action contribute to its therapeutic benefits in patients with stable angina pectoris and chronic heart failure?
Class I: Voltage-gated Na+ Channel Blockers
Class IA
Class IA medications block fast sodium channels and include agents such as quinidine, procainamide, and disopyramide [11]. Quinidine, disopyramide, and procainamide are used in the management of supraventricular tachyarrhythmias, recurrent atrial fibrillation, ventricular tachycardia, ventricular fibrillation, Brugada syndrome, and Short QT Syndrome (SQTS) [4].
These drugs are associated with the highest risk of proarrhythmic among sodium channel blockers due to their capacity to prolong the QTc interval, which restricts their use because of their proarrhythmic potential [11].
For patients with Brugada syndrome, Quinidine is an alternative to implantable cardioverter-defibrillator (ICD) placement [66].
This class has also shown utility in individuals with short QT syndrome experiencing recurrent ventricular arrhythmias (VAs), reducing the frequency of ICD shocks in these patients [66].
Disopyramide is employed in cases of hypertrophic obstructive cardiomyopathy (HOCM), when combined with a beta-blocker or verapamil to alleviate symptoms like angina or dyspnea in patients unresponsive to beta-blockers or verapamil alone [67].
Procainamide is useful for exposing and diagnosing Brugada syndrome in individuals suspected of the condition but without a confirmed diagnosis [68]. Procainamide has shown to reestablish sinus rhythm in patients with Wolff-Parkinson-White (WPW) syndrome who experience atrial fibrillation (AF) without hemodynamic instability, which is marked by a wide QRS complex or a rapid pre-excited ventricular response [69]. Procainamide may also assist in terminating ventricular tachycardia and other arrhythmias [69].

Self Quiz
Ask yourself...
- How might the proarrhythmic potential of these drugs and their ability to prolong the QTc interval influence the decision-making process in selecting an appropriate treatment strategy for patients with conditions like Brugada syndrome, Short QT Syndrome (SQTS), or Wolff-Parkinson-White (WPW) syndrome?
Class IB
Lidocaine and Mexiletine treat ventricular tachycardia and ventricular fibrillation after a myocardial infarction by inducing a mild blockade of sodium channels [4]. These are not effective for treating atrial arrhythmias [4].
In the context of long QT syndrome, mexiletine is capable of reducing the QTc interval and has been employed to decrease the incidence of recurrent arrhythmias and the need for interventions by implantable cardioverter-defibrillators (ICDs) [70].
The effectiveness of Lidocaine diminishes in instances of hypokalemia, necessitating the correction of potassium levels [80].

Self Quiz
Ask yourself...
- How does the mechanism of sodium channel blockade by Lidocaine and Mexiletine contribute to their differential effectiveness in treating ventricular?
- What implications does this have for their use in managing long QT syndrome and conditions of electrolyte imbalance such as hypokalemia?
Top of Form
Class IC
Research recommends Encainide (Enkaid), flecainide (Tambocor), and propafenone (Rythmol SR) for managing supraventricular and ventricular tachyarrhythmias that do not respond to standard treatments, in the absence of underlying structural heart disease. [72].
Propafenone increases the effects of cyclosporin, desipramine, and theophylline [81].
Class IC is used to treat premature ventricular contractions and catecholaminergic polymorphic ventricular tachycardia [18]. These drugs block sodium channels without altering the QT interval and suit ongoing management in individuals with symptomatic supraventricular tachycardia (SVT) who have no structural or ischemic heart disease, and either are unsuitable for or opt against catheter ablation. [11].
In addition, these agents are effective for the pharmacological cardioversion of atrial fibrillation (AF). The “pill in the pocket” strategy involves patients with paroxysmal AF carrying a loading dose of medication to take at the onset of an AF episode. This approach aims for chemical cardioversion to restore normal rhythm, rather than adhering to a regular maintenance dose regime [71].
The Cardiac Arrhythmia Suppression Trials (CAST I and II) showed that patients with a history of myocardial infarction, treated with class IC agents (flecainide, encainide, moricizine) to reduce premature ventricular contractions (PVCs), faced a higher mortality risk compared to those receiving placebo [72].

Self Quiz
Ask yourself...
- How does the balance between the therapeutic benefits in the absence of structural heart disease and the increased mortality risk with a history of myocardial infarction guide clinical decision-making?
- How would you describe the 'pill in the pocket' strategy versus traditional maintenance dosing?
Class ID
Ranolazine presents a potential therapeutic option for managing tachyarrhythmias and ventricular tachycardia [19].

Class II: Autonomic Inhibitors/Activators
The literature recommends beta-blockers (BB) for managing the heart rate in individuals with paroxysmal, persistent, or permanent atrial fibrillation (AF) and atrial flutter [73]. Beta-blockers (BB) are also beneficial for long-term management in patients with symptomatic supraventricular tachycardia (SVT) [73].
Due to their favorable safety profile and efficacy, healthcare professionals can consider beta-blockers as the first-choice therapy for ventricular arrhythmias [39]. Their use is associated with a reduction in adverse cardiac events in conditions such as long QT syndrome and catecholaminergic polymorphic ventricular tachycardia [74].
For patients exhibiting symptomatic premature ventricular contractions (PVCs) in the absence of underlying heart disease, beta-blocker therapy can help decrease the frequency of recurrent arrhythmias and alleviate symptoms [75].

Self Quiz
Ask yourself...
- How does the mechanism of action of beta-blockers contribute to their effectiveness across a spectrum of arrhythmias?
- What factors influence the decision to prioritize beta-blockers as a first-choice therapy in these conditions, considering their impact on reducing adverse cardiac events in long QT syndrome and catecholaminergic polymorphic ventricular tachycardia?
IIa: Inhibitors including pindolol, carvedilol, timolol, nadolol (non-selective beta-blockers), and bisoprolol, atenolol, metoprolol, esmolol (selective beta-1 blockers) treat rate control in atrial fibrillation, atrial flutter, and ventricular tachyarrhythmia [4].
IIb: Activators: The use of Isoproterenol can manage ventricular escape rhythm in cases of complete AV block before pacemaker implantation [4][20].
IIc: Inhibitors: Atropine treats symptomatic sinus bradycardia and conduction block [4][21].
IId: Activators: For the management of supraventricular tachyarrhythmias, Carbachol, methacholine, and digoxin [4].
IIe: Activators: Adenosine for the cessation of paroxysmal supraventricular tachycardia (PSVT) [22].
Class III: K+ Channel Blockers/Openers
Potassium channel blockers decrease potassium efflux out of the cell and prolong the QTc interval [4] Amiodarone displays sympatholytic effects as well as sodium and calcium channel blocking properties, leading to reduced conduction through the AV and sinus nodes [4].
Amiodarone helps maintain sinus rhythm in patients with atrial fibrillation (AF) and those suffering from left ventricular systolic dysfunction [4].
Amiodarone also stands as a viable choice for pharmacological cardioversion and can help manage ventricular rate in critical patients without pre-excitation, though it is less effective than non-dihydropyridine calcium channel blockers [4] [49].
Amiodarone is the preferred antiarrhythmic medication for suppressing ventricular arrhythmias (VA) [4]. Administration of intravenous amiodarone may achieve rhythm stabilization in cases of unstable persistent ventricular arrhythmias (VA) following defibrillation [49]. Administering intravenous amiodarone stabilizes rhythm in cases of unstable persistent ventricular arrhythmias (VA) following defibrillation.
In addition, amiodarone controls ventricular arrhythmias (VA) in patients with ischemic heart disease who are also receiving beta-blocker treatment [4].
Observations show that Dronedarone reduces hospital admissions for atrial fibrillation (AF) in individuals with a history of non-permanent AF who are in sinus rhythm. However, for patients with permanent AF that cannot convert back to normal sinus rhythm, it is contraindicated due to FDA reviews indicating a significant increase in the risk of cardiovascular death, stroke, and heart failure in these cases [76].
Dofetilide is employed in the treatment of atrial arrhythmias and for the acute pharmacological cardioversion of atrial fibrillation or flutter [77].
Sotalol, combining class II beta-blocker properties and class III potassium channel blocker effects, manages both ventricular and supraventricular arrhythmias. [78].
Ibutilide (Corvert) targets the treatment of atrial fibrillation or flutter, underlining its specialized use in managing these specific arrhythmias [79].

Self Quiz
Ask yourself...
- How do these drug mechanisms contribute to its efficacy in managing both atrial and ventricular arrhythmias
- What considerations should be made when choosing Amiodarone for patients with left ventricular systolic dysfunction or those undergoing pharmacological cardioversion?
- How should clinicians navigate the decision-making process for employing Dronedarone in treating AF, considering the patient's AF status and the potential benefits and risks?
IIIA: Voltage-dependent K+ channels
Amiodarone and dronedarone are notable for their role as non-selective potassium (K+) channel blockers, which are crucial in the treatment of unstable ventricular tachycardia and life-threatening recurrent ventricular fibrillation [4].
Kv11.1 (rapid K+ current) blockers: Dofetilide, almokalant, ibutilide, sematilide, and sotalol manage ventricular tachycardia in patients without prior myocardial infarction or underlying structural heart disease. They also treat Wolff-Parkinson-White (WPW) syndrome when associated with atrial fibrillation [4].
Kv1.5 (ultra-rapid K+ current) blockers: Vernakalant serves to convert recent onset atrial fibrillation in patients without structural or ischemic heart disease. It is important to note that the FDA does not approve this specific use of vernakalant [24].
IIIb: Metabolically dependent K+ channels blockers: Nicorandil and pinacidil are employed as second-line treatments for stable angina [25].
Class IV: Ca2+ handling modulators
Non-dihydropyridine calcium channel blockers, such as diltiazem and verapamil, reduce conduction speed and decelerate signal transmission through the AV node [82]. These medications are effective for controlling the ventricular rate in both acute and chronic cases of atrial fibrillation (AF) and atrial flutter [82].
In the acute management of stable patients with supraventricular tachycardia (SVT), including focal and multifocal atrial tachycardias, diltiazem and verapamil serve as viable treatment options [4] [39].

Self Quiz
Ask yourself...
- Given the mechanism by which non-dihydropyridine calcium channel blockers like diltiazem and verapamil slow conduction and decelerate signal transmission through the AV node, why are these medications suited for controlling the ventricular rate in atrial fibrillation and atrial flutter?
- How does this mechanism influence their effectiveness in the acute management of various forms of supraventricular tachycardia?
IVa: Bepridil and falipamil, which block non-selective surface membrane calcium (Ca2+) channels, may manage supraventricular tachyarrhythmias [4]. Verapamil and diltiazem, which block surface membrane L-type calcium (Ca2+) channels, treat supraventricular arrhythmias, and control the rate of atrial fibrillation [4].
IVb: Propafenone and flecainide serve as intracellular calcium channel blockers utilized in addressing catecholaminergic polymorphic ventricular tachycardia (CPVT) [4].
Class V Mechanosensitive channel blockers
Inhibitors: N-(p-amylcinnamoyl) Anthranilic Acid: Under Research and Not Approved by the FDA [26].
Class VI: Gap junction channel blockers
Inhibitors: carbenoxolone (under investigation- not FDA approved) [27].
Class VII: Upstream target modulators
Omega-3 fatty acids: Eicosapentaenoic Acid and Docosahexaenoic Acid: Reduction in Cardiac Death Risk Post-Myocardial Infarction [28].
Statins: Potential for use in atrial fibrillation
ACE inhibitors: Captopril, Enalapril, Ramipril, Lisinopril (ACE Inhibitors), and ARBs (Losartan, Telmisartan): Potential Use in Atrial Fibrillation Associated with Heart Failure [29].
Clinical Prescribing Criteria
Antiarrhythmic medications are pivotal in managing symptoms and safeguarding against the decline of cardiac function caused by conditions such as tachycardia, irregular rhythms, or desynchrony [4]. The primary objective is to reestablish normal cardiac rhythm and conduction, averting the onset of more severe and fatal arrhythmias.
Antiarrhythmics have a narrow therapeutic index, indicating a minimal margin between the effective dosage and the onset of toxicity [4]. There is a tenuous balance between suboptimal treatment and the risk of toxic or proarrhythmic effects, underscoring the importance of precise dosing and monitoring. Clinical attention focuses on the patient's clinical status, underlying structural and functional conditions, and the mechanisms of arrhythmia at both cellular and molecular levels.
The use of antiarrhythmic drugs in therapy seeks to alter conduction velocity, by either slowing down or speeding it up, modify the excitability of cardiac cells via changes in the length of the effective refractory period, and suppress unusual spontaneous activity [14].
Numerous variables influence the effectiveness of antiarrhythmic drugs, such as race, sex, genetics, environmental temperature, drug interactions, precipitating factors, changes in neurohormones, the disease's present condition and severity, and disease-driven structural changes in the body [30]. The complexity increases with some antiarrhythmic drugs (AADs) displaying diverse electrophysiological and pharmacological effects that depend on the administration route, plasma concentration, and the existence of active metabolites.
A variety of factors can influence the efficacy of medications, including racial background, gender, genetic makeup, ambient temperature, interactions between different drugs, initiating triggers, neurohormonal fluctuations, the current state and intensity of the disease, and alterations in the body's structure caused by the disease itself [31].
Complicating the pharmacology, certain antiarrhythmic drugs (AADs) exhibit a wide range of electrophysiological and pharmacological actions, which can vary based on the method of administration, concentration levels in the plasma, and the presence of active metabolites [4] [5].

Self Quiz
Ask yourself...
- Considering the narrow therapeutic index of antiarrhythmic drugs, how does precise dosing and monitoring contribute to optimizing treatment outcomes while minimizing the risk of adverse effects?
- What role do patient-specific factors play in this process?
- Given the complex interplay of factors such as race, sex, genetics, and environmental conditions on the effectiveness of antiarrhythmic drugs, how should clinicians integrate this knowledge into personalized treatment plans?
- With some antiarrhythmic drugs displaying varied electrophysiological and pharmacological effects based on administration route, plasma concentration, and the presence of active metabolites, how do these variables complicate the management of arrhythmias?
The Cardiac Electrical Cycle (Electrical Cascade)
The cardiac action potential represents the sequence of ion exchanges that result in the successive depolarization and repolarization of the cardiac myocyte, culminating in muscle contraction [4]. During its resting phase, a cardiac myocyte maintains a baseline resting membrane potential ranging from negative 80 to negative 90 millivolts [32].
Antiarrhythmic drugs slow down ion movement during various stages of the cardiac action potential [32].
- Phase 0: The “depolarization” phase of the action potential occurs due to the influx of sodium ions (Na+) into the cell, following an electrochemical gradient, leading to a membrane potential of around positive 30 millivolts [33].
- Phase 1: “The notch,” or the early repolarization phase of the action potential, features potassium (K+) ions flowing out. [4] [33].
- Phase 2: “The plateau” phase occurs when the inward movement of calcium ions (Ca2+) balances the outward movement of potassium (K+) ions [4] [34].
- Phase 3: “The repolarization” phase of the action potential occurs through the efflux of potassium (K+) ions along their electrochemical gradient out of the cell. This movement removes the positive charge of the K+ ion from the cell, reinstating the cardiac myocyte's negative potential [33][34].
- Phase 4: Reactivation of the Na/K-ATPase pump, which re-establishes the resting membrane potential in the cardiac myocyte [4][35].

Self Quiz
Ask yourself...
- How do antiarrhythmic drugs alter the ion movement during the different phases of the cardiac action potential to correct arrhythmias?
- What are the potential consequences of these modifications on the overall function of the cardiac myocyte and the heart's rhythm?
Pharmacokinetics of Anti-Arrhythmic Medications
Pharmacokinetics involves the study of drug absorption, distribution, metabolism, and excretion. All antiarrhythmic drugs affect the conductance of membranes and ions, modifying cardiac action potential dynamics either via direct or indirect action.
For example, some medications inhibit fast sodium channels, essential for controlling the rate of membrane depolarization (phase 0) during an action potential [33]. Electrical conduction velocity links to membrane depolarization and blocking sodium channels slows this velocity down [32][33]. Slowing conduction velocity is advantageous for eradicating tachyarrhythmias resulting from reentry circuits [36].
Various antiarrhythmic drug classes affect the duration of action potentials and the effective refractory period [37]. Extending the effective refractory period often eradicates reentry tachyarrhythmias [38]. This effect occurs by inhibiting potassium channels and postponing the repolarization phase (phase 3) of action potentials [38].
Medications that inhibit the slow inward calcium channels aim to diminish pacemaker activity by decelerating the depolarizing pacemaker potential’s rate of rise (phase 4 depolarization) [14].
These drugs also decrease the speed of electrical signal transmission through the atrioventricular (AV) node [14][38]. Similar to sinoatrial (SA) node cells, AV nodal cells rely on the influx of calcium ions for depolarization [34]. Due to the potential for sympathetic nervous system activity to cause arrhythmias, beta1-adrenoceptor blockers are employed to diminish the sympathetic impact on the heart [39]. These beta-adrenoceptors connect to ion channels through specific signal transduction pathways, indicating that beta-blockers modify ion conductance across the membrane, influencing calcium and potassium conductance [39].
In instances of AV block, doctors sometimes use drugs like atropine, a muscarinic receptor antagonist, to counteract vagal effects [40]. AV block can emerge as an adverse effect of beta-blocker medication, and stopping the beta-blocker in such cases may return AV conduction to normal [41].
An increased ventricular rate can be a consequence of atrial flutter or fibrillation [42].
In response, medications that slow down conduction through the atrioventricular (AV) node regulate the ventricular rate. Calcium channel blockers and beta-blockers are particularly effective for this purpose [4].
Digoxin proves advantageous for patients with systolic heart failure, also referred to as heart failure with reduced ejection fraction (HFrEF), characterized by an ejection fraction of less than 40% [43]. Nonetheless, it does not contribute to a reduction in mortality [43]. When standard treatments do not achieve heart rate objectives in atrial fibrillation or atrial flutter, clinicians can deploy Digoxin for heart rate management [44].
Administration of digoxin is contraindicated in instances of pre-excitation due to accessory pathways since it promotes AV blockade and could precipitate ventricular tachyarrhythmias [44]. In conditions of elevated sympathetic activity, digoxin is ineffective, and beta-blockers are the preferred treatment option [44].
Healthcare providers favor oral drug formulations for their improved patient compliance, ease of use, and scalability, which offer economic advantages [45]. However, the oral bioavailability of drugs can vary, influenced by differences in physicochemical characteristics and metabolic activities that impact pharmacokinetics [46]. Challenges such as intestinal metabolism, efflux mechanisms in the gastrointestinal tract, and the hepatic first-pass effect hinder the bioavailability of drugs administered orally [46] [47].
The first-pass effect describes a pharmacokinetic process in which a drug undergoes metabolism at a specific site in the body before reaching the systemic circulation or its intended site of action, which reduces the concentration of the active drug available [47]. The liver, a primary location for drug metabolism, has a direct link to the first-pass effect. This effect can also occur in other active metabolic areas of the body, such as the lungs, blood vessels, gastrointestinal tract, and various tissues [47].

Self Quiz
Ask yourself...
- How do the pharmacokinetic processes of absorption, distribution, metabolism, and excretion influence the clinical efficacy and safety of antiarrhythmic drugs?
- What strategies can healthcare providers employ to mitigate potential adverse effects, including those related to the first-pass effect?
- Given the varied mechanisms by which antiarrhythmic drugs modify cardiac action potentials and conduction velocities to treat arrhythmias, how do these mechanisms align with the selection of specific antiarrhythmic medications for conditions such as atrial flutter, atrial fibrillation, and AV block, considering both the intended therapeutic outcomes and the potential for adverse effects?
Absorption
Antiarrhythmic medications exhibit quick absorption, but the pronounced first-pass effect often reduces their bioavailability [47]. They achieve peak plasma concentrations within 1–3 hours, except for digoxin and dronedarone, which reach their peak in 3–6 hours, and amiodarone, which takes 6–8 hours to peak [11]. In elderly individuals and patients with liver dysfunction, the oral bioavailability of medications tends to be higher [11].
Intestinal bacteria transform digoxin into inactive compounds; antibiotics such as tetracycline and erythromycin eliminate these bacteria, leading to elevated levels of digoxin in the bloodstream [44]. The antiarrhythmic effect of digoxin starts within 2–5 minutes after its intravenous administration [44].
The absorption of drugs through the gastrointestinal tract is crucial for their bioavailability, with meals playing a role that can either enhance or impede this process [46]. For instance, a high-fat meal can increase the oral absorption of dronedarone by fourfold [11].

Self Quiz
Ask yourself...
- How do factors such as age, liver function, intestinal flora, and dietary habits influence the therapeutic levels and efficacy of these drugs in the bloodstream?
Distribution
With the exception of sotalol, antiarrhythmic drugs (AADs) exhibit some degree of binding to plasma proteins [11]. Amiodarone, digoxin, flecainide, and propafenone build up in the heart at concentrations higher than those in plasma and dialysis cannot remove them [11].
The concurrent administration of flecainide and amiodarone increases flecainide plasma concentrations by 50% [48].
Disopyramide, mexiletine, sotalol, and verapamil can cross the placenta and appear in breast milk. High concentrations of Procainamide also occur in breast milk and eliminated by newborns [11].
Oral administration of amiodarone reaches steady-state plasma concentrations after an extended period, except when administered in substantial loading doses; delivering it via intravenous form also delays its maximal effect [49]. This delay is indicative of its distribution across multiple compartments, including the intravascular compartment, which a standard loading dose saturates, a peripheral compartment encompassing various tissues, and a deep compartment represented by adipose tissue, serving as a reservoir for the drug [49].
Amiodarone is known for its distinctive side effects, with a 15% prevalence rate in the first year of use, which can escalate to up to 50% with prolonged treatment [87]. Side effects include pulmonary fibrosis, thyroid dysfunction, photosensitivity, blue-grey skin discoloration, corneal microdeposits, peripheral neuropathy, and elevated liver enzymes [87]. Providers must weigh the potential benefits of amiodarone against its long-term risks [94].

Self Quiz
Ask yourself...
- How does the differential binding of antiarrhythmic drugs to plasma proteins and their accumulation in various body compartments (heart and adipose tissue) affect their pharmacodynamics and pharmacokinetics?
Biotransformation
Antiarrhythmic drugs (AADs) undergo metabolism in the liver through CYP450 isoenzymes, resulting in active metabolites that either block sodium (Na+) channels (such as mexiletine and propafenone), extend action potential duration (APD) [for instance, N-acetylprocainamide (NAPA)], or cause central nervous system (CNS) toxicity (as seen with lidocaine) [11].
Genetics influence the metabolism of CYP2D6 resulting in higher plasma concentrations and extended half-lives (t½) of metoprolol and propafenone in individuals with poor metabolizing capabilities (6% of Caucasians) compared to those who are rapid metabolizers [50]. In similar fashion, the conversion of procainamide to N-acetylprocainamide (NAPA) varies, with 15–20% metabolized in individuals classified as 'slow-acetylators' and 25–33% in 'fast-acetylators' [11].
These metabolic phenotypes are determined by genetics. There are no standard tests available to identify a patient's metabolic phenotype prior to treatment initiation, with the exception of measuring the procainamide/NAPA concentration ratio. It is advisable to decrease dosages for poor or slow metabolizers to two-thirds or less of the standard maintenance dose [11] [51].
Lipophilic beta-blockers, such as bisoprolol, carvedilol, metoprolol, and propranolol, undergo metabolism via CYP2D6, and their bioavailability and half-life (t½) are prolonged in cases of liver dysfunction [11]. The body excretes hydrophilic beta-blockers, including atenolol and sotalol, in their unchanged form through the urine [11].
Following an intravenous loading dose, lidocaine undergoes metabolism with a half-life (t½) of 1.5–2 hours. In patients with liver impairment or decreased hepatic blood flow — including the elderly, those experiencing cardiogenic shock, heart failure, myocardial infarction, or those taking cimetidine and beta-blockers — lidocaine's plasma levels increase, and its half-life extends [51]. In these cases, one should lower both the loading and maintenance doses.
Due to its brief half-life, an initial loading dose of lidocaine requires supplementation with a continuous infusion or repeated administrations to achieve and maintain a consistent plasma concentration [53].
Intravenous Esmolol undergoes rapid hydrolysis in red blood cells, with a half-life of ∼ 9 minutes, and achieves complete reversal of beta-blockade 20–30 minutes after drug cessation [11]. Intravenous adenosine acts within 15–30 seconds, with erythrocytes and vascular endothelial cells absorbing and metabolizing it through adenosine deaminase (ADA), leading to a short half-life (t½) of less than 10 seconds [11].

Self Quiz
Ask yourself...
- How do genetic variations in CYP450 isoenzymes (CYP2D6) affect the metabolism, efficacy, and safety of antiarrhythmic drugs?
Elimination
Antiarrhythmic drugs (AADs) vary in their extent of excretion through urine and feces [11]. The half-life (t½) of these drugs extends in elderly individuals and patients with renal impairment (such as digoxin, disopyramide, dofetilide, flecainide, procainamide, and sotalol) or liver dysfunction (including amiodarone, diltiazem, flecainide, lidocaine, metoprolol, mexiletine, propafenone, propranolol, quinidine, and verapamil) [11]. This prolongation also occurs in congestive heart failure (seen with amiodarone, flecainide, lidocaine, mexiletine, procainamide, and quinidine) or after a myocardial infarction (noted with disopyramide, lidocaine, and mexiletine) [11][54].
For these patients, it is advisable to lower the dosages and to conduct regular ECG monitoring. Amiodarone is subject to extensive metabolism in the liver, excreted through the bile, and has a prolonged half-life (t½) ranging from 25 to 110 days. This extended half-life accounts for the persistence of its effects for weeks or even months following cessation of the drug [11][55].
Due to their short half-life (t½), manufacturers dispense certain antiarrhythmic drugs, including beta-blockers, diltiazem, propafenone, and verapamil, in modified-release formulations [11].
Amiodarone, cimetidine, diltiazem, ketoconazole, procainamide, propranolol, and verapamil elevate plasma concentrations of quinidine [11]. Quinidine acts as a strong inhibitor of CYP2D6 and P-glycoprotein (P-gp), raising the plasma levels of drugs metabolized by this enzyme; it also reduces digoxin clearance, necessitating a 50% reduction in digoxin dosage [11]. Beta-blockers, cimetidine, and halothane cause an increase in plasma concentrations of lidocaine, thereby requiring a reduction in lidocaine dosage [11]. In addition, mexiletine elevates the plasma levels of theophylline, while amiodarone increases the levels of mexiletine [56].
Flecainide and propafenone lead to higher plasma concentrations of digoxin and propranolol. Propafenone (Rythmol) raises the plasma levels of digoxin, metoprolol, propranolol, and warfarin [48]. Mexiletine and quinidine amplify the effects of warfarin; thus, it is advisable to decrease the dosage of warfarin and monitor the prothrombin time/international normalized ratio (INR) [11] [48].
Amiodarone inhibits P-glycoprotein (P-gp) and several cytochrome P450 isoenzymes, such as CYP1A2, CYP2C9, CYP2D6, and CYP3A4, thus increasing the plasma concentrations of drugs metabolized by these pathways or that are substrates of P-gp [94]. Dosage modifications are necessary for medications such as digoxin, flecainide, and warfarin; it is also important to monitor digoxin concentrations and the international normalized ratio (INR) [57][58].
Cholestyramine may decrease the absorption of amiodarone [59]. Since diltiazem and verapamil inhibit both CYP3A4 and P-glycoprotein (P-gp), adjusting the dosages of drugs metabolized by CYP3A4 or are substrates of P-gp becomes necessary [59]. Furthermore, verapamil has the capacity to suppress the liver's metabolism of lipophilic beta-blockers, resulting in elevated plasma concentrations of these medications [60].
There is a significant pharmacokinetic interaction between certain antiarrhythmic/rate controlling medications (such as amiodarone, quinidine, dronedarone, verapamil, digoxin, and diltiazem) and non-vitamin K antagonist oral anticoagulants (NOACs) due to competition for P-glycoprotein (P-gp) or inhibition of CYP3A4 (notably by diltiazem, dronedarone, and verapamil) [61] [62].
Due to these interactions leading to elevated plasma levels of NOACs, experts advise against combining dronedarone with dabigatran and recommend reducing the dose of edoxaban by 50% [62] [63]. Consider reducing the dose of all non-vitamin K antagonist oral anticoagulants (NOACs) when administering amiodarone alongside other P-gp competing substances [64].
Prescribers recommend reducing the dose of dabigatran (Pradaxa) when used with verapamil. Combining edoxaban with verapamil, especially when other P-gp competitors are present, may also necessitate a dose reduction [65].


Self Quiz
Ask yourself...
- Considering the extensive metabolism of antiarrhythmic drugs in the liver and their excretion through bile or urine, how do renal and liver dysfunctions affect the pharmacokinetics of these drugs?
- What principles should guide the adjustment of dosages in patients with such conditions to maintain therapeutic efficacy while minimizing toxicity?
Other Antiarrhythmic Drugs
- Adenosine is effective for both diagnosing and halting supraventricular tachycardia (SVT) arising from atrioventricular nodal reentrant tachycardia (AVNRT) or orthodromic atrioventricular reentrant tachycardia (AVRT) [83]. Adenosine serves as a diagnostic aid by revealing underlying atrial flutter or atrial tachycardia (AT) [83]. Adenosine can also terminate focal AT caused by a triggered mechanism and distinguish focal AT from AVNRT and AVRT [83]. Adenosine, a purine nucleoside, results from the breakdown of adenosine triphosphate [86]. Within cardiomyocytes, it interacts with Gi-protein type 1 receptors, facilitating swift potassium efflux and hyperpolarization, while also inhibiting calcium influx [86]. These actions decrease the heart rate and slow down conduction velocity by targeting the AV node.
- Digoxin is not a first-line therapy for ventricular rate control in patients with AF, a combination of digoxin and beta-blocker/or non-dihydropyridine calcium channel blockers is a reasonable rate control option in patients with AF and heart failure [84].
Treatment of Overdose
In instances of antiarrhythmic drug overdose, medical professionals must ensure the patient has a clear airway, adequate breathing, and support for circulation [4] [87]. Managing cardiac arrest and severe toxicity from poisoning involves the use of specialized interventions, including antidotes and venoarterial extracorporeal membrane oxygenation (VA-ECMO), alongside fundamental and advanced life support techniques [87].
Symptoms such as nausea, vomiting, neurological manifestations, and lethal arrhythmias characterize Digoxin toxicity [88]. To treat ventricular tachyarrhythmias from digoxin toxicity, clinicians can use lidocaine, and atropine serves as an option for bradyarrhythmia’s. In addition, digoxin-specific antibody fragments prove effective in severe toxicity cases [89].
Therapeutic and excessive dosages of dofetilide may induce Torsades de Pointes (TdP), which clinicians manage by reducing or stopping the drug's dosage [90]. If the arrhythmia persists, initial treatment involves activated charcoal if ingestion occurred within the last 15 minutes, followed by intravenous magnesium and correction of any electrolyte imbalances [90].
For persistent arrhythmias, administering isoproterenol/dopamine may serve as a temporary measure until initiating pacing [90].
In cases of beta-blocker poisoning, treatments involve administering catecholamines, applying high-dose insulin euglycemic therapy, and using vasopressors, noting glucagon for its positive effects on hemodynamics [91].
Treating calcium channel blocker (CCB) overdoses involves administering intravenous calcium, dopamine, and norepinephrine. High-dose insulin therapy can reduce mortality in cases of calcium channel blocker poisoning [60]. For severe shock or cardiac arrest resulting from these overdoses, extracorporeal life support is employed [60]. Case reports indicate that clinicians use lipid emulsion therapy to treat overdoses of amiodarone and flecainide [4] [92].

Self Quiz
Ask yourself...
- How do the principles of emergency management in the treatment of severe toxicity from antiarrhythmic drug overdose?
- What factors determine the choice of specific treatments for complications such as lethal arrhythmias, digoxin toxicity, Torsades de Pointes, beta-blocker poisoning, and calcium channel blocker overdoses?
Conclusion
Arrhythmias encompass a broad spectrum of heart rate and rhythm disturbances and present significant clinical challenges. Atrial fibrillation (AF) is the most prevalent arrhythmia, associated with increased morbidity, mortality, and healthcare costs [2][3]. Management involves antiarrhythmic drugs (AADs), which play a fundamental role despite their limitations and the potential for adverse effects.
Antiarrhythmic agents, classified by their primary action mechanism on the cardiac action potential, impact ionic channels or receptors, aiming to suppress arrhythmias [4]. The management objectives for arrhythmias include alleviating significant clinical symptoms and extending life.
Pharmacokinetic aspects, such as drug absorption, distribution, metabolism, and excretion, play crucial roles in the effectiveness and safety of AADs. Pharmacodynamics involves modifying the cardiac action potential and conduction velocity to prevent or terminate arrhythmias. Factors influencing drug efficacy include genetics, environmental conditions, and the patient's specific clinical profile.
Treatment of overdose with antiarrhythmic drugs requires immediate medical intervention, including antidotes and supportive measures like venoarterial extracorporeal membrane oxygenation (VA-ECMO) for severe cases. The management of specific drug toxicities, such as digoxin and beta-blockers, involves targeted therapies and supportive care to mitigate adverse effects and stabilize the patient's condition.
Conclusion
Arrhythmias encompass a broad spectrum of heart rate and rhythm disturbances and present significant clinical challenges. Atrial fibrillation (AF) is the most prevalent arrhythmia, associated with increased morbidity, mortality, and healthcare costs [2][3]. Management involves antiarrhythmic drugs (AADs), which play a fundamental role despite their limitations and the potential for adverse effects.
Antiarrhythmic agents, classified by their primary action mechanism on the cardiac action potential, impact ionic channels or receptors, aiming to suppress arrhythmias [4]. The management objectives for arrhythmias include alleviating significant clinical symptoms and extending life.
Pharmacokinetic aspects, such as drug absorption, distribution, metabolism, and excretion, play crucial roles in the effectiveness and safety of AADs. Pharmacodynamics involves modifying the cardiac action potential and conduction velocity to prevent or terminate arrhythmias. Factors influencing drug efficacy include genetics, environmental conditions, and the patient’s specific clinical profile.
Treatment of overdose with antiarrhythmic drugs requires immediate medical intervention, including antidotes and supportive measures like venoarterial extracorporeal membrane oxygenation (VA-ECMO) for severe cases. The management of specific drug toxicities, such as digoxin and beta-blockers, involves targeted therapies and supportive care to mitigate adverse effects and stabilize the patient’s condition.
Schedule II Controlled Substances and Risks of Addiction
Case Scenario
Mary, a 34-year-old Hispanic female patient you have cared for in the past for management of Type II diabetes, visits after moving furniture over the weekend. She is in apparent pain, holding her lower back and flinching with every movement. She is physically unkept and appears exhausted. Mary states, “I’ve been trying to rest, I’ve iced the area and have been taking Ibuprofen every 6 hours and I can’t get any relief, I need something stronger.” What are your next steps?
Introduction
Every clinician has cared for a patient in pain. Non-maleficence and beneficence, to do no harm and to do good, are the guiding ethical principles in patient care (16). Historically, easing pain and suffering was ethically straight forward- treating the pain was beneficence. Now, with the understanding of opioid misuse, clinicians may ease pain (beneficence), but cause opioid use disorder, abuse, and/or diversion and do more harm (maleficence).
Prescribing pain medications, especially schedule II-controlled substances, comes with overwhelming responsibility and burden to the prescriber. The dual edged sword of schedule II-controlled substances is to ease pain and to prevent misuse. To safely prescribe schedule II-controlled substances, you must be aware of a myriad of facts, as well as clinically assess pain through the individual experience of each patient.

Self Quiz
Ask yourself...
- How do you balance non-maleficence and beneficence regarding pain in your practice?
- How can you help prevent opioid related use disorder, addiction, overdose and/or deaths?
Recent History of Pain in the United States
Pain management in the United States changed acutely when Dr. James Campbell, in his 1995 Presidential Address to the American Pain Society, presented the concept of pain evaluation as the fifth vital sign (2).
Throughout the late 1990’s, opioids were increasingly prescribed, and problems associated with opioids correspondingly increased (5,6).
In 2014, the Agency for Healthcare Research and Quality (AHRQ) published a systematic review lacking evidence to show long-term benefits of prescription opioid treatment for chronic pain (3). The report found that long-term prescription opioid use was related to higher risk for overdose and opioid misuse (3).
Since then, drug overdose deaths have increased five times over the past twenty years (17). In both 2020 and 2021, drug overdose death rates were highest for adults between the ages of 35–44 and adults 65 and older had the largest percentage increase in drug overdose death rates during that same time, with a 28% increase (17).

Self Quiz
Ask yourself...
- How has the opioid crisis affected you?
- How has it changed your prescribing practices?
- Have you known anyone that died from a drug overdose, how did that impact you?
In 2016, the US CDC issued guidelines for the prescription of opioids to treat chronic, non-cancer pain. These more restrictive guidelines were partly in response to the growing number of people using opioids and the AHRQ 2014 findings. The guidelines were adopted by many states, including limiting prescriptions for opioids for the treatment of chronic, non-cancer pain. This event caused inadequate pain control and suffering for many patients who truly needed opioids but couldn’t obtain them through prescription because of many states using the CDC recommendations as law.
In May 2021, California alone saw the sudden closure of twenty-nine pain management centers leaving more than twenty thousand opioid prescribed patients without help or anywhere to go (13).

Self Quiz
Ask yourself...
- Have you been in a situation where you undertreated pain because of the law? If yes, how did you feel about this? If not, what would you do if you were in that situation?
Pain
Pain is a complex, not completely understood experience that is influenced by many components, including biological, psychological, and social factors (19).
Seeking relief from pain is one of the most common reasons patients reach out for medical care (19).
Pain Theory
Pain has existed since humans existed. The cause of pain has been explored for centuries. Even though our understanding of pain is still incomplete, there are many pain theories. A brief, incomplete explanation of each theory is as below (8,11):
Intensity Theory-pain is an emotion.
Cartesian Dualistic Theory-pain is a consequence of committing immoral acts.
Specificity Theory-different sensations take different paths causing pain.
Pattern Theory-each sensation relays a particular pattern of signals to the brain, and then the brain reads the pattern to decipher the pain.
Gate Control Theory- pain travels from the periphery to the spinal cord. When pain gets to a specific magnitude, the “gate” opens. After the spinal gate is open, the pain signal can reach the brain where it is processed, and lastly, the patient feels pain.
Neuromatrix Model- the central nervous system is responsible for painful sensations, not the periphery. Pain messages to the areas of the central nervous system work together to create messages to allow patients to feel pain, called the neurosignature.
Biopsychosocial Model-The biopsychosocial model is a comprehensive pain model encompassing all spheres of our humanness. This theory hypothesizes that pain is not made up of any one cause, but the result of multifarious psychological, biological, and sociological interactions. The theory links psychological and sociological interactions to the biological and helps us understand associated opioid use disorder, abuse, and diversion.
Case Scenario
Mary starts crying and says, “I know I deserve this pain; I had an abortion when I was in high school, and I guess I’m paying for it now.”

Self Quiz
Ask yourself...
- What pain theory drives your clinical practice? Why?
- How would you approach pain with a patient that believed in the Cartesian Dualistic pain theory?
- Do you judge or have bias to others based on the pain theory you ascribe to?
- How do you talk to Mary about her pain beliefs?
Case Scenario
After trying to explain that the pain was caused by the injury, not the abortion, Mary asks more about what actually causes the pain she is experiencing.

Self Quiz
Ask yourself...
- How do you explain the etiology of their pain to your patients in pain?
- Why is it important to share this information with the patient?
Types of Pain by Origin
There are different types of pain, depending on the origin. Determining the origin of the pain is essential in the assessment and treatment of pain. The most common causes of pain (acute and/or chronic) include (8):
Neuropathic Pain
Neuropathic pain can be peripheral or central and the pain is from nerve compression or nerve changes from other pathologies.
Peripheral neuropathic pain- examples include post-herpetic neuralgia and diabetic neuropathy.
Central neuropathic pain – examples include post-cerebral vascular accident.
Nociceptive Pain
Nociceptive pain is from direct tissue injuries, usually from an external force.
Examples include sprains, bruises, burns or dental procedures.
Musculoskeletal Pain
Musculoskeletal pain originates from bones, joints, ligaments, tendons, or muscles.
Examples include arthritis, fractures, or back pain.
Inflammatory Pain
Inflammatory pain is due to the inflammatory response and associated swelling.
Examples include swelling from tissue injury, infection or from autoimmune disorders.
Psychogenic Pain
Psychogenic pain is caused by psychological factors.
Examples include tension headache or stomach pain caused by stress.
Mechanical Pain
Mechanical pain is caused by pressure exerted on body structure or part.
Examples include low back pain, abnormal growth, or tumor.
(8)

Self Quiz
Ask yourself...
- Have you treated a patient with psychogenic pain? How did or would you validate their pain experience?
- What are the non-pharmacological treatments you offer your patients depending on the pain’s origin?
Case Scenario
As you assess Mary and her pain, she tells you about the furniture moving last week-end, and that she fell while carrying a couch down a flight of stairs with another person. “I thought I was going to pass out! I had to stop right there and just cried from the pain. I had my friend take me home and this is the first time I’ve come out of the house since Saturday. I can’t sleep and I’m not hungry. My roommate is mad because I haven’t done a thing.”

Self Quiz
Ask yourself...
- What repercussions to pain Mary is experiencing physically, emotionally, and socially?
Pain Classifications
Determining the classification of pain- acute, chronic or high-impact chronic will help the practitioner decide appropriate treatment options.
Acute
Pain may be classified as acute pain. Acute pain comes on quickly and limited (less than 1 month in duration) (18). Causes of acute pain can be a result of inflammation, injury, or a disease process. Acute pain interferes with daily functioning, but usually subsides as the cause it treated. Acute pain may be described as throbbing, stabbing, or burning. Acute pain can cause physiologic symptoms including elevated heart rate and blood pressure.
Chronic
Pain can become classified as chronic when it lasts more than 3 months (8). Chronic pain is usually a result of injury, inflammation, treatment or a pre-existing medical condition or disease (8). Every aspect of a patient's life may be affected by chronic pain and lead to poor physical and mental health, reduced quality of life, and changes to sleep, libido, and appetite are common (4).
High-impact Chronic Pain
In 2019, about 20% of adults in the US had chronic pain in 2019 and close to 7% had “high-impact” chronic pain, meaning they have pain on every day, or most days during the past 3 months that impacts normal work and life activities (5,6).
Assessing Pain
When contemplating prescribing opioids for chronic pain, thorough patient assessment including risk assessment of opioid use. Assessments of the patient’s pain encompass the origin, type and intensity of the pain, past and present treatments, any underlying problems and how pain affects physical and mental functioning (13). Patient reported outcome (PRO) tools may simplify and organize the documentation of these goals and can be used to track patient progress over time (13).
As nurses, we all have been taught how to assess pain using the OPQRST mnemonic:
- Onset
- Provocation/Palliation
- Quality
- Region/Radiation
- Severity
- Time
And the 7 components of pain assessment:
- Onset/cause of pain
- Location/distribution
- Duration
- Pattern
- Character/quality
- Aggravating factors
- Alleviating factors/associated symptoms
These pain assessments only view the physical aspects of pain and are limited.
When assessing pain, these elements of pain experience need to be taken into consideration and include (11):
Nociception- signal sent to brain from the periphery that injury or damage is present.
What is the origin of the pain?
Pain-subjective experience after brain processed nociception.
What is the patient’s subjective pain experience?
Suffering-emotional response to nociception.
What is the patient’s emotional response to the pain?
Pain behaviors-actions patients have in response to the experience of pain.
What behaviors or changes does the patient have in response to pain?
Looking at these elements of the pain experience allows the practitioner to get a more holistic, individualized view of pain from the patients’ perspective.
Using reliable, validated tools to assess pain is needed to accurately measure and track pain levels. Mental health screening may be used to gather baseline information about and screen for any mental health concerns the clinician may have. Several Patient reported outcome (PRO) patient tools are available and suggested pain screening tools (13):
Pain Intensity and interference (pain scale)
Brief Pain Inventory - Short Form (BPI-SF)
PROMIS Pain Interference
Mental health screening (the type depends on the practitioner’s evaluation and as appropriate)
(13)

Self Quiz
Ask yourself...
- How differently do you view acute verses chronic pain and why?
- Do your personal experiences with pain affect how you perceive and address your patients’ pain?
- What are your pain biases physically, psychologically, and sociologically?
- What repercussions to pain have you experienced physically, emotionally, and socially?
- How were you taught to deal with pain as a child?
2022 CDC Guidelines for the Prescription of Opioids to Treat Chronic Pain
In 2022, the US CDC issued new guidelines for the prescription of opioids to treat chronic pain (5,6). The new guidelines support clinical judgment and individualized patient-centered care. Even though the CDC stresses they are recommendations, many states are again using the guidelines to make changes in state laws about prescribing opioids. It is important that every prescriber reads the guidelines and reviews state prescribing laws from 2022 forward. The guidelines serve as a resource for prescribers and the recommendations should be adhered to with the caveat to always individualize care and do the best for the given situation.
There are five guiding principles when implementing the recommendations into clinical practice. These broad guiding principles should be foremost when dealing with patients’ pain.
Five Guiding Principles of the Guidelines:
- All pain, whether opioids are prescribed or not, needs to be assessed and treated on its own.
This reminds us that pain is pain and needs to be treated if opioids are prescribed or not.
- Flexible, supportive, individualized care should be voluntary in nature and all recommendations are supportive person-centered care.
This reminds us that we are dealing with a unique person and our care should reflect that fact and we need patient input for person-centered care.
- Pain should be managed using a multidisciplinary approach utilizing physical and behavioral health, and long-term services.
This reminds us we aren’t in it alone; a multidisciplinary approach allows the patient to receive services they need from the appropriate provider.
- Make sure the clinical practice guidelines aren’t used beyond their intended use. Incorporate them with clinical judgement and patient-specific needs.
This reminds us that the guidelines are recommendations, and we need to use clinical judgement and the needs of the patient to drive our care.
- All layers of health systems need to be vigilant of health inequities and provide care and communication that is culturally and linguistically appropriate and accessible to all. Nonpharmacologic and pharmacologic pain management regimens should be affordable, diversified, and coordinated.
This reminds us that health inequities exist, and we need to provide care that is appropriate and accessible to all people to the best of our ability.
(5,6)
Case Scenario
While explaining to Mary that you will give her printed care instructions before she leaves, Mary states, “Don’t bother, I can’t understand those instructions, they are way over my head, just tell me what I need to know.”

Self Quiz
Ask yourself...
- How do you stay vigilant of health inequities?
- What can you do to prevent health inequities in your practice?
- How will you help Mary with understanding her care?
Four Key Issues Addressed By the Guidelines
The four key issues addressed by the new guidelines include specifics on opioid prescribing (5,6). The first issue, whether to start opioids for pain should be addressed with every patient that is experiencing pain, the other three are after and if opioids are prescribed.
- Deciding whether to start opioids for pain.
Many factors need to be considered when making the decision to prescribe opioids or not. Many times, pain can be controlled using nonpharmacological interventions and nonopioid medications.
- Choosing an opioid and the appropriate opioid dose.
There is no perfect way to decide an initial opioid dose. In general, starting with a low dose is safer.
- Determining the length of time for the opioid prescription and conducting follow-up assessments.
Make sure to only prescribe the number of pills needed and schedule follow-up assessments.
- Assessing the risk for and educating on the potential harms of opioids.
Each patient taking opioids needs to understand the harms of opioids and be assessed for harm before, during and after opioid therapy (6).
Case Scenario
As you explore treatment options with Mary, she tells you, “I told you I need something strong, the good stuff, the rest won’t do a thing for my pain!”

Self Quiz
Ask yourself...
- Do you think opioids are the best treatment for pain? Why or why not?
- Do your patients have the preconceived notion that opioids are “best” for pain control? How do you approach this notion?
Non-pharmacological Treatments for Pain
Nonpharmacological treatments for pain should be suggested as appropriate to patients in pain (6). These are usually cost effective and have minimal downsides.
Examples of non-pharmacological treatments include:
Exercise (aquatic, aerobic and/or resistance)
Application of heat/cool
Elevation of affected body part
Weight loss (for osteoarthritis or back pain)
Massage
Mindfulness-based stress reduction
Yoga
Acupuncture/acupressure
Cognitive behavioral therapy
Physical therapy
Tai Chi
Qigong
(6).
Case Scenario
While exploring non-pharmacological pain management options with Mary, she states, “My grandmother used to swear by hot baths with Epson salts for all pain! But why bother when you can take a pill, right?”

Self Quiz
Ask yourself...
- What non-pharmacological pain therapies have you seen patients use that are specific to their country of origin or passed down by generations?
- What non-pharmacological pain therapies have you used and why? Science or family history?
- How will you respond to Mary’s statement?
Non-schedule II-Controlled Medications for Pain
As a prescriber of controlled II medications, remember that there are many non-opioid medications that treat pain effectively (as or more effectively than opioids in many cases) including:
NSAIDS
Example: Ibuprofen
200 to 400 mg PO every 4 to 6 hours as needed. Max: 1,200 mg/day. Discontinue use if pain gets worse or lasts more than 10 days (15).
SNRI antidepressants
Example: Venlafaxine
37.5 mg PO once daily for 1 week, then 75 mg PO once daily for 1 week, and then 150 mg PO once daily. Doses up to 225 mg/day have been used. Guidelines state this medication is most likely effective and should be considered for the treatment of diabetic neuropathy (15).
Gabapentin
Example: Gabapentin
300 mg PO 3 times daily, at first. Titrate dose is based on clinical response and tolerance. Max: 3,600 mg/day. Guidelines suggest gabapentin is most likely effective for diabetic neuropathy (15).
Case Scenario
You tell Maria that the ibuprofen she is taking is an excellent pain reliever and to continue taking it for the pain. She states, “I guess you haven’t been listening to me here, I still have pain so that means ibuprofen is useless to me.”

Self Quiz
Ask yourself...
- How do you use pain adjuncts in your practice?
- How do you respond when patients react as if you aren’t validating their pain when you prescribe non-opioids for pain relief?
- How do you explain to Mary why she should continue the ibuprofen?
Controlled Substance Act
Title II of the Controlled Substance Act (CSA) established federal regulation of controlled substances in 1970 (9). It was largely created to make a legal foundation to combat drug abuse.
This act also gave power to the Food and Drug Administration (FDA) and the Drug Enforcement Agency (DEA), to determine classification schedules. Mandatory registration through the US Attorney General controls and restricts who may import/export, manufacture, distribute or dispense controlled substances.
Currently, there are five schedules of Controlled Substances (20). A brief description of each follows with emphasis on schedule II-controlled substances.
Prescribers need to be aware of the specific, current information regarding each medication they prescribe and how that information relates to the individual patient receiving care.
Schedule I-V Controlled Substances
Schedule I Controlled Substances
These drugs have a high potential for abuse. Marijuana is the only schedule I product that may be obtained legally in certain states in the US.
Examples:
Heroin
Lysergic acid diethylamide (LSD)
Marijuana (cannabis)
Peyote
Methaqualone
3,4-methylenedioxymethamphetamine ("Ecstasy")
(20).
Case Study
Mary asks, “What about smoking pot for the pain? My neighbor told me I should and that it works great for his arthritis.”

Self Quiz
Ask yourself...
- How do you incorporate the discussion of legal cannabis for pain with your patients?
- How do you respond to Mary in this situation?
Schedule II/IIN Controlled Substances
Drugs in this schedule have a high potential for abuse that may lead to serious psychological or physical dependence. Oxycodone, hydrocodone, and hydromorphone tablets are all derived from poppy plants and are morphine derivatives whereas fentanyl is synthetic and much more potent (15). Most opioids go through first-pass metabolism in the liver before entering the systemic circulation and reaching target tissues. There are individual differences on how opioids are metabolized because there are differences in patients CYP-450 and UGT liver enzymes which are part of the metabolizing process (15).
Examples of drugs in this class:
Morphine- opioid agonist
Example: Morphine Tablets 15 mg PO every 8 to 12 hours, at first. Titrate dose every 1 to 2 days to achieve adequate analgesia. While discontinuing, decrease dose 25% to 50% every 2 to 4 days to prevent withdrawal symptoms. Extended-release tablets are only prescribed for opioid tolerant patients (15).
Hydromorphone-opioid agonist
Example-Dilaudid Give 2 to 4 mg PO every 4 to 6 hours PRN initially (15).
Fentanyl-opioid agonist
Examples: Duragesic -follow the FDA-approved conversion chart to convert 24-hour oral morphine equivalents dose to the corresponding transdermal fentanyl system dose. To start, apply at minimum a 25 mcg/hour transdermal patch for patients receiving at least 60 mg/day oral morphine equivalents. All other opioids should be stopped with transdermal fentanyl initiation (15).
Methadone-opioid agonist
Example-Dolophine- 0.05 to 0.1 mg/kg PO every 6 hours, to start. Titrate dose by 0.05 mg/kg/dose until symptoms are managed. Taper dosage 10% to 20% of initial dose every 1 to 2 days, lengthening interval before discontinuation (15).
Meperidine-opioid agonist
Example-Demerol tablets- 50 to 150 mg PO every 3 to 4 hours PRN (15).
Oxycodone-opioid agonist
Example-OxyContin- 5 to 15 mg PO every 4 to 6 hours PRN (15).
Hydrocodone-opioid agonist
Example-Norco- 2.5 to 5 mg hydrocodone/325 to 650 mg acetaminophen (1 to 2 tablets) Q 4 to 6 PRN. Max: 30 mg hydrocodone/3,900 mg acetaminophen (12 tablets)/day (15).
Side Effects of Schedule II Narcotics
Common side effects of schedule II narcotics include:
Nausea and vomiting-may also increase aspiration. Patients may need to be prescribed anti-emetics.
Pruritus-may cause skin irritation. Patients may need to be to take Benadryl to decrease itching.
Dizziness-this is a safety concern, and the patient must know not to drive or be weary of falls.
Dry Mouth-hard candy or gum may alleviate this symptom.
Sedation- this is a safety concern, and the patient must know not to drive or be weary of falls.
Euphoria-patients must understand not to drive, sign legal documents, or purchase items while “high.”
Constipation-counsel to increase fluids and fiber in the diet, over the counter stool softeners may be recommended.
(6, 20).

Self Quiz
Ask yourself...
- What are the most common side effects of opioids you see in your clinical practice and how do you individualize interventions for your patients?
Schedule IIN stimulants examples:
Amphetamine (Dexedrine, Adderall)
Methamphetamine (Desoxyn)
Methylphenidate (Ritalin)
Amobarbital
Glutethimide
Pentobarbital
(20).
Schedule III/IIIN Controlled Substances
These drugs have less potential for abuse than Schedules I or II drugs.
Examples:
Medication with 90mg or less of codeine per dose
Buprenorphine (Suboxone)
Examples of Schedule IIIN:
Benzphetamine (Didrex)
Phendimetrazine
Ketamine
Anabolic steroids
(20).
Schedule IV Controlled Substances
These drugs have even less potential for abuse compared to schedule III drugs.
Examples:
Alprazolam (Xanax)
Carisoprodol (Soma)
Clonazepam (Klonopin)
Clorazepate (Tranxene)
Diazepam (Valium)
Lorazepam (Ativan)
Midazolam (Versed)
Temazepam (Restoril)
Triazolam (Halcion)
(20).
Case Study
After reviewing Mary’s current medications, you see that she has been prescribed Triazolam by another provider.

Self Quiz
Ask yourself...
- How does having a patient on a schedule IV-controlled substance affect your decision whether or not to prescribe opioids for pain relief?
Schedule V Controlled Substances
Drugs in this schedule have the least potential for abuse.
Examples:
Cough preparations containing no more than 200 mg. of codeine per 100 ml.
Ezogabine
(20).
How Opioids Work
Opioids work by sending chemical signals that bind and activate opioid receptors. There are four known opioid receptors, and they include DOP, KOP, NOP, and MOP. A brief list of effects elicited by each receptor follows (10):
DOP-spinal and supraspinal analgesia and decreased gastric mobility.
KOP- spinal analgesia, diuresis, and dysphoria (like MOP without the vital sign changes)
NOP- hyperalgesia, allodynia, and analgesia
MOP-sedation, respiratory depression, analgesia, bradycardia, nausea, and vomiting, and decreased gastric mobility.
Opioids used in practice wield actions at the MOP receptor (James & Williams, 2020). The MOP receptor effects are the classic opioid effects that most clinicians see when caring for a patient taking opioids.
Opioids are highly addictive simply because they make you feel good. The release of endorphins triggered from opioids causes a sense of pleasure and well-being. As an opioid wears off, patients may find themselves craving the feel-good feeling again and take more opioids- not for pain, but to gain the good feeling back. There are psychological, genetic, and environmental factors that make patients at higher risk for abuse. They are also addictive because drug tolerance occurs and requires higher doses for the same effect.
The odds are a patient will still be on opioids a year after only five days on opioids (12).
Dosing
The first daily dose is a clinical decision made with the patient to provide individualized, appropriate care. When dosing opioids, use a Morphine Milligram Equivalent (MME) calculator. Use this tool to calculate the total daily opioid dose and document the MME. The total daily dose helps clinicians and patients figure out who may need additional monitoring, when tapering may be needed and the risk of overdose (13). Patient risks increase as the daily MME increases (13).

Self Quiz
Ask yourself...
- How do you use the MME as a clinical tool in your practice?
- What changes in your practice when you increase the MME for a patient?
Assessing the Effectiveness of Opioids
Suggestions on assessing the response to opioids for pain and include:
Assessing the 4 A’s
Analgesia-are they getting pain relief, what are the pain scores?
Adverse effects-what side effects are they experiencing from the drug?
Activity-how has the drug affected their activities of daily living including work and sleep?
Aberrancies-anything out of the ordinary (such as asking for a refill early, taking the drug other than prescribed)?
(13)
Definitions Regarding Opioid Misuse
A bit about definitions. These terms about the consequences of opioid use are often used interchangeably and incorrectly (7). Note the differences in each and make sure the patients know them too.
Addiction - the continual need for a drug despite harmful repercussions.
Example- a patient buys opioids off the street illegally because they physiologically need the medication and will go to any lengths to receive it.
Pseudo-addiction – the persistent fear of pain, hypervigilance; it may go away when the pain resolves.
Example- a patient will not go anywhere without their medications in hand because they are afraid of being without pain treatment and limit many activities due to the fear of having more pain.
Dependence – the body needs medication to function normally, and physiologic withdrawal symptoms occur without the medication.
Example- a patient becomes anxious and starts sweating an hour before a medication is due.
Tolerance – the body needs more of the medication to achieve the same response caused by the CNS adjusting to a medication over a period of time.
Example- a patient's pain rating was between 4 and 5 (out of 10) on an opioid and after a week of therapy the pain rating increases (between 6 and 8) on the same dose, and they come to their prescriber requesting more drugs to get the same effect.
Opioid Use Disorder (OUD)- encompasses dependence and addiction specifically to opioid use. OUD is defined in the DSM-5 as a problematic pattern of opioid use leading to clinically significant impairment or distress, as manifested by at least two DSM-5 criteria occurring within a 12-month period. This disorder is on a continuum and can be measured as mild, moderate, or severe.
Example- A patient has been on oxycodone for 3 years for back pain and their MME is 80. They admit physical addiction and emotional dependence on opioids.
Drug Diversion-the unlawful use or distribution of a drug.
Example-A patient comes in with complaints of severe pain with the intention of getting opioids to sell for cash.
Drug Misuse-taking the drug differently than prescribed.
Example-A patient was prescribed 20 Tylenol #3 tablets to take Q6 hours PRN and comes to the clinic asking for more drugs in 3 days because they took them more often than prescribed.
(7)

Self Quiz
Ask yourself...
- How do you respond if/ when a patient or a colleague uses one of these terms incorrectly?
- What are the effects of incorrectly labeling someone as addicted?
Case Scenario
You decide to prescribe short term opioid therapy for acute pain to Mary. What are your next steps?

Self Quiz
Ask yourself...
- How do you decide what and how much to prescribe?
- What are the state requirements to prescribe schedule II medications in California?
- What does Mary need to know about her prescription?
Steps for Prescribing
1. Establish a diagnosis and medical necessity.
The diagnosis should support opioid use, and the assessments support the medical necessity of the medication.
2. Explore non-controlled medication treatment.
Again, many pain situations are controlled using non-controlled medications.
3. If using schedule II-controlled medications: Use a patient-specific protocol for schedule II medications.
Patient-specific protocols may differ depending on the state in which you practice. Treatment plan should include a plan to discontinue or taper opioids as appropriate.
- Make a treatment plan.
Treatment goals and objectives should be created when initiating an opioid trial. The goal of pain treatment should include documented improvement in pain, functioning, and a decrease in disturbances caused by pain. The plan should include an exit strategy of when and how the opioids will be discontinued.
- Obtain consent.
By creating a formal agreement and obtaining consent for opioid use, prescribers include their patients and can document their participation and enter the discussion of the gravity of opioid use.
- Enter a pain agreement.
The pain agreement can be tailored to the individual patient. Pain agreements can include safe medication administration and storage, what to do if medications are lost or if the pain increases. It can also include monitoring and compliance issues such as urine drug testing and pill counting. By entering a formal agreement, the patient realizes their accountability and responsibility in their pain management. Pain agreements can also be used to document teaching related to opioid use.
- Counsel on overdose and OUD prevention.
The eleven criteria for OUD should be discussed with the patient prior to prescribing opioids. This item is key to safety education. Beyond that, signs, and symptoms of overdose, how to use naloxone, and when to call 911 or seek medical attention are lifesaving instructions that patients need to know.
- Ongoing assessments (pain, risk for misuse, risk of OUD).
While on opioid therapy, documentation of ongoing assessments is necessary to support your treatment plan and may prompt changes to the plan as assessments change.
- Compliance monitoring.
There are many aspects to compliance monitoring:
- Drug testing
Urine drug testing can monitor if the patient is taking the opioids as described and detect if they are concurrently using substances that may make them at higher risk for OUD.
- Pill counting
Having the patient bring their prescription to visits and verifying that the number of pills in the container correspond to the number that should be there based on the prescribing frequency is an active form of compliance monitoring and can be a part of a pain agreement or consent to treat.
- Drug diversion
If a patient is found to be participating in drug diversion, depending on the nature of the diversion, appropriate reporting to the DEA or legal actions may be necessary.
- Tapering and Discontinuing Opioid Therapy
Tapering or discontinuing opioids should be a part of the patient-specific plan and planned accordingly.
(13).
Case Study
Mary appears overwhelmed and anxious as you go through prescribing steps. She asks you, “Are you doing all this because you think I’m a drug addict? I bet you don’t do all this for your rich, white patients!”

Self Quiz
Ask yourself...
- How do you respond to Marys’ claim of bias against her?
- How do you explain “all this” is required by law and consistent with good practices, not to accuse her of being an addict?
Case Scenario
Mary asks for a paper copy of her prescription because it’s easy for her to drop it off at the pharmacy in the store where she does her grocery shopping.

Self Quiz
Ask yourself...
- How do you explain to Mary that you can’t give her a paper prescription without eroding her thin trust in you?
Patient Communication and Education
When prescribing opioids, patient education, and an open discussion on the potential harms from opioids is paramount for patient safety. Providers must effectively communicate with every patient, without bias and with cultural competence. By effectively listening and assessing each patient’s pain experience, the practitioner can more effectively and safely treat pain.
Essential topics for dialogue and discussion with patients before and during opioid treatment for acute and chronic pain include many aspects. Communication topics and suggestions from the 2022 CDC guidelines include (6):
- Make a plan to discontinue when prescribing opioids.
- Let a patient know whom and how to contact and protocols to follow for uncontrolled pain, so it can be quickly reassessed and managed.
- Explain respiratory depression and opioid use disorder-how to avoid, how to recognize, and how to treat. Teach that taking opioids with taken benzodiazepines, sedatives, alcohol, or illicit drugs increases the chance of respiratory depression.
- Advise patients of side effects and how to treat- dry mouth, nausea, constipation, vomiting, drowsiness, and confusion.
- Initiate an opioid tapering plan if opioids are prescribed more than a few days.
- Teach that medication should only be taken as needed, not as often as prescribed. Encourage non-pharmacological treatments.
- Remind the patient the medication is to make the pain tolerable, not to eliminate it, not to make you “feel good.”
- Remind patients not to drive or operate machinery when taking opioids.
- Talk to patients about safe medication handling, storage, and no sharing. Include how to dispose of medications safely and naloxone for an overdose.
- Explain workplace toxicology testing and its potential to check the amount of opioids they are taking. Discuss using state prescription drug monitoring program (PDMP) data to evaluate the patients’ risk for an overdose.
- Explain why opioid prescriptions should only contain the quantity needed for the anticipated period of severe pain.
- Explain why within a month of prescribing opioids, the patient should be re-evaluated and prescription changes (escalating, de-escalating, or discontinuing opioids) made accordingly. If continued, re-evaluating will need to be performed at least monthly.
- Let the patient know if they show signs of opioid use disorder or addiction, you will offer, or arrange evidence-based treatment. Educate the patient that stopping on their own can be deadly, and they need medical help.
- Remind the patient there are no reliable ways to predict which patient will benefit from opioid prescriptions and which will be harmed (6).
Using these recommendations in your practice is essential for safely prescribing opioids. Review the complete CDC Guidelines for further information (6).

Self Quiz
Ask yourself...
- How do the 2022 CDC recommendations contrast to your current practice for opioid prescriptions?
- How can the 2022 CDC recommendations decrease opioid use disorder?
- How will you incorporate the guidelines in Mary’s case?
Risk Factors for Opioid Misuse
As a prescriber of opioids, you are responsible for understanding and recognizing opioid misuse, diversion, and OUD.
There are risk factors associated with the misuse of opioids. These risk factors increase the likelihood of opioid misuse or taking the medication differently than intended.
Risk factors of opioid misuse include (12):
- Being poor
- Unemployed
- History of substance abuse
- Environment that is high risk for misuse
- Adventurous or dangerous behaviors
- History of any mental disorder
- Stressful life
- History of drug or alcohol rehabilitation
- Female gender
(12)
When patients have identifiable risk factors, prescribers should share this information with their patients, so they understand they are at risk for misusing opioids.
Tools for Assessment of Opioid Misuse Behavior
There are several reliable and valid tools to assess opioid misuse behaviors:
TAPS
SOAPP-R
CRAFFT for adolescents
(13)

Self Quiz
Ask yourself...
- What changes in your care when you have a patient that is at high-risk for misuse?
- Do you trust your patients that have opioid misuse behaviors and how does that impact your care?
Diversion
Drug diversion, or the illegal use of distribution of a drug, may lead to accidental overdose and a myriad of illegal activities (7). Prescribers need to be careful not to fall victim to drug diversion by protecting their prescribing information and watching out for patients that visit solely to receive narcotics.
Common Drug Diversion Activities include:
Doctor shopping
Prescription pad theft
Selling drugs for money
Giving drugs to someone other than to whom they are prescribed (7).

Self Quiz
Ask yourself...
- What would you do if you found out your patient was selling their controlled-II medications for money?
- Have you experienced a patient that visited you only to try to get narcotics (doctor shopping)? How did or would you respond in that situation?
Opioid Use Disorder
The term Opioid Use Disorder (OUD) encompasses opioid dependence and addiction and can be mild to severe. Opioid use disorder (OUD) currently affects over three million people in the United States (1).
The diagnosis of OUD is made by meeting at least two DSM-5 criteria of the eleven in a year time according to the DSM-5 (1). The key 11 criteria are as follows:
- Increasing dose/tolerance
- Wishing to cut down
- Excessive time spent getting or using the medication
- Strong want to use
- Use interferes with normal daily obligations
- Continued use despite life disruption
- Use of opioids in hazardous situations like driving
- Reduced interest in important activities
- Continued use despite physical and/or psychological problems
- Need for more of the medication for the same effect
- Withdrawal symptoms occur when the dose is decreased
Estimates support that less than 20% of people in the United States with OUD are receiving effective available treatment (7). Screening for OUD is part of prescribing. If OUD is found, start treatment, or arrange for the patient to receive treatment and further care from a substance use disorder treatment specialist certified by SAMHSA. Practitioners should not terminate care with a patient because of OUD, as this event could represent patient abandonment and is unsafe for the patient (6).
Case Scenario
Mary returns to the clinic for follow-up five days after she was prescribed opioids. She tells you, “I’m really happy you went through all that stuff when you prescribed those pain killers to me. On that list of 11 things that I was supposed to watch for, and I can see I have a couple already-like I don’t want to do anything on these drugs. There is no way I can be on these and live a normal life on these. I also found myself almost not wanting to take the pills or wishing I was off them because they messed up my life so badly. I want off.”

Self Quiz
Ask yourself...
- How did being proactive and following good prescribing practices impact Mary?
- How to you respond to Mary in this situation?
Tapering and Discontinuing Opioid Therapy
Discontinuing opioids can be achieved rapidly, as in the case of someone that was prescribed a 3-day course of opioids for an acute injury and healing has reduced the pain so opioids aren’t warranted, or slowly through tapering. Tapering is the reduction of the daily opioid dose or daily MME. Tapering should be used as an exit strategy for opioids for patients that have been on long term opioids or anyone that has withdrawal symptoms when trying to discontinue. Tapering about 10% per month or slower is usually better tolerated than rapid tapers, especially when patients have been taking opioids for a year or longer (6).
Reasons for tapering include:
Implementing the planned opioid exit as part of the patients’ treatment plan
Pain resolution
Pain not resolved and a new treatment plan without opioids is introduced
Patient is experiencing impairment to daily functioning
Patient is showing signs of OUD, misuse, or diversion
Patient experienced an overdose or event leading to hospitalization
(6).
Adjunct drugs can be co-prescribed to help withdrawal symptoms and making the taper more tolerable (6).
Examples of Adjunct Medications include (15):
Clonidine- Alpha-2-Agnonist for sedative and antihypertension effects
Hydroxyzine-Antihistamine for nausea, vomiting, anxiety, and itching
Loperamide-Antidiarrheal, for diarrhea (15).
Opioid Withdrawal symptom severity can be measured by the clinician using the Clinical Opioid Withdrawal Scale (COWS) or patients may self-report severity using the Subjective Opiate Withdrawal Scale (SOWS) (13). Treatments can be based on the severity of the symptoms (13).
If a patient has OUD, start treatment immediately. The use of Buprenorphine is appropriate and is within the NP’s prescribing privileges as a schedule III providers (6, 13).
Buprenorphine is a schedule III-controlled substance, a partial opioid agonist with pain relieving and addiction reliving properties. It reduces pain, withdrawal symptoms and craving (15).
Depending on the severity of withdrawal symptoms, the NP may also arrange for the patient to get treatment from a substance use disorder treatment specialist certified by SAMHSA (6).

Self Quiz
Ask yourself...
- How have you implemented care with patients with OUD?
- What resources do you have and use to support and guide you with caring for patients with OUD?
Conclusion
Prescribing controlled substances is cumbersome, loaded with paperwork and forms and full of legal caveats and ethical considerations. It is that way on purpose. When discouraged about the process, please remember all the people that have died or have had their lives destroyed by opioids before these laws and guidelines existed. The steps are taken to allow medication to do what it is supposed to do and address opioid misuse before it becomes a problem.
Antipsychotic Prescribing
Introduction
There are many intricacies surrounding mental health conditions, notably psychosis. Can you imagine being placed into an anti-gravity machine without holding on to any sort of anchor? I imagine this loss of control would be terrifying. Similarly, psychosis can disorient and destabilize patients, as they have lost their grip on reality and their perceptions are no longer reliable. Psychosis is characterized by hallucinations, delusions, disorganized thinking, and impaired social functioning.
The use of antipsychotics has resulted in decreased mortality and improved patient’s quality-of-life; however, adverse drug reactions from these drugs are major challenges in treating patients with psychotic disorders (2).
In this course, we will review conditions associated with psychosis and explore the crucial role of antipsychotic medications in restoring stability and fostering recovery. Through a comprehensive understanding of antipsychotics and their uses, mechanisms of action, adverse reactions, and contraindications, prescribers can play a pivotal role in administering these treatments with precision and compassion. The ultimate goal is to empower patients to regain their footing and take back control over their lives.
Case Study
Lilly, age 36, presents to the urgent care clinic with complaints of a large and bleeding laceration on her tongue, which she got from biting her tongue while eating earlier this evening. She reports, “I guess you have to take the good with the bad”, which puzzles you, but you decide not to further question what this means right now. While reviewing her chart, you note a diagnosis of schizoaffective disorder.
Medical History: Lilly's medical history is significant for a family history of mental illness. Her paternal grandmother was diagnosed with schizophrenia. Lilly has been on antipsychotic medication since her diagnosis 10 years ago, with varying degrees of success in managing her symptoms. Lilly has struggled with medication adherence, often discontinuing the medication due to side effects or a belief that she no longer needed treatment.
Current Presentation: At present, Lilly reports strict adherence to her medication regimen with exacerbation of her symptoms for four years, and grateful that her paranoia and auditory hallucinations have reduced in frequency. She also states, “I cannot remember what the physician told me about the medication side effects, but it cannot be as frightening as my life had become.” She has a history of delusions and hallucinations, accompanied by long-term depression and anxiety.

Overview of Psychosis
Instead of reviewing all mental health conditions, we will examine psychosis, as treatment of this symptom is one of the main goals for antipsychotics. Psychosis can be described as a burden of interruption to an individual’s thoughts and perceptions that make it difficult for them to recognize what is real and what isn’t. Most people who have experienced psychosis describe it as frightening and confusing.
Psychosis is actually a symptom, not an illness. As many as 3 in 100 people will have an episode of psychosis at some point in their lives (8). This symptom can be present in different conditions.
Early or first-episode psychosis (FEP) refers to the initial occurrence of the symptom of distortion of reality.
Psychopathology
Mental health professionals need the ability to carefully assess and precisely describe signs and symptoms in a qualified manner. Psychopathology is a discipline that applies systematic methods to study abnormal functioning of psychic activity, with the intent to elaborate subdivisions, classifications, and theories, to identify the causes of mental illnesses.
Psychopathology applies direct observation of the clinical manifestations of mental illnesses to diagnostic systems (e.g., Diagnostic and Statistical Manual of Mental Disorders-5) which outlines signs, symptoms, and features that are required to establish a diagnosis.
Psychopathology can be broadly divided into two main subgroups (3):
- Descriptive psychopathology - involves the description and denomination of mental states and abnormal behaviors of the patient, carefully removing theoretical assumptions and personal interpretations.
- Interpretative psychopathology - the understanding of a human subjective experiences and attempting to explain these features using theoretical models.
Early Warning Signs Before Psychosis
Early psychosis or FEP rarely has a sudden onset. Typically, an individual has gradual, non-specific changes in thoughts and perceptions.
Early warning signs include the following (8):
- Difficulty concentrating
- Suspiciousness or uneasiness with others
- Poor self-care or personal hygiene
- Isolation
- A worrisome change in job performance completion of typical duties
- Strong, inappropriate emotions or the absence of response or emotions at all
Signs Of Early or First-Episode Psychosis
It can be difficult to pinpoint the initial episode of psychosis, but these signs and symptoms strongly indicate an episode of psychosis:
- Hearing, seeing, tasting, or believing things that others don’t
- Persistent, unusual thoughts or beliefs that can’t be set aside regardless of what others believe
- Strong and inappropriate emotions or no emotions at all
- Withdrawing from family or friends
- A sudden decline in self-care
- Trouble thinking clearly or concentrating
Such warning signs often point to a person’s deteriorating health, and a physical and neurological evaluation can help find the problem. A mental health professional performing a psychological evaluation can determine if a mental health condition is involved and discuss next steps. If the psychosis is a symptom of a mental health condition, early action helps to keep lives on track.
Psychosis
Psychosis includes a range of symptoms but typically involves one of these two major experiences (6):
Hallucinations - seeing, hearing, or feeling things that aren’t there, such as the following:
- Hearing voices (auditory hallucinations)
- Seeing glimpses of objects or people that are not there or distortions
Delusions - strong beliefs that are not consistent and unlikely to be true, and may seem irrational to others, such as the following (6):
- Believing external forces are controlling thoughts, feelings, and behaviors
- Believing that trivial remarks, events, objects have personal meaning or significance
- Thinking you have special powers or even that you are God
Causes of Psychosis
The medical and research community is still learning about how and why psychosis develops, but several factors are thought to be involved.
Several factors that can contribute to psychosis:
- Genetics
- Many genes can contribute to the development of psychosis, but just because a person has a gene doesn’t mean they will experience psychosis. Ongoing studies will help us better understand which genes play a role in psychosis.
- Trauma
- A traumatic event such as a death, war, or sexual assault can trigger a psychotic episode. The type of trauma—and a person’s age—affects whether a traumatic event will result in psychosis.
- Substance use
- The use of marijuana, LSD, amphetamines, and other substances can increase the risk of psychosis in people who are already vulnerable.
- Physical illness or injury
- Traumatic brain injuries, brain tumors, strokes, HIV, and some brain diseases such as Parkinson’s, Alzheimer’s and dementia can sometimes cause psychosis.
- Mental health conditions
- Sometimes psychosis is a symptom of a condition like schizophrenia, schizoaffective disorder, bipolar disorder, or depression.
These mental health problems have catastrophic effects on an individual’s life. Schizophrenia (SCZ) and bipolar disorder (BPD) are two of the most relevant mental disorders when focusing on functional impairment (3). There are significant implications for poor long-term functional outcomes, such as lower rates of employment, difficulties in living independently, and harming or preventing stable relationships. The World Psychiatry Association explains that cognition is highly related to functional outcome.

Self Quiz
Ask yourself...
- Have you ever cared for a patient experiencing psychosis?
- Can you name some factors that can contribute to the development of psychosis?
- What are some possible long-term outcomes that untreated psychosis can lead to?
- How would you describe signs of early or first-episode psychosis?
Types of Antipsychotics
Antipsychotic drugs tend to fall into one of two categories:
- First Generation Antipsychotics
-
- Referred to as “Typical” antipsychotics
- Older
- Second Generation Antipsychotics
- Referred to as “Atypical” antipsychotics.
- Newer
- In general, they cause less severe neuromuscular side effects than first generation antipsychotics.
- Second generation antipsychotics may be more likely to cause serious metabolic side effects such as rapid weight gain and changes to blood sugar levels.
Both types can potentially work for different people. Remember, the different types of antipsychotics have different side effects.
Indications for Antipsychotic Prescribing
Schizophrenia is the primary indication for antipsychotic agents (2). However, the use of antipsychotics in the treatment of mood disorders such as type 1 bipolar disorder (BD-1), depression with psychosis, and treatment-resistant depression have become widely used in the last decade. The following are descriptions of the indications for antipsychotic use.
Schizophrenia and Schizoaffective disorders
Schizophrenia is a chronic disorder that affects less than one percent of the U.S. population (1). When schizophrenia is active, symptoms can include delusions, hallucinations, disorganized speech, difficulty thinking and lack of motivation. Antipsychotic drugs are the mainstay of treatment for this condition.
Antipsychotic drugs are also indicated for schizoaffective disorders, which show characteristics of both schizophrenia and affective disorders. The psychotic aspects of the illness require treatment with antipsychotic drugs, along with other drugs such as antidepressants, lithium, or valproic acid (2).
Acute Mania
The manic phase in bipolar affective disorder often requires treatment with antipsychotic agents. First-generation antipsychotics are effective in the treatment of acute mania with psychotic symptoms and all second-generation antipsychotics (except clozapine) can also be used as a treatment of symptoms of acute mania.
Major Depressive Disorder with Psychotic features
First or second-generation antipsychotics, along with an antidepressant, is typically the treatment of choice for depression with psychotic features.
Personality Disorders
First-generation antipsychotics are indicated in the treatment of paranoia and delusions associated with personality disorders. Borderline personality disorder is a type of personality disorder that can have symptoms of psychosis and paranoia; both first and second-generation antipsychotics are used for the treatment of these symptoms.
Dementia and Delirium
A low dose of high potency first-generation antipsychotics like haloperidol is recommended for the treatment of agitation in delirium and dementia. It is essential to use caution in elderly patients as the antimuscarinic effects can cause significant adverse effects in this population. Second-generation antipsychotics can also be used for treating behavioral disturbances in dementia. Off-Label use of second-generation antipsychotics is acquired immunodeficiency syndrome-related dementia.
Substance-Induced Psychotic Disorder
In cases of severe psychosis secondary to substance use, antipsychotics can be used to control agitation symptoms. Caution is necessary when using first-generation antipsychotics in alcohol withdrawal and phencyclidine intoxication.
Non-Psychiatric Indications
- Antiemetic effect: Older first-generation antipsychotic drugs (with the exception of thioridazine).
-
- This action is due to dopamine-receptor blockade
- Some drugs, such as prochlorperazine and benzquinamide, are used solely as antiemetics.
- H1-receptor-blocking
-
- Phenothiazines have been used for relief of pruritus or, in the case of promethazine, as preoperative sedatives.

Figure 1. Indications for Antipsychotics (Designed by course author, 2024)

Self Quiz
Ask yourself...
- Can you describe the symptoms of schizophrenia?
- What are some conditions that antipsychotic drugs may be used for?
- What are some precautions needed when using antipsychotic drugs to treat substance-induced psychotic disorders?
- Can you describe the antiemetic effect of these drugs?
How Do Antipsychotics Work on the Brain?
There are several researched reasons why antipsychotic drugs may help to reduce psychotic symptoms, these include:
Blocking the Action of Dopamine
It is a common perception that many psychotic experiences are caused by the brain producing too much dopamine. Dopamine is a neurotransmitter, which means that it passes messages around in your brain. Most antipsychotic drugs are known to block some of the dopamine receptors in the brain. This reduces the flow of these messages and ultimately helps to reduce your psychotic symptoms.
Dopamine is an important central nervous system neurotransmitter in the body. It belongs to the catecholamine family, which includes dopamine, norepinephrine, and epinephrine. Dopamine is the first catecholamine made in the biosynthetic pathway, produced by the decarboxylation of L-3, 4-dihydroxyphenylalanine (dopa) by aromatic amino acid decarboxylase (15).
Dopamine is stored in vesicles that are released into the synaptic cleft; dopamine binds to dopamine receptors at the synapse (15). There are five different types of dopamine receptors (D1, D2, D3, D4, and D5), all receptors have different pharmacological, biochemical, and physiological functions.
These receptors are divided into two receptor families (15):
- D1-like receptor family: D1 and D5 receptors
- D2-like receptor family: D2, D3, and D4 receptors
Dopamine pathways are neuronal connections in which dopamine travels to areas of the brain and body to relay messages such as executive thinking, cognition, feelings of reward and pleasure, and voluntary motor movements. Dopamine is responsible for many functions in the brain, including actions and perceptions, voluntary movements, motivation, punishment and reward, sleep, mood, attention, memory, and learning (15).
Affecting Other Brain Chemicals
Most antipsychotics are known to affect other brain chemicals too. This may include the neurotransmitters serotonin, noradrenaline, and glutamate. These chemicals are thought to be involved in regulating your mood.
Parkinsonism
Some of the medical and research community believe that certain antipsychotics work by causing Parkinsonism, which is a movement disorder. This means they can induce the physical symptoms of Parkinsonism as side effects; they are also known to produce psychological symptoms of Parkinsonism, such as lacking appropriate emotions or losing interest in activities. These effects are more common with first-generation, or 'typical' antipsychotics.

Self Quiz
Ask yourself...
- How would you describe the role of dopamine in the central nervous system?
- Can you name examples of messages that dopamine transports within the brain?
- Do all dopamine receptors have the same physiological functions?
- Do you think elevated levels of dopamine could negatively impact mood, attention, sleep, and voluntary movements?
Case Study (Continued)
Following the treatment for Lilly’s oral laceration, she asks if this report will be sent to her primary care provider (PCP). She states she is not followed by a psychiatrist, but she gets her routine prescription for Chlorpromazine from her PCP.
Previous medical history:
- Schizoaffective disorder, diagnosed 2013
- Hypothyroidism, diagnosed 2010
Her medication list includes the following:
- Chlorpromazine 400mg, PO, Twice Daily
- Synthroid 75mcg, PO, Daily
Lilly reports taking Benadryl every night to help her sleep (which is not on her medication list), as she struggles with ongoing insomnia.
Pharmacokinetics of First-Generation Antipsychotics: Dopamine Receptor Antagonists (DRA)
First-generation antipsychotics are dopamine receptor antagonists (DRA). They are known as typical antipsychotics. The first-generation antipsychotics work by inhibiting dopaminergic neurotransmission, with the peak effectiveness when 72% of the D2 dopamine receptors in the brain are blocked (4). They also have noradrenergic, cholinergic, and histaminergic blocking action.
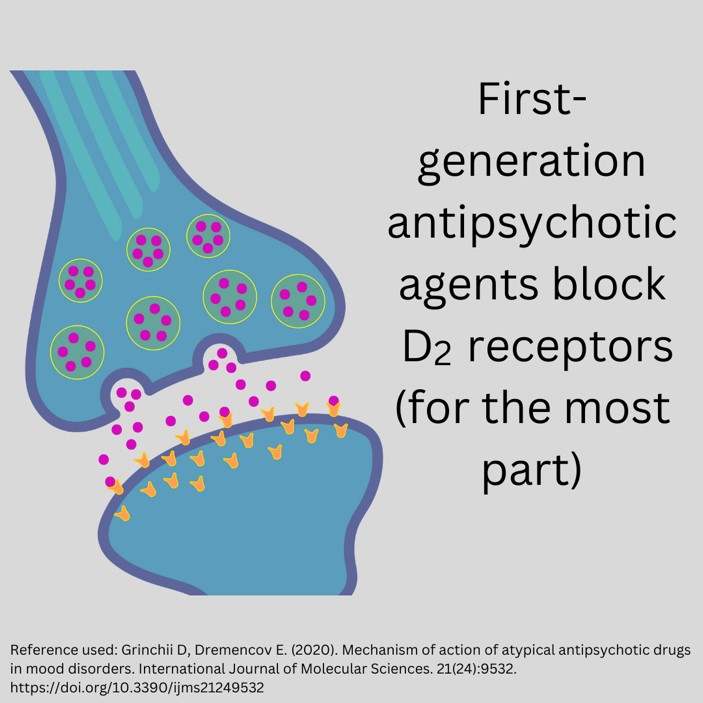
Figure 2. Blocking Action of First-Generation Antipsychotics (Designed by course author, 2024)
First-generation antipsychotics have the following approved uses:
- Psychotic disorders
- Schizophrenia
- Sedation for psychosis
- Acute agitation
- Behavioral disorders
- Schizophrenia and bipolar I agitation
- Non-psychotic anxiety
- Tourette syndrome (a nervous system disorder involving repetitive movements or unwanted sounds)
- Nausea and vomiting
It is important to note that adverse reactions of first-generation antipsychotics are highly prevalent in a significant number of patients. This warrants prescribers to perform risk minimization strategies including staff training on identification, management, and prevention, revision of treatment guidelines and algorithms, and laboratory protocol set-up to enable therapeutic and adverse event monitoring (2).
Examples of dopamine receptor antagonists (DRA) include (4):
- Butyrophenones (haloperidol)
- Phenothiazines (trifluoperazine, perphenazine, prochlorperazine, acetophenazine, triflupromazine, mesoridazine)
- Thioxanthenes (thiothixene, chlorprothixene),
- Dibenzoxazepines (loxapine)
- Dihydroindoles (molindone)
- Diphenylbutylpiperidines (pimozide)
Mechanism of Action of Dopamine Receptor Antagonists (DRA)
There are five dopaminergic pathways that are important for understanding schizophrenia and the mechanism of action of antipsychotic drugs.
- Mesolimbic-mesocortical pathway
-
- Projects from cell bodies in the ventral tegmentum in separate bundles of axons to the limbic system and neocortex
-
- The most closely related to behavior and psychosis.
- Nigrostriatal pathway
-
- Neurons that project from the substantia nigra to the dorsal striatum
- Involved in the coordination of voluntary movement
- Tuberoinfundibular system
-
- Occurs in the arcuate nuclei and periventricular neurons and releases dopamine into the pituitary portal circulation.
- Dopamine released by these neurons inhibits prolactin secretion from the anterior pituitary.
- Medullary-periventricular pathway
-
- Consists of neurons in the motor nucleus of the vagus
- This system is thought to be involved in eating patterns and behavior.
- Incertohypothalamic pathway
-
- Connections from the medial zona incerta to the hypothalamus and the amygdala
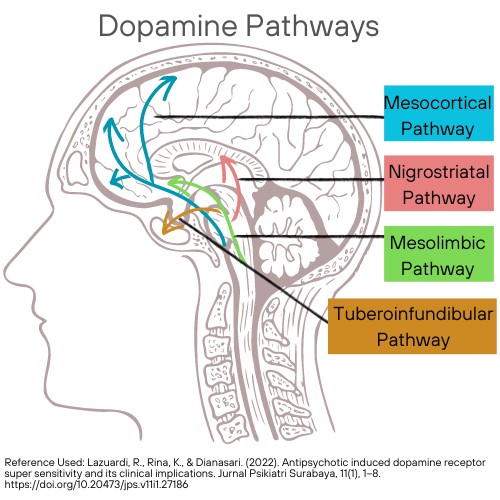
Figure 3. Dopamine Pathways. (This design is copyrighted by Abbie Schmitt, RN, MSN, 2024, and may not be reproduced without permission from Nursing CE Central)
At present, five dopamine receptors have been described, consisting of two separate families, the D1-like (D1, D5) and D2-like (D2, D3, D4) receptor groups.
The antipsychotic action is thought to be mostly produced by their ability to block the effect of dopamine; D2 receptors inhibit the activity of adenylyl cyclase in the mesolimbic system (4).
The D1-like receptor is coded by a gene on chromosome 5, increases cAMP by Gs-coupled activation of adenylyl cyclase, and is located mainly in the putamen, nucleus accumbens, and olfactory tubercle and cortex (2). D5, is coded by a gene on chromosome 4, also increases cAMP, and is found in the hippocampus and hypothalamus.
The D2 decreases cAMP (by Gi-coupled inhibition of adenylyl cyclase), and inhibits calcium channels, but opens potassium channels (2). It is found both pre- and postsynaptically on neurons in the caudate-putamen, nucleus accumbens, and olfactory tubercle. A second member of this family, the D3 receptor is thought to also decrease cAMP and is located in the frontal cortex, medulla, and midbrain. D4 receptors also decrease cAMP and are concentrated in the cortex (2).
The first-generation antipsychotic agents block D2 receptors for the most part, and their binding affinity is correlated with clinical antipsychotic and extrapyramidal potency. The degree of this blockade in D2 receptors and relation to other actions varies considerably among drugs (2).
The first-generation antipsychotics also depress the release of hormones by the hypothalamus and pituitary gland. Loxapine, in addition to D2 receptors, blocks the activity of serotonin 5-HT2A receptors (4).

Self Quiz
Ask yourself...
- Can you describe the five dopaminergic pathways?
- Does the degree D2 receptor blocking vary among first-generation antipsychotic drugs?
- Are there commonly known side effects of these drugs?
- What are some risk minimization strategies that prescribers can use?
Case Study (Continued)
You ask Lilly to describe what happened when she bit her tongue. You note scars from deep lacerations in the mucosa of her tongue, gums, and lips at varying degrees of healing.
Lilly states, “my face and mouth stiffen and jerk for several minutes at a time”. When you ask about the frequency, she reports “almost daily.” She explains that her family gets aggravated at her when she “smacks her lips” or “sticks out her tongue,” but she cannot control these movements. Her elderly mother tells her she is possessed by a demon, which Lilly says causes her significant emotional distress and embarrassment.
- What other assessment data would you collect from Lilly?
- How would you assess psychosocial factors, such as coping and support systems?
Drug-Specific Pharmacokinetics of First-Generation Antipsychotics
Butyrophenones (Haloperidol)
The following are pharmacokinetics of butyrophenones (10):
Class: Antipsychotic, neuroleptic
Black Box Warning: Risk for death in dementia-related psychosis
Uses: Psychotic disorders, control of vocal utterances in Gilles de la Tourette’s syndrome, short-term treatment of hyperactive children showing excessive motor activity, prolonged parenteral therapy in chronic schizophrenia, organic mental syndrome with psychotic features, emergency sedation of severely agitated or delirious patients.
- Metabolized by liver
- Excreted in urine, bile
- Crosses placenta; enters breast milk
- Protein binding: 92%
- Terminal half-life 12-36 hr. (metabolites)
- PO: Onset erratic, peak 2-6 hr.; half-life 24 hr.
- IM: Onset 15-30 min, peak 15-20 min; half-life: 21 hr.
- IM (Decanoate): Peak 4-11 days; half-life: 3 weeks
Contraindications: Hypersensitivity, coma, Parkinson’s disease
Precautions: Pregnancy, breastfeeding, geriatric patients, seizure disorders, hypertension, pulmonary or cardiac conditions, hepatic disease, QT prolongation, torsades de pointes, prostatic hypertrophy, hyperthyroidism, thyrotoxicosis, pediatrics, blood dyscrasias, neurological injury, bone marrow depression, alcohol and barbiturate withdrawal states, angina, epilepsy, urinary retention, closed-angle glaucoma, and CNS depression.
Phenothiazines
The following are pharmacokinetics of phenothiazines (7):
Group: Nitrogen and sulfur-containing heterocyclic compounds
Uses: Treatment of schizophrenia, bipolar disorders, control nausea and vomiting, and other psychotic disorders with delusional manifestations.
Examples of phenothiazines (10):
- Chlorpromazine
- Black Box Warning: Beers Criteria: Avoid in older adults (except in schizophrenia, bipolar disorder, or short-term use as antiemetic in chemotherapy); increased risk for stroke and cognitive decline and mortality if used in dementia (10).
- Metabolized by liver, excreted in urine (metabolites)
- Crosses placenta; Enters breast milk
- 95% bound to plasma proteins
- Elimination half-life: 23-37 hr.
- PO: Absorption variable, widely distributed, onset erratic 30-60 min, duration 4-6 hr.
- PO/extended release (ER): Onset 30-60 min, peak unknown, duration 10-12 hr
- IM: Well absorbed, peak 15-20 min, duration 4-8 hr. IV: Onset 5 min
- Fluphenazine
- Black Box Warning: Increased mortality reported with dementia-related psychosis.
- Metabolized by liver
- Excreted in urine (metabolites)
- Crosses placenta; Enters breast milk
- Protein binding >90%, not dialyzable
- PO/IM (HCl): Onset 1 hr, peak 90-120 min, duration 6-8 hr, half-life 15 hr
- IM/SUBCUT (decanoate): Onset 1-3 days; peak 1-2 days, duration over 4 wk, single-dose half-life 7-10 days, multiple dose 14.3 days
Phenothiazines produce the most optimal results when combined with non-pharmacological psychotherapeutic therapy, such as narrative, meta-cognitive, and mindfulness therapy (7)
Thioxanthenes (thiothixene, chlorprothixene)
The following are pharmacokinetics of thioxanthenes:
Thioxanthenes reduce antipsychotic activity by postsynaptic blocking of central nervous system (CNS) dopamine receptors, resulting in inhibition of dopamine-mediated effects; also has alpha-adrenergic blocking activity.
Example of thioxanthenes:
- Thiothixene
- Thiothixene is not approved for the treatment of patients with dementia-related psychosis (13).
- Absorption: Erratic; high lipophilicity
- Protein binding: 90%
- Metabolism: Hepatic; substrate of CYP1A2
- Half-life elimination: 34 hours
Dibenzoxazepines (Loxapine)
The following are pharmacokinetics of dibenzoxazepines (10):
Uses: Schizophrenia, bipolar disorder, Unlabeled uses: Anxiety
Black Box Warning: Acute bronchospasm, asthma, chronic obstructive pulmonary disease (COPD), emphysema
Loxapine inhalation must be administered only in a health care facility (14).
- Loxapine
- Onset of action: Oral, IM: Within 30 minutes; Inhalation: 2 minutes
- Peak effect: Oral, IM: 1.5 to 3 hours
- Duration: Oral, IM: ~12 hours
- Absorption: Oral, inhalation, IM: Rapid and complete
- Protein binding: Inhalation: ~97%
- Metabolism: Hepatic to glucuronide conjugates
- Bioavailability: Inhalation: 91% (de Berardis 2017)
- Half-life elimination: Oral: Biphasic: Initial: 5 hours; Terminal: 19 hours; Inhalation: 6 to 8 hours
- Excretion: Urine (as metabolites); feces (as metabolites)
Diphenylbutylpiperidines (Pimozide)
- Pimozide
- Onset of action: Within 1 week; Maximum effect: 4 to 6 weeks
- Duration of action: Variable
- Absorption: ≥50%
- Protein binding: 99%
- Metabolism: Hepatic
- Half-life elimination: Children 6 to 13 years (n=4): Mean ± SD: 66 ± 49 hours; Adults 23 to 39 years (n=7): Mean ± SD: 111 ± 57 hours
- Time to peak, serum: 6 to 8 hours; Range: 4 to 12 hours
- Excretion: Urine

Self Quiz
Ask yourself...
- What are the black box warnings for these drugs?
- How do the onset of action and elimination differ among these drugs?
- Are these drugs considered older or newer agents?
- What are some populations that are contraindicated for use of first-generation antipsychotics?
Adverse Effects of First-Generation Antipsychotics
Possible side effects and adverse effects of first-generation antipsychotics include (1, 2, 5, 6, 7, 8):
- Extrapyramidal symptoms, which are drug-induced movement disorders such as:
- Akathisia
- Dystonia
- Dyskinesia
- Akinesia
- Muscle stiffness
- Tardive dyskinesia
- Anticholinergic effects such as:
- Constipation
- Urinary retention
- Blurred vision
- Sedation
- Confusion
- Drowsiness
- Dizziness
- Headache
- Agitation
- Seizure
- Cerebral edema
- Peripheral edema
- Impaired regulation of body temperature
- Hypotension
- Orthostatic hypotension
- Abnormal ECG results or cardiac abnormalities
- Retinal disorders such as:
- Pigmentary retinopathy
- Retinitis pigmentosa
- Nausea
- Diarrhea
- Hypersensitivity reactions such as:
- Rash
- Pruritus (itching)
- Light sensitivity
- Abnormal liver function test results
- Sexual disfunction
Neurological Effects
First-generation antipsychotics are associated with significant extrapyramidal (EPS) side effects. The mild to severe EPS, including akathisia, sleepiness, restlessness, and autonomic effects, are unlike any associated sedatives or hypnotics. It is important that prescribers recognize this possibility and educate patients on these effects.
Tardive dyskinesia, as the name implies, is a late-occurring syndrome of abnormal choreoathetoid movements and is the most important unwanted effect of antipsychotic drugs (2). It is suspected to be caused by cholinergic deficiency secondary to sensitivity of dopamine receptors in the caudate-putamen (2). Tardive dyskinesia is estimated to have occurred in 20–40% of chronically treated patients before the introduction of the newer atypical antipsychotics, which caused the prevalence to vary greatly (11).
The majority of the medical community agree that the first step after tardive dyskinesia is noted should be to discontinue or slowly reduce the dose of the current antipsychotic agent or switch to one of the newer atypical agents, followed by elimination of all drugs with central anticholinergic action (2)
Neuroleptic malignant syndrome is a life-threatening disorder that occurs in patients who are extremely sensitive to the extrapyramidal effects of antipsychotic agents. This syndrome is believed to result from an excessively rapid blockade of postsynaptic dopamine receptors. The initial symptom is marked muscle rigidity, then fever may ensue and often reaches dangerous levels (2). The leukocytosis and high fever that is present with this syndrome may erroneously be considered as infectious process.
Altered blood pressure and pulse rate are also common (2). Muscle-type creatine kinase levels are usually elevated, which reflects the muscle damage.
Switching to an atypical antipsychotic drug following recovery is indicated.
Anticholinergic Effects
Anticholinergic adverse effects like dry mouth, constipation, and urinary retention are common with dopamine receptor antagonists like chlorpromazine and thioridazine (4). The action of H1 histamine blocking causes sedation; chlorpromazine is the most sedating, while fluphenazine, haloperidol, and pimozide are less sedating (4).
Cardiac Effects
Haloperidol can cause abnormal heart rhythm, ventricular arrhythmia, torsades de pointes, and even sudden death if injected intravenously (2). Other adverse effects include prolongation of QTc interval, prolonged atrial and ventricular contraction, and other cardiac conduction abnormalities (2).
Thioridazine has an FDA-backed warning for sudden cardiac death. Low-potency first-generation antipsychotics, like chlorpromazine or thioridazine, commonly cause orthostatic hypotension (2).
Additional Effects
First-generation antipsychotics can also lower the seizure threshold, and chlorpromazine and thioridazine are more epileptogenic than others (2).
Increased serum prolactin concentrations, with breast enlargement, amenorrhea, changes in sexual function are known adverse effects due to the action of the dopamine receptor block in the tuberoinfundibular tract (2).
Use In Pregnancy
Although antipsychotic drugs appear to be relatively safe in pregnancy, there is a small increase in teratogenic risk. If a pregnant woman could manage to be free of antipsychotic drugs during pregnancy, this would be desirable because of their effects on the neurotransmitters involved in neurodevelopment.
Drug Interactions for Antipsychotic Drugs
Antipsychotics are contraindicated in combination with other drugs that have sedative effects, including α-adrenoceptor-blocking action, anticholinergic effects, and—for thioridazine and ziprasidone—quinidine-like action (2).
Parenteral Preparations
There are parenteral forms of haloperidol and fluphenazine that are available for rapid initiation of treatment, as well as for maintenance treatment in noncompliant patients (2). It is important for prescribers to closely follow the manufacturer’s literature because the parenterally administered drugs may have much greater bioavailability (2). Fluphenazine decanoate and haloperidol decanoate are appropriate for long-term therapy in patients who cannot or will not take oral medication.
Dosage Schedules
Antipsychotic drugs are often given in divided daily doses, titrating to an effective dosage (2). The low end of the dosage range is recommended for at least several weeks. After an effective daily dosage has been defined for an individual patient, doses can be given less frequently. Once-daily doses (usually given at night) are feasible for many patients during chronic maintenance treatment. Research shows that simplification of dosage schedules often leads to better compliance.

Self Quiz
Ask yourself...
- Can you describe common adverse effects of these drugs?
- Have you ever witnessed extrapyramidal (EPS) side effects in a patient?
- Are extrapyramidal (EPS) side effects more common in first- or second-generation antipsychotics?
- What are some of the cardiac abnormalities reported with specific first-generation antipsychotic agents?
Pharmacokinetics of Second-Generation Antipsychotics
Second-generation antipsychotics are serotonin-dopamine antagonists and are also known as atypical antipsychotics.
Typical antipsychotics act almost exclusively on the dopamine system; these atypical drugs, however, regulate serotonin (5-HT), norepinephrine, and/or histamine neurotransmission as well (5).
The Food and Drug Administration (FDA) has approved the following atypical antipsychotics:
(* indicates the pharmacokinetics will be discussed in the next section)
- Aripiprazole (marketed as Abilify)*
- Asenapine Maleate (marketed as Saphris)
- Clozapine (marketed as Clozaril)*
- Risperidone (marketed as Risperdal)*
- Quetiapine (marketed as Seroquel)*
- Olanzapine (marketed as Zyprexa)
- Olanzapine/Fluoxetine (marketed as Symbyax)
- Iloperidone (marketed as Fanapt)
- Lurasidone (marketed as Latuda)
- Paliperidone (marketed as Invega)
- Ziprasidone (marketed as Geodon)
Second-generation antipsychotics are approved for treatment of the following:
| Generic Name | Indications |
| Aripiprazole |
|
| Asenapine |
|
| Clozapine |
|
| Iloperidone |
|
| Olanzapine |
|
| Paliperidone |
|
| Quetiapine |
|
| Risperidone |
|
| Ziprasidone |
|
Mechanism of Action of Second-Generation Antipsychotics: Serotonin-Dopamine Antagonists (SDA)
Typical antipsychotics act almost exclusively as blockers of dopamine-2 (D2) receptors. In contrast, second-generation atypical antipsychotic drugs are characterized by serotonin-2A (5-HT2A) antagonistic property (5). Targeting these receptors can lead to altering the excitability of 5-HT neurons.
Some atypical antipsychotics are also potent serotonin-1A (5-HT1A; aripiprazole), serotonin-1C (5-HT1C; clozapine, olanzapine, risperidone), histamine-1 (H1; olanzapine, quetiapine) and α1-(aripiprazole, clozapine, olanzapine, paliperidone, quetiapine) and α2-adrenergic (clozapine, olanzapine, paliperidone, quetiapine, risperidone) receptor blockers (5).
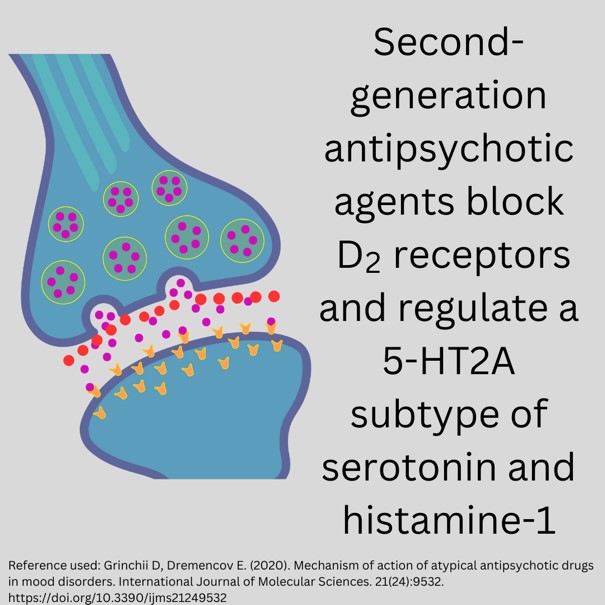
Figure 4. Blocking Action of Second-Generation Antipsychotics (Designed by course author, 2024)

Self Quiz
Ask yourself...
- Can you explain the difference in mechanism of action between first- and second-generation antipsychotic medications?
- Are you familiar with any atypical antipsychotics?
- What would be an overall benefit of targeting more than one specific neurotransmitter receptor?
- Can you name some uses for second-generation antipsychotics?
Drug-Specific Pharmacokinetics of Second-Generation Antipsychotics
These antipsychotics are identified in the Beers Criteria as potentially inappropriate medications to be avoided in patients 65 years and older due to an increased risk of cerebrovascular accidents (stroke) and a greater rate of cognitive decline and mortality in patients with dementia (12).
Aripiprazole
The following are pharmacokinetics of aripriprazole (12):
- Onset of action: Initial: 1 to 3 weeks.
- Absorption:
- IM: Extended-release: Slow, prolonged.
- Oral: Well absorbed.
- Distribution: Vd: 4.9 L/kg.
- Protein binding: ≥99%, primarily to albumin.
- Metabolism: Hepatic dehydrogenation, hydroxylation and N-dealkylation via CYP2D6, CYP3A4
- Bioavailability: Tablet: 87%
- Half-life elimination:
- Aripiprazole: 75 hours; dehydro-aripiprazole: 94 hours
- IM, extended release (terminal): ~30 to 47 days (dose-dependent).
- Time to peak, plasma:
- IM: Extended release (after multiple doses): 4 days (deltoid administration); 5 to 7 days (gluteal administration).
- Tablet: 3 to 5 hours; high-fat meals delay time to peak by 3 hours for aripiprazole and 12 hours for dehydro-aripiprazole.
- Excretion: Feces (55%; 18% of the total dose as unchanged drug; 37% of the total dose as changed drug); urine (25%)
Risperidone
The following are pharmacokinetics of risperidone (10):
- Black Box Warning: Increased mortality in elderly patients with dementia-related psychosis (10).
- PO: Extensively metabolized by liver
- Plasma protein binding: 90%
- Peak 1-2 hr.
- Excreted 90% in urine
- Terminal half-life 3-24 hr.
Quetiapine
The following are pharmacokinetics of quetiapine (10):
- Black Box Warning: Increased risk for mortality in elderly patients with dementia-related psychosis; Increased suicidal ideation in pediatric patients (10)
- Extensively metabolized by liver
- Half-life: ≥ 6 hr.
- Peak: 1.5 hr.
- Protein binding: 83%
- Excretion: <1% unchanged urine
Clozapine
The following are pharmacokinetics of clozapine (10):
- Black Box Warning: Bone marrow suppression, hypotension, myocarditis, orthostatic hypotension, geriatric patients with dementia-related psychosis, seizures, syncope (10).
- Patient-specific registration required before administration
- If WBC <3500 cells/mm 3 or ANC <2000 cells/mm 3, therapy should not be initiated
- Prescribers can only dispense 7-, 14-, 28-day supply upon receipt of lab report that is within appropriate levels.
- Completely metabolized by liver enzymes
- Bioavailability 27%-47%
- Protein binding: 97%
- Excreted in urine (50%), feces (30%) (metabolites)
- Half-life 8-12 hr.
- Clozapine can cause the following adverse effects:
- Hypersalivation
- Tachycardia
- Hypotension
- Anticholinergic side effects
- Agranulocytosis and leukopenia; therefore, requires monitoring of white blood cells and absolute neutrophil count. The FDA guidelines indicate monitoring absolute neutrophil count weekly for the first six months and, if normal, can be monitored every two weeks after that (10). Clozapine should be discontinued if absolute neutrophil count is below 1000 cells per cubic millimeter or below 500 cells per cubic millimeter in those with benign ethnic neutropenia.

Self Quiz
Ask yourself...
- Can you describe common pharmacokinetics of these second-generation antipsychotic drugs?
- What is a major adverse effect of Clozapine that must be closely monitored before prescribing?
- Can you name the black box warnings for Clozapine?
- How would you compare the pharmacokinetics of these drugs to the first-generation antipsychotics?
Adverse Effects of Second-Generation Antipsychotics
Second-generation antipsychotics (SGAs) have a decreased risk of extrapyramidal side effects as compared to first-generation antipsychotics. However, SGAs are associated with significant weight gain and the development of metabolic syndrome (2).
The FDA recommends that prescribers closely consider the following:
- Diabetes mellitus (personal and family history)
- Dyslipidemia
- BMI
- Blood pressure
- Fasting plasma glucose
- Fasting lipid profile
Risperidone is associated with dizziness, anxiety, sedation, and extrapyramidal side effects.
Paliperidone can cause temperature sensitivity to hot or cold temperatures and QTc prolongation (QTc = corrected QT interval).
Olanzapine has been associated most frequently with weight gain, increased appetite, and somnolence.
Quetiapine is the least likely to cause extrapyramidal side effects. The most common side effects of quetiapine are somnolence, orthostatic hypotension, and dizziness.
Ziprasidone has almost no weight gain but can cause prolongation of QTc. Aripiprazole is the most common side effect of agitation, headache, and akathisia-like restlessness.
Asenapine can cause an increase in serum prolactin concentrations, weight gain, and prolongation of QTc.

Self Quiz
Ask yourself...
- What does the FDA recommend to closely monitor when prescribing second-generation antipsychotics?
- What are some adverse effects of Risperidone?
- What is the most common adverse effect of Quetiapine?
- What lab work would be helpful in monitoring metabolic alterations in patients taking these drugs?
Drug Choice
Choice among antipsychotic drugs is based mainly on differences in adverse effects and possible differences in efficacy. In addition, cost and the availability of a given agent on drug formularies also influence the choice of a specific antipsychotic.
For approximately 70% of patients with psychotic features, first- and second-generation antipsychotic drugs have similar efficacy for treating positive symptoms; however, evidence shows that second-generation drugs may have fewer negative symptoms, poor cognition, risk of tardive dyskinesia, and lesser increases in prolactin levels (2).
Some of the second-generation antipsychotic drugs cause increased weight gain and increased lipid levels compared to some first-generation drugs, and a small percentage of patients develop diabetes mellitus, most often seen with clozapine and olanzapine (2). Thus, these drugs should be considered as second-line drugs unless there is a specific indication.
Uncontrollable behavior may respond equally well to all potent antipsychotics but is still frequently treated with older drugs that offer intramuscular formulations for acute and chronic treatment. The low cost of the older first-generation antipsychotic drugs contributes to their widespread use despite their risk of adverse EPS effects (2).
The best guide for selecting a drug for an individual patient is the patient history of past responses to drugs.
At present, clozapine is limited to patients who have failed to respond to substantial doses of conventional antipsychotic drugs (2). Aripiprazole is one of the most commonly prescribed second-generation antipsychotics in the U.S. due to lower side effect profile and aggressive marketing (2).


Self Quiz
Ask yourself...
- Do both types of antipsychotics (typical and atypical) have similar efficacy for treating positive symptoms?
- Which type of antipsychotics (first or second generation) are more likely to cause tardive dyskinesia?
- Which types of antipsychotics (first or second generation) are more likely to cause an increase in lipid levels?
- How would you explain the benefits and risks of each antipsychotic to a patient who has recently been prescribed one of these drugs?
Case Study (Continued)
After providing education to Lilly on the side effects of the first-generation antipsychotic medications, you note that Lilly appears anxious. However, you quickly explain that research supports that changing to a second-generation antipsychotic agent can lead to less of these involuntary muscle movements. Lilly seems relieved and hopeful.
She agrees to the mental health referral for psychiatry establishment, verbalizes understanding of the gradual taper instructions from the psychologist that consulted while at your facility, and is thankful for the counseling, outpatient therapy, and community resources. All lab work is normal at this time. The social worker at the hospital is also consulted and begins case management for Lilly, making sure she has support for areas such as housing, financial resources, and employment stability.

Self Quiz
Ask yourself...
- What type of antipsychotic agent was Lilly taking?
- What condition do her symptoms align with?
- What resources and referrals would you incorporate if you were involved in her care?
- Do you think the muscle movements and facial twitching is chronic and incurable for Lilly?
Psychosocial Treatment & Cognitive Remediation
Patients with schizophrenia need psychosocial support based around activities of daily living, including housing, social activities, returning to school, obtaining the optimal level of work they may be capable of, and restoring social interactions.
Case management and therapy services are a vital part of the treatment program that should be provided to patients with schizophrenia. First-episode patients may be particularly needful of this support because they may be more likely to deny their illness and display noncompliance with medication.

Self Quiz
Ask yourself...
- Why is it important that a case manager be involved with patients experiencing symptoms of psychosis?
- Are you familiar with social determinants of health (SDOH)?
- How can psychosis interfere with an individual’s daily activities?
- Do you think there is a stigma attached to mental health conditions?
Research
Considering that most second-generation and some first-generation antipsychotic agents are at least as potent in inhibiting 5-HT2 receptors as they are in inhibiting D2 receptors, current research is directed toward developing antipsychotic compounds that are more selective for the mesolimbic system (2). This increased selectivity would reduce their effects on the extrapyramidal system and hopefully decrease undesirable side effects.
The differences in receptor effects of various antipsychotics can explain many of their toxicities and are shown to be consistently associated with high D2 potency (2). Essentially, a significant goal of research is to find more effective blockage of dopamine activity with less involuntary movement or toxic effects.
There is also research on non-pharmacological therapies such as psychotherapy, cognitive behavioral therapy, and physical activity in improving these conditions.

Self Quiz
Ask yourself...
- What is a major goal of research when considering involuntary movement or toxic effects?
- What are non-pharmacological areas of research for treatment of psychosis?
Conclusion
As we have discussed, the use of antipsychotics has resulted in improved quality-of-life for many patients; however, prescribers must be aware of the adverse drug reactions from these drugs. Treating patients with psychotic disorders requires a holistic care plan for each patient. The pharmacokinetics of each drug, specifically the mechanism of action and side effects, can be used as an effective tool for designing a holistic care plan.
References + Disclaimer
- Agarwal, P., Kukrele, R., & Sharma, D. (2019). Vacuum assisted closure (VAC)/ negative pressure wound therapy (NPWT) for difficult wounds: A review. Journal of clinical orthopaedics and trauma, 10(5), 845–848. https://doi.org/10.1016/j.jcot.2019.06.015
- Chen, C. Y., Kuo, S. M., Tarng, Y. W., & Lin, K. C. (2021). Immediate application of negative pressure wound therapy following lower extremity flap reconstruction in sixteen patients. Scientific reports, 11(1), 21158. https://doi.org/10.1038/s41598-021-00369-5
- Doenges, M.E., Moorhouse, M.F., & Alice C. Murr, A.C. (2019). Nursing care plans: guidelines for individualizing client care across the life span: Vol. 10th edition. F.A. Davis.
- Gold, C., Kournoutas, I., Seaman, S. C., & Greenlee, J. (2021). Bone flap management strategies for postcraniotomy surgical site infection. Surgical neurology international, 12, 341. https://doi.org/10.25259/SNI_276_2021
- Hong, J.P., Kwon, J.G. (2022). Flaps in Plastic Surgery. In: Maruccia, M., Giudice, G. (eds) Textbook of Plastic and Reconstructive Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-82335-1_9
- Mazine, A., Hofer, S.O.P., & Yau, T.M. (2021). Infection, sternal debridement and muscle flap. In: Cheng, D.C., Martin, J., David, T. (eds) Evidence-Based Practice in Perioperative Cardiac Anesthesia and Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-47887-2_57
- McNichol, Ratliff, C., & Yates, S. (2021). Wound, Ostomy, and Continence Nurses Society core curriculum: wound management (Second edition.). Wolters Kluwer Health.
- Rohrer, Cook, J. L., & Kaufman, A. J. (Eds.). (2018). Flaps and grafts in dermatologic surgery (Second edition.). Elsevier.
- Saber AY, Hohman MH, Dreyer MA. (2022). Basic flap design. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563252/
- Strauch, B. (2016). Grabb’s encyclopedia of flaps. Volume II, Upper extremities, torso, pelvis, and lower extremities (4th ed.). Wolters Kluwer.
- Vincent, A., Sawhney, R., & Ducic, Y. (2019). Perioperative care of free flap patients. Seminars in plastic surgery, 33(1), 5–12. https://doi.org/10.1055/s-0038-1676824
- World Health Organization: WHO. (2019, May 13). Diabetes. https://www.who.int/health-topics/diabetes#tab=tab_
- Lizzo, J. M., Goyal, A., & Gupta, V. (2023, July 10). Adult diabetic ketoacidosis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560723/
- Diabetic ketoacidosis: MedlinePlus Medical Encyclopedia. (n.d.). https://medlineplus.gov/ency/article/000320.htm
- UpToDate. (n.d.). UpToDate. https://www.uptodate.com/contents/diabetic-ketoacidosis-in-children-clinical-features-and-diagnosis/print
- Virdi, N., Poon, Y., Abaniel, R., & Bergenstal, R. M. (2023). Prevalence, cost, and burden of diabetic ketoacidosis. Diabetes Technology & Therapeutics, 25(S3), S-84. https://doi.org/10.1089/dia.2023.0149
- Matthews, S., Coates, M. M., Bukhman, A., & Trujillo, C. (2023). Health system capacity to manage diabetic ketoacidosis in nine low-income and lower-middle income countries: a cross-sectional analysis of nationally representative survey data. eClinical Medicine, 55(101759). https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00488-6/fulltext#%20
- Virdi, N., Poon, Y., Abaniel, R., & Bergenstal, R. M. (2023b). Prevalence, cost, and burden of diabetic ketoacidosis. Diabetes Technology & Therapeutics, 25(S3), S-84. https://doi.org/10.1089/dia.2023.0149
- Lizzo, J. M., Goyal, A., & Gupta, V. (2023b, July 10). Adult diabetic ketoacidosis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560723/
- Ghimire, P., & Dhamoon, A. S. (2023, August 8). Ketoacidosis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK534848/#:~:text=Introduction-,Ketoacidosis%20is%20a%20metabolic%20state%20associated%20with%20pathologically%20high%20serum,individual%20cells%20in%20the%20body
- Hamdy, O., MD PhD. (n.d.). Diabetic Ketoacidosis (DKA): practice essentials, background, pathophysiology. https://emedicine.medscape.com/article/118361-overview?form=fpf
- JDRF. (2023, February 24). Signs and symptoms of Type 1 diabetes in Children – JDRF. https://www.jdrf.org/t1d-resources/about/symptoms/children/
- Diabetic ketoacidosis: MedlinePlus Medical Encyclopedia. (n.d.-b). https://medlineplus.gov/ency/article/000320.htm#:~:text=People%20with%20type%202%20diabetes,a%20severe%20illness%20or%20infection.
- Diabetic ketoacidosis – Symptoms & causes – Mayo Clinic. (2022, October 6). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/diabetic-ketoacidosis/symptoms-causes/syc-20371551
- Chukwuka, E., Johnson, D., Udoyen, A.-O., Egbunu, E. O., & Ogbuiyi-Chima, I. C. (2023). Comprehensive Review of Diabetic Ketoacidosis: An update. PMC PubMed Central, 85(6), 2802–2807. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10289692/
- Cavataio, M. M., & Packer, C. D. (2022). Steroid-Induced Diabetic Ketoacidosis: A case Report and Review of the literature. Cureus, 14(4), e24372. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9124452/#:~:text=Mechanisms%20for%20steroid%2Dinduced%20diabetes,translocation%20%5B9%2D10%5D.
- Mfa, J. H. M. M. (2021, August 18). What to know about Steroid-Induced Diabetes. Healthline. https://www.healthline.com/health/diabetes/steroid-induced-diabetes
- Professional, C. C. M. (n.d.). Hyperosmolar hyperglycemic state (HHS). Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/21147-hyperosmolar-hyperglycemic-state
- Ghimire, P., Dhamoon, A. S., & Doerr, C. (2023, August 8). Ketoacidosis (Nursing). StatPearls – NCBI Bookshelf. Retrieved March 22, 2024, from https://www.ncbi.nlm.nih.gov/books/NBK568717/
- Anion gap blood test. (n.d.). https://medlineplus.gov/lab-tests/anion-gap-blood-test/#:~:text=A%20high%20anion%20gap%20test,Dehydration
- Ghimire, P., Dhamoon, A. S., & Doerr, C. (2023b, August 8). Ketoacidosis (Nursing). StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK568717/
- Besen, B. a. M. P., Boer, W., & Honoré, P. M. (2021). Fluid management in diabetic ketoacidosis: new tricks for old dogs? Intensive Care Medicine, 47(11), 1312–1314. https://doi.org/10.1007/s00134-021-06527-7
- Jayashree, M., Williams, V., & Iyer, R. (2019). Fluid therapy for pediatric patients with diabetic ketoacidosis: current perspectives. PMC PubMed Central, 12, 2355–2361. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6858801/
- Lizzo, J. M., Goyal, A., & Gupta, V. (2023c, July 10). Adult diabetic ketoacidosis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560723/
- Professional, C. C. M. (n.d.-a). Cerebral edema (Brain swelling). Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/cerebral-edema-brain-swelling
- Hamdy, O., MD PhD. (n.d.-b). Diabetic Ketoacidosis (DKA) treatment & management: approach considerations, correction of fluid loss, insulin therapy. https://emedicine.medscape.com/article/118361-treatment#d13
- Intracranial pressure monitoring. (2024, January 1). PubMed. https://pubmed.ncbi.nlm.nih.gov/31194438/#:~:text=Excerpt,20%20to%2025%20mm%20Hg
- Ahmad, F., & Joshi, S. H. (2023). Self-Care practices and their Role in the control of Diabetes: A Narrative review. PubMed Central Cureus, 15 (7), e41409. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10402910/#:~:text=To%20reduce%20the%20burden%20of,glucose%20levels%20in%20the%20patients.
- Diabetes self-management training. (n.d.). Medicare. https://www.medicare.gov/coverage/diabetes-self-management-training
- Diabetic ketoacidosis. (2021, March 25). Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/diabetic-ketoacidosis.html
- Managing sick days | Diabetes | CDC. (n.d.). https://www.cdc.gov/diabetes/managing/flu-sick-days.html
- Mental Health and Diabetes | ADA. (n.d.). https://diabetes.org/health-wellness/mental-health
- Diabetes Distress Assessment & Resource Center. (n.d.). https://diabetesdistress.org/learn-about-dd/
- Dealing with diabetes burnout. (2022, June 20). Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/library/spotlights/diabetes-burnout.html
- Professional, C. C. M. (n.d.-b). Diabulimia. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/22658-diabulimia
- Bulimia nervosa – Symptoms and causes – Mayo Clinic. (2024, February 29). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/bulimia/symptoms-causes/syc-20353615
- Anniversary of the Inflation Reduction Act: Update on CMS Implementation | CMS. (2023, August 16). https://www.cms.gov/newsroom/fact-sheets/anniversary-inflation-reduction-act-update-cms-implementation#:~:text=Specifically%2C%20the%20Inflation%20Reduction%20Act,Traditional%20Medicare%2C%20or%20Medicare%20Advantage.
- International Diabetes Federation. (2024, February 7). Diabetes Facets and Figures | International Diabetes Federation. https://idf.org/about-diabetes/diabetes-facts-figures/
- Brown, A. (2021, August 14). Diabetes distress: why it’s common and what we can do about it! diaTribe. https://diatribe.org/diabetes-distress-why-it%E2%80%99s-common-and-what-we-can-do-about-it
- Luo, F., Chapel, G., Ye, Z., Jackson, S. L., & Roy, K. (2023). Labor income losses associated with heart disease and stroke from the 2019 panel Study of Income Dynamics. JAMA Network Open, 6(3), e232658. https://doi.org/10.1001/jamanetworkopen.2023.2658
- Coronary artery disease – symptoms and causes | Penn Medicine. (2023). https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/coronary-artery-disease
- Heart disease resources | Cdc.gov. (2023, May 15). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/about.htm
- NHS inform. (2023, November 9). Common heart conditions – Illnesses & conditions | NHS inform. NHS Inform. https://www.nhsinform.scot/illnesses-and-conditions/heart-and-blood-vessels/conditions/common-heart-conditions/
- Casas, R., Castro‐Barquero, S., Estrus, R., & Sacanella, E. (2018). Nutrition and cardiovascular health. International Journal of Molecular Sciences, 19(12), 3988. https://doi.org/10.3390/ijms19123988
- Coronary artery disease – Symptoms and causes – Mayo Clinic. (2022, May 25). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/coronary-artery-disease/symptoms-causes/syc-20350613
- NHS inform. (2023, November 9). Cardiovascular disease – Illnesses & conditions | NHS inform. NHS Inform. https://www.nhsinform.scot/illnesses-and-conditions/heart-and-blood-vessels/conditions/cardiovascular-disease/
- Data and Statistics on Congenital Heart Defects | CDC. (2023, September 19). Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/heartdefects/data.html
- Valvular heart Disease | Cdc.gov. (2019, December 9). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/valvular_disease.htm
- What is heart failure? | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/heart-failure
- Heart failure signs and symptoms. (2023, October 18). www.heart.org. https://www.heart.org/en/health-topics/heart-failure/warning-signs-of-heart-failure
- What is cardiomyopathy? | NHLBI, NIH. (2022, April 19). NHLBI, NIH. https://www.nhlbi.nih.gov/health/cardiomyopathy
- Cardiomyopathy | cdc.gov. (2023, February 21). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/cardiomyopathy.htm
- Khandaker, M. H., Espinosa, R. E., Nishimura, R. A., Sinak, L. J., Hayes, S. N., Melduni, R. M., & Oh, J. K. (2010). Pericardial Disease: Diagnosis and management. Mayo Clinic Proceedings, 85(6), 572–593. https://doi.org/10.4065/mcp.2010.0046
- What is an arrhythmia? | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/arrhythmias
- Atrial fibrillation | Cdc.gov. (2022, October 14). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- Harris, C., Croce, B., & Cao, C. (2016). ACS Patient Page Type A aortic dissection. Annals of Cardiothoracic Surgery, 5(4), 256. https://doi.org/10.21037/acs.2016.05.04
- Milner, R., MD. (2022, January 25). What is aortic dissection: What you need to know about the causes, types and more. UChicago Medicine. https://www.uchicagomedicine.org/forefront/heart-and-vascular-articles/silent-killer-everything-you-need-to-know-about-aortic-dissection
- World Health Organization: WHO. (2021, June 11). Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Gaziano, T. A., Bitton, A., Anand, S., Abrahams‐Gessel, S., & Murphy, A. (2010). Growing epidemic of coronary heart disease in Low- and Middle-Income countries. Current Problems in Cardiology, 35(2), 72–115. https://doi.org/10.1016/j.cpcardiol.2009.10.002
- Fuchs, F. D., & Whelton, P. K. (2020). High blood pressure and cardiovascular disease. Hypertension, 75(2), 285–292. https://doi.org/10.1161/hypertensionaha.119.14240
- Martín-Timón, I., Sevillano-Collantes, C., Segura-Galindo, A., & Del Cañizo-Gómez, F. J. (2014). Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World Journal of Diabetes, 5(4), 444. https://doi.org/10.4239/wjd.v5.i4.444
- Hajar, R. (2017). Risk factors for coronary artery disease: Historical perspectives. Heart Views, 18(3), 109. https://doi.org/10.4103/heartviews.heartviews_106_17
- Yazdanyar A., Newman A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009;25:563–577. doi: 10.1016/j.cger.2009.07.007
- Graham, G. (2015). Disparities in cardiovascular disease risk in the United States. Current Cardiology Reviews, 11(3), 238–245. https://doi.org/10.2174/1573403×11666141122220003
- Multifactorial inheritance and genetic disease | Learn Science at Scitable. (2023). https://www.nature.com/scitable/topicpage/multifactorial-inheritance-and-genetic-disease-919/
- Saheera, S., & Krishnamurthy, P. (2020). Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transplantation, 29, 096368972092083. https://doi.org/10.1177/0963689720920830
- What is Blood Cholesterol? | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/blood-cholesterol
- Diabetes, Heart Disease, & Stroke. (2023, June 7). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/heart-disease-stroke
- How smoking affects the heart and blood vessels | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/heart/smoking
- Benefits | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/heart/physical-activity/benefits
- Family Health History of Heart Disease | CDC. (2023). https://www.cdc.gov/genomics/disease/fh/history_heart_disease.htm
- Diab, A., Dastmalchi, L. N., Gulati, M., & Michos, E. D. (2023). A Heart-Healthy Diet for cardiovascular Disease Prevention: Where are we now? Vascular Health and Risk Management, Volume 19, 237–253. https://doi.org/10.2147/vhrm.s379874
- Increase the proportion of adults who do enough aerobic physical activity for substantial health benefits — PA02 – Healthy People 2030 | health.gov. (2023). https://health.gov/healthypeople/objectives-and-data/browse-objectives/physical-activity/increase-proportion-adults-who-do-enough-aerobic-physical-activity-substantial-health-benefits-pa-02
- Dineen-Griffin, S., García-Cárdenas, V., Williams, K., & Benrimoj, S. I. (2019). Helping patients help themselves: A systematic review of self-management support strategies in primary health care practice. PLOS ONE, 14(8), e0220116. https://doi.org/10.1371/journal.pone.0220116
- Moudatsou, M., Stavropoulou, A., Philalithis, Α., & Koukouli, S. (2020). The role of empathy in health and social care professionals. Healthcare, 8(1), 26. https://doi.org/10.3390/healthcare8010026
- Boyde, M., Tuckett, A. G., Peters, R., Thompson, D. R., Turner, C., & Stewart, S. (2009). Learning style and learning needs of heart failure patients (The Need2Know-HF patient study). European Journal of Cardiovascular Nursing, 8(5), 316–322. https://doi.org/10.1016/j.ejcnurse.2009.05.003
- Ricci, L., Villegente, J., Loyal, D., Ayav, C., Kivits, J., & Rat, A. (2021). Tailored patient therapeutic educational interventions: A patient‐centred communication model. Health Expectations, 25(1), 276–289. https://doi.org/10.1111/hex.13377
- Zhou, Q., & Liao, J. K. (2009). Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Current Pharmaceutical Design, 15(5), 467–478. https://doi.org/10.2174/138161209787315684
- Medical treatments for carotid artery disease. (2017, September 11). Stanford Health Care. https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/carotid-artery-disease/treatments/medical.html
- Types of blood pressure medications. (2023, June 7). www.heart.org. https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/types-of-blood-pressure-medications
- Coronary artery bypass graft Surgery. (2021, August 8). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/coronary-artery-bypass-graft-surgery
- Conditions treated by heart surgery | NHLBI, NIH. (2022, June 1). NHLBI, NIH. https://www.nhlbi.nih.gov/health/heart-surgery/conditions
- Community-based organizations encourage action to prevent heart disease and stroke. (2023). American Heart Association. https://newsroom.heart.org/news/community-based-organizations-encourage-action-to-prevent-heart-disease-and-stroke
- Medina, E. L., Filho, O. L. M., & Mesquita, C. T. (2013). Health Social Networks asOnlineLife Support Groups for Patients With Cardiovascular Diseases. Arquivos Brasileiros De Cardiologia. https://doi.org/10.5935/abc.20130161
- Corbett, J. A., Opladen, J., & Bisognano, J. D. (2020). Telemedicine can revolutionize the treatment of chronic disease. International Journal of Cardiology: Hypertension, 7, 100051. https://doi.org/10.1016/j.ijchy.2020.100051
- Kang, H. S., & Exworthy, M. (2022). Wearing the Future—Wearables to Empower users to take Greater responsibility for their health and care: Scoping review. Jmir Mhealth and Uhealth, 10(7), e35684. https://doi.org/10.2196/35684
- Research at AHA. (2023). https://professional.heart.org/en/research-programs
- National Heart, Lung, and Blood Institute (NHLBI). (2018, June 14). National Institutes of Health (NIH). https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-heart-lung-blood-institute-nhlbi
- Lee, J., & Cooke, J. P. (2011). The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis, 215(2), 281–283. https://doi.org/10.1016/j.atherosclerosis.2011.01.003
- Diab, A., Dastmalchi, L. N., Gulati, M., & Michos, E. D. (2023). A Heart-Healthy Diet for cardiovascular Disease Prevention: Where are we now? Vascular Health and Risk Management, Volume 19, 237–253. https://doi.org/10.2147/vhrm.s379874
- Cena, H., & Calder, P. C. (2020). Defining a Healthy Diet: Evidence for the role of contemporary dietary patterns in Health and disease. Nutrients, 12(2), 334. https://doi.org/10.3390/nu12020334
- Diab, A., Dastmalchi, L. N., Gulati, M., & Michos, E. D. (2023). A Heart-Healthy Diet for cardiovascular Disease Prevention: Where are we now? Vascular Health and Risk Management, Volume 19, 237–253. https://doi.org/10.2147/vhrm.s379874
- World Health Organization: WHO. (2019, June 11). Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
- Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004; 364:937–952. doi: 10.1016/S0140-6736(04)17018-9.
- Akesson A., Weismayer C., Newby P.K., Wolk A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch. Intern. Med. 2007; 167:2122–2127. doi: 10.1001/archinte.167.19.2122
- Ford E.S., Bergmann M.M., Kröger J., Schienkiewitz A., Weikert C., Boeing H. Healthy living is the best revenge: Findings from the European Perspective Investigation into Cancer and Nutrition-Potsdam study. Arch. Intern. Med. 2009; 169:1355–1362. doi: 10.1001/archinternmed.2009.237.
- Chiuve S.E., McCullough M.L., Sacks F.M., Rimm E.B. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: Benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006; 114:160–167. doi: 10.1161/CIRCULATIONAHA.106.621417
- National Library of Medicine. (2022). Heart diseases. https://medlineplus.gov/heartdiseases.html
- Sharifi‐Rad, J., Rodrigues, C. F., Sharopov, F., Docea, A. O., Karaça, A. C., Sharifi‐Rad, M., Karıncaoglu, D. K., Gülseren, G., Şenol, E., Demircan, E., Taheri, Y., Suleria, H. a. R., Özçelik, B., Kasapoğlu, K. N., Gültekin-Özgüven, M., Daşkaya-Dikmen, C., Cho, W. C., Martins, N., & Calina, D. (2020). Diet, Lifestyle and Cardiovascular Diseases: Linking pathophysiology to cardioprotective effects of natural bioactive compounds. International Journal of Environmental Research and Public Health, 17(7), 2326. https://doi.org/10.3390/ijerph17072326
- Chacko, M., Sarma, P. S., Harikrishnan, S., Zachariah, G., & Jeemon, P. (2020). Family history of cardiovascular disease and risk of premature coronary heart disease: A matched case-control study. Welcome Open Research, 5, 70. https://doi.org/10.12688/wellcomeopenres.15829.2
- Holvoet P. Stress in obesity and associated metabolic and cardiovascular disorders. Scientifica. 2012; 2012:205027. doi: 10.6064/2012/205027
- Casas, R., Castro‐Barquero, S., Estruch, R., & Sacanella, E. (2018). Nutrition and cardiovascular health. International Journal of Molecular Sciences, 19(12), 3988. https://doi.org/10.3390/ijms19123988
- Coronary artery disease – Symptoms and causes – Mayo Clinic. (2022, May 25). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/coronary-artery-disease/symptoms-causes/syc-20350613
- NHS inform. (2023, November 9). Cardiovascular disease – Illnesses & conditions | NHS inform. NHS Inform. https://www.nhsinform.scot/illnesses-and-conditions/heart-and-blood-vessels/conditions/cardiovascular-disease/
- Data and Statistics on Congenital Heart Defects | CDC. (2023, September 19). Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/heartdefects/data.html
- Valvular heart Disease | Cdc.gov. (2019, December 9). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/valvular_disease.htm
- What is heart failure? | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/heart-failure
- Heart failure signs and symptoms. (2023, October 18). www.heart.org. https://www.heart.org/en/health-topics/heart-failure/warning-signs-of-heart-failure
- What is cardiomyopathy? | NHLBI, NIH. (2022, April 19). NHLBI, NIH. https://www.nhlbi.nih.gov/health/cardiomyopathy
- Cardiomyopathy | cdc.gov. (2023, February 21). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/cardiomyopathy.htm
- Khandaker, M. H., Espinosa, R. E., Nishimura, R. A., Sinak, L. J., Hayes, S. N., Melduni, R. M., & Oh, J. K. (2010). Pericardial Disease: Diagnosis and management. Mayo Clinic Proceedings, 85(6), 572–593. https://doi.org/10.4065/mcp.2010.0046
- What is an arrhythmia? | NHLBI, NIH. (2022, March 24). NHLBI, NIH. https://www.nhlbi.nih.gov/health/arrhythmias
- Atrial fibrillation | Cdc.gov. (2022, October 14). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- Harris, C., Croce, B., & Cao, C. (2016). ACS Patient Page Type A aortic dissection. Annals of Cardiothoracic Surgery, 5(4), 256. https://doi.org/10.21037/acs.2016.05.04
- Milner, R., MD. (2022, January 25). What is aortic dissection: What you need to know about the causes, types and more. UChicago Medicine. https://www.uchicagomedicine.org/forefront/heart-and-vascular-articles/silent-killer-everything-you-need-to-know-about-aortic-dissection
- Yu, E., Rimm, E. B., Qi, L., Rexrode, K. M., Chen, A., Sun, Q., Willett, W. C., Hu, F. B., & Manson, J. E. (2016). Diet, lifestyle, biomarkers, genetic factors, and risk of cardiovascular disease in the nurses’ health studies. American Journal of Public Health, 106(9), 1616–1623. https://doi.org/10.2105/ajph.2016.303316
- Lichtenstein, A. H., Appel, L. J., Vadiveloo, M., Hu, F. B., Kris‐Etherton, P. M., Rebholz, C. M., Sacks, F. M., Thorndike, A. N., Van Horn, L., Wylie‐Rosett, J., & Biology, V. (2021). 2021 Dietary Guidance to Improve Cardiovascular Health: A scientific statement from the American Heart Association. Circulation, 144(23). https://doi.org/10.1161/cir.0000000000001031
- Schwingshackl L, Schwedhelm C, Hoffmann G, Boeing H. Potatoes, and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur J Nutr. 2019; 58:2243–2251. doi: 10.1007/s00394-018-1774-2
- Preventing heart disease. (2023, February 2). The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/disease-prevention/cardiovascular-disease/preventing-cvd/
- Tosh SM, Bordenave N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr Rev. 2020; 78(suppl 1):13–20. doi: 10.1093/nutrit/nuz085
- Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020; 382:20–28. doi: 10.1056/NEJMoa1817591
- Hardy DS, Garvin JT, Xu H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe, and Asia: a dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2020; 30:853–871. doi: 10.1016/j.numecd.2019.12.050
- Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, et al; American Heart Association. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017; 136: e1–e23. doi: 10.1161/CIR.0000000000000510
- Chiavaroli, L., Viguiliouk, E., Nishi, S. K., Mejía, S. B., Rahelić, D., Kahleová, H., Salas‐Salvadó, J., Kendall, C. W., & Sievenpiper, J. L. (2019). DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients, 11(2), 338. https://doi.org/10.3390/nu11020338
- Satija, A., & Hu, F. B. (2018). Plant-based diets and cardiovascular health. Trends in Cardiovascular Medicine, 28(7), 437–441. https://doi.org/10.1016/j.tcm.2018.02.004
- Wang DD, Hu FB. Dietary fat and risk of cardiovascular disease: recent controversies and advances. Annu Rev Nutr. 2017; 37:423–446. doi: 10.1146/annurev-nutr-071816-064614
- Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY; Trans Fat Conference Planning Group. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: report of the Trans Fat Conference Planning Group. Circulation. 2007; 115:2231–2246. doi: 10.1161/CIRCULATIONAHA.106.181947
- Heart-healthy diet: 8 steps to prevent heart disease. (2022, April 28). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/heart-disease/in-depth/heart-healthy-diet/art-20047702
- Bhattad, P. B., & Pacifico, L. (2022). Empowering patients: Promoting patient education and health literacy. Cureus. https://doi.org/10.7759/cureus.27336
- Pem, D. (2015, October 1). Fruit and Vegetable Intake: Benefits and Progress of Nutrition Education Interventions- Narrative Review article. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4644575/
- Marcus, C. (2014). Strategies for improving the quality of verbal patient and family education: a review of the literature and creation of the EDUCATE model. Health Psychology and Behavioral Medicine, 2(1), 482–495. https://doi.org/10.1080/21642850.2014.900450
- Woodgate, R. L., & Sigurdson, C. (2015). Building school-based cardiovascular health promotion capacity in youth: a mixed methods study. BMC Public Health, 15(1). https://doi.org/10.1186/s12889-015-1759-5
- Trichopoulou, A., Martínez‐González, M. A., Tong, T. Y., Forouhi, N. G., Khandelwal, S., Prabhakaran, D., Mozaffarian, D., & De Lorgeril, M. (2014). Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Medicine, 12(1). https://doi.org/10.1186/1741-7015-12-112
- Challa, H. J. (2023, January 23). DASH Diet to Stop Hypertension. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482514/
- Childs, C. E., Calder, P. C., & Miles, E. A. (2019). Diet and immune function. Nutrients, 11(8), 1933. https://doi.org/10.3390/nu11081933
- Centers for Disease Control and Prevention (CDC) (2023). Fast Facts: Preventing Adverse Childhood Experiences. https://www.cdc.gov/violenceprevention/aces/fastfact.html
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., Koss, M. P., & Marks, J. S. (1998). Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. https://doi.org/10.1016/S0749-3797(98)00017-8.
- Starecheski, L. (2015). Take the ACE quiz—And learn what it does and doesn’t mean. NPR. https://www.npr.org/sections/health-shots/2015/03/02/387007941/take-the-ace-quiz-and-learn-what-it-does-and-doesnt-mean
- Center on the Developing Child at Harvard University (n.d.). What are ACEs? And how do they relate to toxic stress? https://developingchild.harvard.edu/resources/aces-and-toxic-stress-frequently-asked-questions/
- CDC, (2022). About the CDC-Kaiser ACE study. https://www.cdc.gov/violenceprevention/aces/about.html
- CDC. (2021). Preventing adverse childhood experiences. https://www.cdc.gov/vitalsigns/aces/index.html
- CDC (2023). Risk and protective factors. https://www.cdc.gov/violenceprevention/aces/riskprotectivefactors.html
- Cleveland Clinic (2023). Adverse childhood experiences (ACEs) & childhood trauma. (2023). https://my.clevelandclinic.org/health/symptoms/24875-adverse-childhood-experiences-ace
- Merrick, M. T. (2019). Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention — 25 States, 2015–2017. Morbidity and Mortality Weekly Report, 68. https://doi.org/10.15585/mmwr.mm6844e1
- Child Trends. (2018). American Indians and Alaska Natives must be included in research on adverse childhood experiences. https://www.childtrends.org/blog/american-indians-alaska-natives-adverse-childhood-experiences
- Hughes, K., Bellis, M.A., Hardcastle, K.A., Sethi, D., Butchart, A., Mikton, C., Jones, L., Dunne, M.P. (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health; 2(8): e356-e366. doi: 10.1016/S2468-2667(17)30118-4.
- CDC (2023). Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Merrick, M. T., Ports, K. A., Ford, D. C., Afifi, T. O., Gershoff, E. T., & Grogan-Kaylor, A. (2017). Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse & Neglect, 69, 10–19. https://doi.org/10.1016/j.chiabu.2017.03.016
- Center on the Developing Child at Harvard University (n.d.). Take the ACE quiz – And learn what it does and doesn’t mean. https://developingchild.harvard.edu/media-coverage/take-the-ace-quiz-and-learn-what-it-does-and-doesnt-mean/
- National Conference of State Legislatures (2022). Adverse Childhood Experiences. https://www.ncsl.org/health/adverse-childhood-experience
- National Center for Missing & Exploited Children (2023). CyberTipline http://www.missingkids.org/content/ncmec/en/gethelpnow/cybertipline.html
- Dowdell, E. B., & Speck, P. M. (2022). Ce: Trauma-informed care in nursing practice. AJN, American Journal of Nursing, 122(4), 30–38. https://doi.org/10.1097/01.naj.0000827328.25341.1f
- Eilers, H., aan het Rot, M., & Jeronimus, B. F. (2023). Childhood trauma and adult somatic symptoms. Psychosomatic Medicine, 85(5), 408–416. https://doi.org/10.1097/psy.0000000000001208
- Fleishman, J., Kamsky, H., & Sundborg, S. (2019). Trauma-informed nursing practice. OJIN: The Online Journal of Issues in Nursing, 24(2). https://doi.org/10.3912/ojin.vol24no02man03
- Purkey, E., Patel, R., & Phillips, S. P. (2018). Trauma informed care better care for everyone. Canadian Family Physician, 64(3), 170–172.
- Schulman, M., & Menschner, C. (2018, November 9). Laying the groundwork for trauma-informed care [PDF]. Trauma-Informed Care Implementation Resource Center. https://www.traumainformedcare.chcs.org/wp-content/uploads/2018/11/Brief-Laying-the-Groundwork-for-TIC.pdf
- Sweeney, A., & Taggart, D. (2018). (Mis)understanding trauma-informed approaches in mental health. Journal of Mental Health, 27(5), 383–387. https://doi.org/10.1080/09638237.2018.1520973
- National Institute on Aging. (2021) What is Lewy Body Dementia? Causes, Symptoms, and Treatments. Retrieved on December 5, 2023, from https://www.nia.nih.gov/health/lewy-body-dementia/what-lewy-body-dementia-causes-symptoms-and-treatments
- Medline Plus [Internet] National Library of Medicine. (2021). Dementia with Lewy Bodies. Retrieved on December 5, 2023, from https://medlineplus.gov/genetics/condition/dementia-with-lewy-bodies/#causes
- Haider, A., Spurlin, B., and Sanchez-Manso, J. (2023). Lewy Body Dementia. In StatPearls. StatPearls [Internet]. Retrieved on December 7, 2023, from https://www.ncbi.nlm.nih.gov/books/NBK482441/
- Prasad, S., Katta, M., Abhishek, S., Sridhar, R., Valisekka, S., Hameed, M., Kaur, J., and Walia, N. (2022). Disease-a-Month. Vol. 69, Issue 5, May 2023, 101441. Retrieved from https://www.sciencedirect.com/science/article/pii/S0011502922001250?via%3Dihub
- Medline Plus [Internet] National Library of Medicine. (2019). Lewy Body Dementia. Retrieved on December 7, 2023, from https://medlineplus.gov/lewybodydementia.html
- Hershey, L. and Coleman-Jackson, R. (2019). Pharmacological Management of Dementia with Lewy Bodies. Drugs Aging. 2019; 36(4) 309-319. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6435621/
- National Institute on Aging. (2018). Caring for a Person with Lewy Body Dementia. Retrieved on December 21, 2023, from https://www.nia.nih.gov/health/lewy-body-dementia/caring-person-lewy-body-dementia
- Capouch, S., Farlow, M., and Brosch, J. (2018). A Review of Dementia with Lewy Bodies’ Impact, Diagnostic Criteria and Treatment. Neurology and Therapy. 2018 Dec; 7(2): 249-263. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6283803/
- Lewy Body Dementia Resource Center. (2021) Our Mission & Vision. Retrieved on December 21, 2023, from https://lewybodyresourcecenter.org/about-us/mission/
- Lewy Body Dementia Association. (2023). About LBDA. Retrieved on December 20, 2023 from https://www.lbda.org/about-lbda/
- National Institute of Neurological Disorders and Stroke. (2023) Focus on Lewy BOdy Dementia (LBD). Retrieved on December 20, 2023, from https://www.ninds.nih.gov/current-research/focus-disorders/alzheimers-disease-and-related-dementias/focus-lewy-body-dementia-lbd-research
- National Institute of Neurological Disorders and Stroke. (2018). PDBP About. Retrieved on December 21, 2023, from https://pdbp.ninds.nih.gov/about
- Bousiges, O. and Blanc, F. (2022). Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. International Journal of Molecular Sciences. 2022 Jun; 23 (12): 6371. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9223587/
- Howell, M. and Schenk, C. Rapid Eye movement sleep behavior disorder. 2023) UpToDate. Retrieved on December 21, 2023 from https://www.uptodate.com/contents/rapid-eye-movement-sleep-behavior-disorder/print#:~:text=INTRODUCTION%20Rapid%20eye%20movement%20(REM,thrashing%2C%20punching%2C%20and%20kicking.
- Arevalo, I., Smailagic, N., Roque I Figuls, M., Ciapponi, A., Sanchez-Perez, E., Giannakou, A., Pedraza, O., Cosp, X., and Cullum, S. (2015). Mini-Mental State Examination (MMSE) FOR THE DETECTION OF Alzheimer’s disease and other dementia in people with milk cognitive impairment (MCI). Cochrane Library. 2015 Mar; 2015(30): CD010783. Retrieved from https://doi.org/10.1002/14651858.CD010783.pub2
- National Institute on Aging. (2021). Diagnosing Lewy Body Dementia: For Professionals. Retrieved on December 22, 2023, from https://www.nia.nih.gov/health/lewy-body-dementia/diagnosing-lewy-body-dementia-professionals
- Anders, H., Ramesh, S., Ming-hui, Z., Ioannis, P., Salmon, J. E., & Mohan, C. (2020). Lupus nephritis (Primer). Nature Reviews: Disease Primers, 6(1). https://doi.org/10.1038/s41572-019-0141-9
- Arnaud, Laurent., & Vollenhoven, R. van. (2018). Advanced handbook of systemic lupus erythematosus. Adis. https://doi.org/10.1007/978-3-319-43035-5
- Hailemariam, F., Falkner, B. (2022). Renal Physiology for Primary Care Clinicians. In: McCauley, J., Hamrahian, S.M., Maarouf, O.H. (eds) Approaches to chronic kidney disease. Springer, Cham. https://doi.org/10.1007/978-3-030-83082-3_1
- Jaryal, A., & Vikrant, S. (2017). Current status of lupus nephritis. The Indian journal of medical research, 145(2), 167–178. https://doi.org/10.4103/ijmr.IJMR_163_16
- Kalantar-Zadeh, K., Mitch, W.E., Fadem, S.Z. (2022). Diet to preserve kidney function. In: Fadem, S.Z. (eds) Staying Healthy with Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-93528-3_7 Maarouf, O.H. (2022). Lupus nephritis. In: McCauley, J., Hamrahian, S.M., Maarouf, O.H. (eds) Approaches to Chronic Kidney Disease . Springer, Cham. https://doi.org/10.1007/978-3-030-83082-3_10
- Parodis, I., & Sjöwall, C. (Eds.). (2023). Clinical Features and Long-Term Outcomes of Systemic Lupus Erythematosus. MDPI – Multidisciplinary Digital Publishing Institute.
- Pryor, K. P., Barbhaiya, M., Costenbader, K. H., & Feldman, C. H. (2021). Disparities in Lupus and Lupus Nephritis Care and Outcomes Among US Medicaid Beneficiaries. Rheumatic diseases clinics of North America, 47(1), 41–53. https://doi.org/10.1016/j.rdc.2020.09.004
- Usman N, Annamaraju P. (2023). Type III hypersensitivity reaction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559122/
- Harris, J.C., Leggio, L., Farokhnia, M. (2021). Blood biomarkers of alcohol use: a scoping review. Current Addiction Reports, 8(4), 500-508. https://doi.org/10.1007/s40429-021-00402-7
- Johns Hopkins Medicine. (2023). CAGE substance abuse screening tool. Retrieved from: https://www.hopkinsmedicine.org/-/media/johns-hopkins-health-plans/documents/all_plans/cage-substance-screening-tool.pdf
- Nathan, P.E., Wallace, J., Zweben, J., Horvath, A. T. (2018). Understanding alcohol use disorders and their treatment. Retrieved from: https://www.apa.org/topics/substance-use-abuse-addiction/alcohol-disorders.
- National Cancer Institute. (2021). Alcohol and cancer risk. Retrieved from: https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet.
- National Institute on Alcohol Abuse and Alcoholism. (2023). Alcohol’s effects on the body. Retrieved from: https://www.niaaa.nih.gov/alcohols-effects-health/alcohols-effects-body.
- National Institute on Alcohol Abuse and Alcoholism. (2023). Goal 3: prevention. Retrieved from: https://www.niaaa.nih.gov/strategic-plan/prevention.
- National Institute on Alcohol Abuse and Alcoholism. (2023). Understanding alcohol use disorder. Retrieved from: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder.
- National Institute on Drug Abuse. (2023). AUDIT. Retrieved from: https://nida.nih.gov/sites/default/files/files/AUDIT.pdf.
- Nehring, S.M., Chen, R. J., Freeman A.M. (2023). Alcohol use disorder. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK436003/.
- Substance Abuse and Mental Health Services Administration, National Institute on Alcohol Abuse and Alcoholism. (2015). Medication for the treatment of alcohol use disorder: a brief guide. Retrieved from: https://store.samhsa.gov/sites/default/files/sma15-4907.pdf.
- Thompson, W., Lande, R. G., Kalapatapu, R.K. (2020). What are the DSM-5 criteria for alcohol use disorder? Retrieved from: https://www.medscape.com/answers/285913-41535/what-are-the-dsm-5-criteria-for-%20alcohol-use-disorder?form=fpf.
- World Health Organization. (2023). AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care. Retrieved from: https://www.who.int/publications/i/item/WHO-MSD-MSB-01.6a.
- American Society of Health-System Pharmacists. (2022, January 1). Fluvoxamine: Medlineplus drug information. MedlinePlus. https://medlineplus.gov/druginfo/meds/a695004.html#:~:text=Fluvoxamine%20is%20used%20to%20treat,that%20interferes%20with%20normal%20life).
- Chu, A. (2023, May 1). Selective serotonin reuptake inhibitors. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Cleveland Clinic. (2024). Vilazodone (Viibryd®): Uses & Side effects. https://my.clevelandclinic.org/health/drugs/20831-vilazodone-tablets
- DrugBank. (2024, March 6). Fluvoxamine. Uses, Interactions, Mechanism of Action | DrugBank Online. https://go.drugbank.com/drugs/DB00176
- Fornaro, M., Anastasia, A., Valchera, A., Carano, A., Orsolini, L., Vellante, F., Rapini, G., Olivieri, L., Di Natale, S., Perna, G., Martinotti, G., Di Giannantonio, M., & De Berardis, D. (2019). The FDA “black box” warning on antidepressant suicide risk in young adults: More harm than benefits? Frontiers in Psychiatry, 10. https://doi.org/10.3389/fpsyt.2019.00294
- Gasanli, E. (2023, December 12). Luvox (fluvoxamine): Uses, side effects, dosage, & more. Choosing Therapy. https://www.choosingtherapy.com/luvox-fluvoxamine/
- Huddart, R., Hicks, J. K., Ramsey, L. B., Strawn, J. R., Smith, D. M., Bobonis Babilonia, M., Altman, R. B., & Klein, T. E. (2020, February). PharmGKB summary: Sertraline pathway, pharmacokinetics. Pharmacogenetics and genomics. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7008964/
- Landy, K. (2023, November 10). Escitalopram. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557734/#article-60.s5
- Mauney, M. (2023, September 5). Paxil: Uses, black box warning, Recall & Alternatives. Drugwatch.com. https://www.drugwatch.com/ssri/paxil/#:~:text=The%20FDA%20issued%20a%20black,risks%20with%20the%20clinical%20need
- Mayo Clinic. (2024a, February 1). Fluoxetine (oral route) description and brand names. https://www.mayoclinic.org/drugs-supplements/fluoxetine-oral-route/description/drg-20063952
- Mayo Clinic. (2024b, February 1). Citalopram (oral route) description and brand names. https://www.mayoclinic.org/drugs-supplements/citalopram-oral-route/description/drg-20062980
- Mayo Clinic. (2024c, March 1). Paroxetine (oral route) description and brand names. https://www.mayoclinic.org/drugs-supplements/paroxetine-oral-route/description/drg-20067632
- Mayo Clinic. (2024d, March 1). Sertraline (oral route) precautions. https://www.mayoclinic.org/drugs-supplements/sertraline-oral-route/precautions/drg-20065940?p=1
- Mayo Clinic. (2024e, March 1). Escitalopram (oral route) description and brand names. https://www.mayoclinic.org/drugs-supplements/escitalopram-oral-route/description/drg-20063707
- Mayo Clinic. (2024f, March 1). Fluvoxamine (oral route) precautions. https://www.mayoclinic.org/drugs-supplements/fluvoxamine-oral-route/precautions/drg-20066874?p=1
- National Institutes of Health. (2023, December 20). Fluvoxamine. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/therapies/miscellaneous-drugs/fluvoxamine/#:~:text=Fluvoxamine%20is%20a%20selective%20serotonin,for%20other%20conditions%2C%20including%20depression.
- Plenge, P., Yang, D., Salomon, K., Laursen, L., Kalenderoglou, I. E., Newman, A. H., Gouaux, E., Coleman, J. A., & Loland, C. J. (2021, August 20). The Antidepressant Drug Vilazodone is an allosteric inhibitor of the serotonin transporter. Nature News. https://www.nature.com/articles/s41467-021-25363-3
- Shoar, N. S. (2023, November 7). Citalopram. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Shrestha, P. (2023, July 17). Paroxetine. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Singh, H. K. (2023, February 13). Sertraline. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- Sohel, A. J. (2022, July 4). Fluoxetine. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Ulrich, A. (2022, December 13). 7 fluoxetine (Prozac) interactions to watch out for. GoodRx. https://www.goodrx.com/fluoxetine/interactions
- UpToDate. (2024). Vilazodone: Drug information. Vilazodone: Drug Information. https://medilib.ir/uptodate/show/16102
- Agrawal, A., Kerndt, C.C., & Manna, B. (Updated 2023, April 19). Apixaban. In Stat Pearls. Stat Pearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK507910/
- American Heart Association. (2023, November 12). New Anti-clotting Medication Reduces Bleeding Among People with Atrial Fibrillation. Retrieved from https://newsroom.heart.org/news/new-anti-clotting-medication-reduces-bleeding-among-people-with-atrial-fibrillation
- Bentounes, N. K., Melicine, S., Martin, A. C., Smadja, D. M., & Gendron, N. (2023). Development of new anticoagulant in 2023: Prime time for anti-factor XI and XIa inhibitors. Journal de medecine vasculaire, 48(2), 69–80. https://doi.org/10.1016/j.jdmv.2023.04.002
- Centers for Disease Control and Prevention. (2023, June 28). Data and Statistics on Venous Thromboembolism. Retrieved from https://www.cdc.gov/ncbddd/dvt/data.html
- Centers for Disease Control and Prevention. (2023, June 28). Diagnosis and Treatment of Venous Thromboembolism. Retrieved from https://www.cdc.gov/ncbddd/diagnosis-treatment.html
- Chen, A., Stecker, E., & A Warden, B. (2020). Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. Journal of the American Heart Association, 9(13), e017559. https://doi.org/10.1161/JAHA.120.017559
- Hull, R.D., Garcia, D.A., Vazquez, S.R. (Updated 2023, January 24). Biology of Warfarin and Modulators of INR Control. UpToDate. Retrieved from https://www.uptodate.com/contents/biology-of-warfarin-and-modulators-of-inr-control
- Iqbal, A.M., Lopez, R.A., & Hai, O. (Updated 2022, November 7). Antiplatelet Medications. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537062/
- Leung, L.LK. (Updated 2023, July 27). Direct oral anticoagulants (DOACs) and parenteral direct-acting anticoagulants: Dosing and adverse effects. UpToDate. Retrieved from https://www.uptodate.com/contents/direct-oral-anticoagulants-doacs-and-parenteral-direct-acting-anticoagulants-dosing-and-adverse-effects/print
- Mahat, K.C., Sedhair, Y.R., Krishnan, P. (Updated 2023, August 4). Argatroban. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK555971/
- Patel, S., Singh, R., Preuss, C.V., and Patel, N. (Updated 2023, March 24). Warfarin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470313/
- Singh, R., & Emmady, P.D. (Updated 2023, April 17). Rivaroxaban. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557502/
- Solari, F., & Varacallo, M. (Updated 2023, August 4). Low-Molecular-Weight Heparin (LMWH). In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK525957/
- Umerah, C.O., & Momodu, I.I. (Updated 2023, July 17). Anticoagulation. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Warnock, L.B., & Huang, D. (Updated 2023, July 10). Heparin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK538247/
- Weitz, J.I. (Updated 2023, August 17). Investigational Anticoagulants. UpToDate. Retrieved from https://www.uptodate.com/contents/investigational-anticoagulants
- Bamalan, O.A., Moore, M.J., Al Khalili Y. (2024). Physiology, serotonin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK545168/
- Dhaliwal JS, Spurling BC, Molla M. (2023). Duloxetine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Fanelli, D., Weller, G., & Liu, H. (2021). New Serotonin-Norepinephrine Reuptake Inhibitors and Their Anesthetic and Analgesic Considerations. Neurology international, 13(4), 497–509. https://doi.org/10.3390/neurolint13040049
- Hussain LS, Reddy V, Maani CV. (2023) Physiology, Noradrenergic Synapse. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK540977/
- Li, J., Lu, C., Gao, Z, Feng, Y., Luo, H., Lu, T., Sun, X., Hu, J., & Luo, Y. (2020). SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology, 177, 108237, ISSN 0028-3908. https://doi.org/10.1016/j.neuropharm.2020.108237.
- Naseeruddin, R., Rosani, A., & Marwaha, R. (2023). Desvenlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534829/
- National Institute of Mental Health (NIH). (2022). Depression. Retrieved from https://www.nimh.nih.gov/health/publications/depression.
- Singh, D., & Saadabadi, A. (2022). Venlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Vallerand, A. H., & Sanoski, C. A. (2023). Davis’s drug guide for nurses (Eighteenth edition.). Philadelphia, PA. F. A. Davis Company. ISBN-10: 1-7196-4640-6. eISBN-10: 1-7196-4811-5.
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Venlafaxine. Access Pharmacy. Retrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426975.
- American Society of Health-System Pharmacists (ASHP). (2023). Dexamethasone (monograph). https://www.drugs.com/monograph/dexamethasone.html
- Dutt, M., Wehrle, C.J., & Jialal, I. (2024). Physiology, adrenal gland. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK537260/
- Chaudhuri, D., Nei, A. M., Rochwerg, B. et at. (2024). 2024 Focused update: Guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Critical Care Medicine ():10.1097/CCM.0000000000006172 https://journals.lww.com/ccmjournal/fulltext/9900/2024_focused_update__guidelines_on_use_of.275.aspx
- U.S. Food and Drug Administration. (2016). FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-label-changes-warn-rare-serious-neurologic-problems-after
- Hannoodee, S., & Nasuruddin, D.N. (2024). Acute inflammatory response. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK556083/
- Merck Manual. (2024). Drug information. Elsevier. https://www.merckmanuals.com/professional/drug-names-generic-and-brand
- Merck Manual. (2024). Corticosteroids: Uses and side effects. Elsevier. https://www.merckmanuals.com/home/multimedia/table/corticosteroids-uses-and-side-effects
- National Institutes of Health, U.S. Department of Health and Human Services. (2020). Autoimmunity may be rising in the United States. https://www.nih.gov/news-events/news-releases/autoimmunity-may-be-rising-united-states
- National Institutes of Health, U.S. Department of Health and Human Services. (2023). Therapeutic management of hospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults–therapeutic-management/
- National Institutes of Health, U.S. Department of Health and Human Services. (2023). Therapeutic management of nonhospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults–therapeutic-management/
- Oronsky, B., Caroen, S., & Reid, T. (2022). What exactly is inflammation (and what is it not?). International Journal of Molecular Sciences, 23(23), 14905. https://doi.org/10.3390/ijms232314905
- Sen, S. K. (2024). Messenger RNA (MRNA). National Human Genome Research Institute. https://www.genome.gov/genetics-glossary/messenger-rna
- World Health Organization. (2020). Corticosteroids for COVID-19: Living guidance. https://iris.who.int/bitstream/handle/10665/334125/WHO-2019-nCoV-Corticosteroids-2020.1-eng.pdf?sequence=1%26isAllowed=y
- Desai, D. S. (2023, June 5). Arrhythmias. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK558923/
- Benjamin, E. J., Wolf, P. A., D’Agostino, R. B., Silbershatz, H., Kannel, W. B., & Levy, D. (1998). Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation, 98(10), 946-952.
- Atrial fibrillation | Cdc.gov. (2022, October 14). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- King, G. S. (2023, February 19). Antiarrhythmic medications. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482322/
- Saljic, A., Heijman, J., & Dobrev, D. (2023). Recent Advances in Antiarrhythmic Drug Therapy. Drugs, 83(13), 1147–1160. https://doi.org/10.1007/s40265-023-01923-3
- Kowey, P. R., & Robinson, V. M. (2020). The relentless pursuit of new drugs to treat cardiac arrhythmias. Circulation, 141(19), 1507–1509. https://doi.org/10.1161/circulationaha.119.045149
- Wright, K. N. (2009). Antiarrhythmic agents. In Elsevier eBooks (pp. 807–810). https://doi.org/10.1016/b978-1-4160-2591-7.10190-0
- Geng, M., Lin, A., & Nguyen, T. P. (2020). Revisiting Antiarrhythmic Drug Therapy for Atrial Fibrillation: Reviewing Lessons Learned and Redefining Therapeutic Paradigms. Frontiers in pharmacology, 11, 581837. https://doi.org/10.3389/fphar.2020.581837
- Sharma, A.K., Singh, S., Bhat, M. et al. new drug discovery of cardiac anti-arrhythmic drugs: insights in animal models. Sci Rep 13, 16420 (2023). https://doi.org/10.1038/s41598-023-41942-4
- Heart arrhythmias. (2024). Physiopedia. https://www.physio-pedia.com/Heart_Arrhythmias
- Dan, G., Martínez‐Rubio, A., Agewall, S., Boriani, G., Borggrefe, M., Gaïta, F., Van Gelder, I. C., Görenek, B., Kaski, J. C., Kjeldsen, K., Lip, G. Y., Merkely, B., Okumura, K., Piccini, J. P., Potpara, T., Poulsen, B. K., Saba, M., Savelieva, I., Tamargo, J., & Wolpert, C. (2018). Antiarrhythmic drugs–clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace, 20(5), 731–732an. https://doi.org/10.1093/europace/eux373
- Heart arrhythmia – Symptoms and causes – Mayo Clinic. (2023, October 13). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/heart-arrhythmia/symptoms-causes/syc-20350668
- Arrhythmias and congenital defects. (2023, June 15). www.heart.org. https://www.heart.org/en/health-topics/congenital-heart-defects/the-impact-of-congenital-heart-defects/arrhythmias-and-congenital-defects
- CV Pharmacology | Vaughan-Williams Classification of Antiarrhythmic Drugs. (2024). https://cvpharmacology.com/antiarrhy/vaughan-williams
- Lei, M., Wu, L., Terrar, D. A., & Huang, C. L. (2018). Modernized classification of cardiac antiarrhythmic drugs. Circulation, 138(17), 1879–1896. https://doi.org/10.1161/circulationaha.118.035455
- Custodis F, Schirmer SH, Baumhäkel M, et al. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 2010; 56:1973–83. 10.1016/j.jacc.2010.09.014
- Kaski, J. C., Gloekler, S., Ferrari, R., Fox, K., Lévy, B., Komajda, M., Vardas, P., & Camici, P. G. (2018). Role of ivabradine in management of stable angina in patients with different clinical profiles. Open Heart, 5(1), e000725. https://doi.org/10.1136/openhrt-2017-000725
- Salvage, S. C., Chandrasekharan, K. H., Jeevaratnam, K., Dulhunty, A. F., Thompson, A. J., Jackson, A. P., & Huang, C. L. (2018). Multiple targets for flecainide action: implications for cardiac arrhythmogenesis. British journal of pharmacology, 175(8), 1260–1278. https://doi.org/10.1111/bph.13807
- Bazoukis, G., Tse, G., Letsas, K. P., Thomopoulos, C., Naka, K. K., Korantzopoulos, P., Bazoukis, X., Michelongona, P., Papadatos, S. S., Vlachos, K., Liu, T., Efremidis, M., Baranchuk, A., Stavrakis, S., & Tsioufis, C. (2018). Impact of ranolazine on ventricular arrhythmias – A systematic review. Journal of arrhythmia, 34(2), 124–128. https://doi.org/10.1002/joa3.12031
- Rahman, M. F. F. (2023, July 24). Atrioventricular dissociation. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK563205/
- Perera, R. K., Fischer, T. H., Wagner, M., Dewenter, M., Vettel, C., Bork, N. I., Maier, L. S., Conti, M., Wess, J., El-Armouche, A., Hasenfuß, G., & Nikolaev, V. O. (2017). Atropine augments cardiac contractility by inhibiting cAMP-specific phosphodiesterase type 4. Scientific reports, 7(1), 15222. https://doi.org/10.1038/s41598-017-15632-x
- Singh, S. (2023, August 28). Adenosine. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK519049/
- Patti, L. (2023, August 7). Supraventricular tachycardia. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK441972/
- McIntyre, W. F., Healey, J. S., Bhatnagar, A. K., Wang, P., Gordon, J. A., Baranchuk, A., Deif, B., Whitlock, R. P., & Belley-Côté, É. P. (2019). Vernakalant for cardioversion of recent-onset atrial fibrillation: a systematic review and meta-analysis. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 21(8), 1159–1166. https://doi.org/10.1093/europace/euz175
- Ahmed L. A. (2019). Nicorandil: A drug with ongoing benefits and different mechanisms in various diseased conditions. Indian journal of pharmacology, 51(5), 296–301. https://doi.org/10.4103/ijp.IJP_298_19
- PubChem. (2024). N-(p-Amylcinnamoyl) anthranilic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/N-_p-Amylcinnamoyl_anthranilic-acid
- Salcher, S., Spoden, G. A., Hagenbuchner, J., Führer, S., Kaserer, T., Tollinger, M., Huber-Cantonati, P., Gruber, T., Schuster, D., Gust, R., Zwierzina, H., Müller, T., Kiechl‐Kohlendorfer, U., Ausserlechner, M. J., & Obexer, P. (2019). A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene, 39(5), 1080–1097. https://doi.org/10.1038/s41388-019-1044-7
- Le, V. T., Knight, S., Watrous, J. D., Najhawan, M., Dao, K., McCubrey, R. O., Bair, T. L., Horne, B. D., May, H. T., Muhlestein, J. B., Nelson, J. R., Carlquist, J. F., Knowlton, K. U., Jain, M., & Anderson, J. L. (2023). Higher docosahexaenoic acid levels lower the protective impact of eicosapentaenoic acid on long-term major cardiovascular events. Frontiers in cardiovascular medicine, 10, 1229130. https://doi.org/10.3389/fcvm.2023.1229130
- Alcocer, L., Bryce, A., Brasil, D. P., Terán, J. L., Cortés, J. E., Quesada, D., & Rodríguez, P. (2023). The pivotal role of Angiotensin-Converting enzyme inhibitors and Angiotensin II receptor blockers in hypertension management and cardiovascular and renal Protection: A Critical appraisal and comparison of international guidelines. American Journal of Cardiovascular Drugs, 23(6), 663–682. https://doi.org/10.1007/s40256-023-00605-5
- Kornej, J., Börschel, C. S., Benjamin, E. J., & Schnabel, R. B. (2020). Epidemiology of Atrial fibrillation in the 21st century. Circulation Research, 127(1), 4–20. https://doi.org/10.1161/circresaha.120.316340
- O’Brien, T. J., Fenton, K., Sidahmed, A., Barbour, A., & Harralson, A. F. (2021). Race and Drug Toxicity: A Study of Three Cardiovascular Drugs with Strong Pharmacogenetic Recommendations. Journal of Personalized Medicine, 11(11), 1226. https://doi.org/10.3390/jpm11111226
- Joukar S. (2021). A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and humans: extrapolation of experimental insights to clinic. Laboratory animal research, 37(1), 25. https://doi.org/10.1186/s42826-021-00102-3
- Chrysafides, S. M. (2023, April 10). Physiology, resting potential. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538338/
- Wei, X. (2023, April 17). Physiology, cardiac repolarization dispersion and reserve. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537194/
- Pirahanchi, Y. (2023, March 13). Physiology, Sodium potassium pump. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537088/
- Goyal, A. (2023, July 4). Reentry arrhythmia. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537089/
- An, Z., Yang, G., Liu, X., Zhang, Z., & Liu, G. (2018). New progress in understanding the cellular mechanisms of anti-arrhythmic drugs. Central European Journal of Biology, 13(1), 335–339. https://doi.org/10.1515/biol-2018-0041
- Osadchii, O. E. (2017). Effects of Na+ channel blockers on the restitution of refractory period, conduction time, and excitation wavelength in perfused guinea-pig heart. PLOS ONE, 12(2), e0172683. https://doi.org/10.1371/journal.pone.0172683
- Wołowiec, Ł., Grześk, G., Osiak, J., Wijata, A., Mędlewska, M., Gaborek, P., Banach, J., Wołowiec, A., & Głowacka, M. (2023). Beta-blockers in cardiac arrhythmias–Clinical pharmacologist’s point of view. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.1043714
- Naji, A. (2023, May 8). Muscarinic antagonists. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK557541/
- Marcu, D., Adam, C. A., Dorobantu, D. M., Șalaru, D. L., Sascău, R. A., Balasanian, M., Macovei, L., Arsenescu-Georgescu, C., & Stătescu, C. (2022). Beta-Blocker-Related Atrioventricular Conduction Disorders—A single tertiary referral Center experience. Medicina-lithuania, 58(2), 320. https://doi.org/10.3390/medicina58020320
- Westergaard, L. M., Alhakak, A., Rørth, R., Fosbøl, E. L., Kristensen, S. L., Svendsen, J. H., Graff, C., Jb, N., Gislason, G., Køber, L., Torp‐Pedersen, C., Lee, C. J., & Weeke, P. (2023). Ventricular rate in atrial fibrillation and the risk of heart failure and death. Europace, 25(5). https://doi.org/10.1093/europace/euad088
- Grubb A, Mentz RJ. Pharmacological management of atrial fibrillation in patients with heart failure with reduced ejection fraction: review of current knowledge and future directions. Expert Rev Cardiovasc Ther. 2020 Feb;18(2):85-101
- David, M. N. V. (2023, January 19). Digoxin. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK556025/
- Alqahtani, M. S., Kazi, M., Alsenaidy, M. A., & Ahmad, M. Z. (2021). Advances in oral drug delivery. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.618411
- Cheng, L., & Wong, H. (2020). Food effects on oral drug absorption: Application of Physiologically Based Pharmacokinetic Modeling as a predictive tool. Pharmaceutics, 12(7), 672. https://doi.org/10.3390/pharmaceutics12070672
- Herman, T. F. (2023, November 3). First-Pass effect. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK551679/
- Mar, P. L., Horbal, P., Chung, M. K., Dukes, J. W., Ezekowitz, M. D., Lakkireddy, D., Lip, G. Y. H., Miletello, M., Noseworthy, P. A., Reiffel, J. A., Tisdale, J. E., Olshansky, B., & Gopinathannair, R. (2022). Drug interactions affecting antiarrhythmic drug use. Circulation: Arrhythmia and Electrophysiology, 15(5). https://doi.org/10.1161/circep.121.007955
- Florek, J. B. (2023, November 12). Amiodarone. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482154/
- Dean, L. (2017, April 4). Propafenone therapy and CYP2D6 genotype. Medical Genetics Summaries – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK425391/
- Rollason, V., Lloret‐Linares, C., Lorenzini, K. I., Daali, Y., Gex‐Fabry, M., Piguet, V., Besson, M., Samer, C. F., & Desmeules, J. A. (2020). Evaluation of phenotypic and genotypic variations of drug metabolizing enzymes and transporters in chronic pain patients facing adverse drug reactions or Non-Response to analgesics: a retrospective study. Journal of Personalized Medicine, 10(4), 198. https://doi.org/10.3390/jpm10040198
- Chu, R. (2020, October 10). Intravenous lidocaine infusion for the management of early postoperative pain: A Comprehensive review of controlled trials. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7901134/
- Weibel, S., Jelting, Y., Pace, N. L., Helf, A., Eberhart, L., Hahnenkamp, K., Hollmann, M. W., Poepping, D., Schnabel, A., & Kranke, P. (2018). Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. The Cochrane Library, 2018(6). https://doi.org/10.1002/14651858.cd009642.pub3
- Larson, J., Rich, L., Deshmukh, A., Judge, E. C., & Liang, J. J. (2022). Pharmacologic Management for Ventricular arrhythmias: Overview of Anti-Arrhythmic Drugs. Journal of Clinical Medicine, 11(11), 3233. https://doi.org/10.3390/jcm11113233
- Tun, M. M., Pandey, S., Adhikari, S., Mainali, A., Thapa, A., Bisural, R., Bista, P. B., Htet, S. Y., Chhetri, B., & Panigrahi, K. (2023). Amiodarone-Induced liver attenuation on CT scan: alarming signal for toxicity and prompt discontinuation. Cureus. https://doi.org/10.7759/cureus.39844
- Sasi, N., MD. (2024). Medscape Registration. https://emedicine.medscape.com/article/813046-overview?form=fpf
- Ohyama, K., Nakajima, M., Suzuki, M., Shimada, N., Yamazaki, H., & Yokoi, T. (2000). Inhibitory effects of amiodarone and its N‐deethylated metabolite on human cytochrome P450 activities: Prediction of in vivo drug interactions. British Journal of Clinical Pharmacology, 49(3), 244–253. https://doi.org/10.1046/j.1365-2125.2000.00134.x
- Beavers, C. J., Rodgers, J. E., Bagnola, A., Beckie, T. M., Campia, U., Di Palo, K. E., Okwuosa, T. M., Przespolewski, E., & Dent, S. (2022). Cardio-Oncology Drug Interactions: A scientific statement from the American Heart Association. Circulation, 145(15). https://doi.org/10.1161/cir.0000000000001056
- Kamaraju, S., Mohan, M., Zaharova, S., Wallace, B., McGraw, J., Lokken, J., Tierney, J. F., Weil, E., Fatunde, O., & Brown, S. (2021). Interactions between cardiology and oncology drugs in precision cardio-oncology. Clinical Science, 135(11), 1333–1351. https://doi.org/10.1042/cs20200309
- Chakraborty, R. K. (2023, July 28). Calcium channel blocker toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537147/
- Konieczny, K. M., & Dorian, P. (2019). Clinically important Drug–Drug interactions between antiarrhythmic drugs and anticoagulants. The Journal of Innovations in Cardiac Rhythm Management, 0(3), 3552–3559. https://doi.org/10.19102/icrm.2019.100304
- Ferri, N., Colombo, E., Tenconi, M., Baldessin, L., & Corsini, A. (2022). Drug-Drug interactions of direct oral anticoagulants (DOACs): From pharmacological to clinical practice. Pharmaceutics, 14(6), 1120. https://doi.org/10.3390/pharmaceutics14061120
- Hügl, B., Horlitz, M., Fischer, K., & Kreutz, R. (2023). Clinical significance of the rivaroxaban–dronedarone interaction: insights from physiologically based pharmacokinetic modelling. European Heart Journal Open, 3(1). https://doi.org/10.1093/ehjopen/oead004
- Lucà, F., Oliva, F., Abrignani, M. G., Di Fusco, S. A., Parrini, I., Canale, M. L., Giubilato, S., Cornara, S., Nesti, M., Rao, C. M., Pozzi, A., Binaghi, G., Maloberti, A., Ceravolo, R., Bisceglia, I., Rossini, R., Temporelli, P. L., Amico, A., Calvanese, R., . . . Gulizia, M. M. (2023). Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. Journal of Clinical Medicine, 12(18), 5955. https://doi.org/10.3390/jcm12185955
- Wiggins, B. S., Dixon, D. L., Neyens, R., Page, R. L., & Gluckman, T. J. (2020). Select Drug-Drug interactions with direct oral anticoagulants. Journal of the American College of Cardiology, 75(11), 1341–1350. https://doi.org/10.1016/j.jacc.2019.12.068
- Tovia-Brodie, O. (2020). Pharmacological therapy in Brugada syndrome. Radcliffe Cardiology. https://www.aerjournal.com/articles/pharmacological-therapy-brugada-syndrome
- Sanchez‐Nadales, A., Anampa-Guzmán, A., & Khan, A. (2019). Disopyramide for hypertrophic cardiomyopathy. Cureus. https://doi.org/10.7759/cureus.4526
- Blotner, M., Betageri, O., Miles, W. M., & Kong, X. (2022). WorkUp for Suspected Brugada Syndrome: Two case reports for the general practitioner. Cureus. https://doi.org/10.7759/cureus.21921
- Chhabra, L. (2023, August 7). Wolff-Parkinson-White Syndrome. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK554437/
- Bohnen, M. S., Peng, G., Robey, S., Terrenoire, C., Iyer, V., Sampson, K. J., & Kass, R. S. (2017). Molecular Pathophysiology of Congenital Long QT Syndrome. Physiological Reviews, 97(1), 89–134. https://doi.org/10.1152/physrev.00008.2016
- Narayanan, K. (2020). Strategies for rhythm control in Atrial fibrillation. Indian Journal of Clinical Cardiology, 1(2), 94–107. https://doi.org/10.1177/2632463620936018
- Arunachalam, K. (2023, August 8). Flecainide. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK542291/
- Sreenivas, S. (2021, March 3). Beta-Blocker Medications for AFIB. WebMD. https://www.webmd.com/heart-disease/atrial-fibrillation/beta-blocker-medications-afib
- Viskin, S., Chorin, E., Viskin, D., Hochstadt, A., Schwartz, A. L., & Rosso, R. (2021). Polymorphic Ventricular Tachycardia: Terminology, mechanism, diagnosis, and emergency therapy. Circulation, 144(10), 823–839. https://doi.org/10.1161/circulationaha.121.055783
- Muser, D., Tritto, M., Mariani, M. V., Di Monaco, A., Compagnucci, P., Accogli, M., De Ponti, R., & Guarracini, F. (2021). Diagnosis and treatment of idiopathic Premature ventricular contractions: A stepwise approach based on the site of origin. Diagnostics, 11(10), 1840. https://doi.org/10.3390/diagnostics11101840
- Khachatryan, A., Merino, J. L., De Abajo, F. J., Botto, G. L., Kirchhof, P., Breithardt, G., Stambler, B. S., Abenhaim, L., & Grimaldi-Bensouda, L. (2021). International cohort study on the effectiveness of dronedarone and other antiarrhythmic drugs for atrial fibrillation in real-world practice (EFFECT-AF). Europace, 24(6), 899–909. https://doi.org/10.1093/europace/euab262
- Clinical use of dofetilide – UpToDate. (2024). UpToDate. https://www.uptodate.com/contents/clinical-use-of-dofetilide
- Mubarik, A. (2022, June 10). Sotalol. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK534832/
- Szymanski, M. W. (2023, April 3). Ibutilide. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK526021/
- Phillips, C. T., Wang, J., Celi, L. A., Zhang, Z., & Feng, M. (2019). Association of hypokalemia with an increased risk for medically treated arrhythmias. PLOS ONE, 14(6), e0217432. https://doi.org/10.1371/journal.pone.0217432
- PROPAFENONE HYDROCHLORIDE TABLETS. (2024). https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a7c8f090-c48c-44f7-9973-2cf4b491e35c&type=display
- Intro_to_antiarrhythmics [TUSOM | Pharmwiki]. (2024). https://tmedweb.tulane.edu/pharmwiki/doku.php/intro_to_antiarrhythmics
- Singh, S. (2023, August 28). Adenosine. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK519049/
- Alobaida, M., & Alrumayh, A. (2021). Rate control strategies for atrial fibrillation. Annals of Medicine, 53(1), 682–692. https://doi.org/10.1080/07853890.2021.1930137
- Fatima, N., Mandava, K., Khatoon, F., Badar, J., Begum, S. F., Narasimhan, C., Daljeet, K., & Mohammed, W. (2022). CLInical Profile and Side Effects of chronic use of oral Amiodarone in cardiology outpatient department (CLIPSE-A Study)- A prospective observational study. Annals of Medicine and Surgery, 80. https://doi.org/10.1016/j.amsu.2022.104167
- Dunn, J. (2023, February 13). Physiology, adenosine triphosphate. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK553175/
- Lavonas, E. J., Akpunonu, P., Arens, A. M., Babu, K. M., Cao, D., Hoffman, R. S., Hoyte, C., Mazer‐Amirshahi, M., Stolbach, A., St‐Onge, M., Thompson, T. M., Wang, G. S., Hoover, A. V., & Drennan, I. R. (2023). 2023 American Heart Association Focused Update on the Management of Patients with Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation, 148(16). https://doi.org/10.1161/cir.0000000000001161
- Rehman, R. (2023, May 1). Digitalis toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK459165/
- Pincus M. (2016). Management of digoxin toxicity. Australian prescriber, 39(1), 18–20. https://doi.org/10.18773/austprescr.2016.006
- Crosby, J., Bhopalwala, H., Kharawala, A., Dewaswala, N., Ganti, S. S., & Bhopalwala, A. (2021). Refractory Torsades de Pointes Due to Dofetilide Overdose. Journal of Investigative Medicine High Impact Case Reports, 9, 232470962110564. https://doi.org/10.1177/23247096211056492
- Khalid, M. M. (2023, July 28). Beta-Blocker toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK448097/
- Bologa, C., Lionte, C., Popescu, A., Sorodoc, V., & Sorodoc, L. (2021). First Case of Acute Poisoning with Amiodarone and Flecainide in Attempted Suicide Successfully Managed with Lipid Emulsion Therapy in the Emergency Department: Case Report and Literature Review. Healthcare (Basel, Switzerland), 9(6), 671. https://doi.org/10.3390/healthcare9060671
- Lei, M., Wu, L., Terrar, D. A., & Huang, C. L. (2018). Modernized Classification of Cardiac Antiarrhythmic Drugs. Circulation, 138(17), 1879–1896. https://doi.org/10.1161/CIRCULATIONAHA.118.035455
- Hakkola, J., Hukkanen, J., Turpeinen, M., & Pelkonen, O. (2020). Inhibition and induction of CYP enzymes in humans: an update. Archives of Toxicology, 94(11), 3671–3722. https://doi.org/10.1007/s00204-020-02936-7
- Azadfard , M., Huecker , M., & Leaming , J. (2023, April 29). Opioid addiction – statpearls – NCBI bookshelf. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448203/
- Campbell, J. N. (1996, March). APS 1995 Presidential address. APS 1995 Presidential address. In Pain Forum (Vol. 5, No. 1, pp. 85-88). .
- Chou , R., Deyo , R., Devine, B., Hansen, R., Sullivan, S., Jarvik , J., Blazina , I., Dana, T., Bougatsos, C., & Turner, J. (2014, September). The effectiveness and risks of long-term opioid treatment of chronic pain. The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain | Effective Health Care (EHC) Program. https://effectivehealthcare.ahrq.gov/products/chronic-pain-opioid-treatment
- Department of Health and Human Services Pain Management Best Practices Inter-Agency Task Force. (2019) Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. Final Report. Washington, DC: Content created by Assistant Secretary for Health (ASH); 2019. https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html;
- Dowell, D., Ragan, K. R., Jones, C. M., Baldwin , G. T., & Chou, R. (2022, December 1). Prescribing opioids for pain — the new CDC clinical practice guideline … New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMp2211040
- Dowell, D., Ragan, K. R., Jones, C. M., Baldwin, G. T., & Chou, R. (2022). CDC Clinical Practice Guideline for prescribing opioids for pain, United States, 2022. MMWR. Recommendations and Reports, 71(3), 1–95. https://doi.org/10.15585/mmwr.rr7103a1
- Dydyk AM, Sizemore DC, Haddad LM, Lindsay L, & Porter BR. (2023, January 29). NP safe prescribing of controlled substances while avoiding drug diversion. National Center for Biotechnology Information. https://pubmed.ncbi.nlm.nih.gov/33232099/
- Dydyk, A. M., & Conermann, T. (2023, July 23). Chronic pain – statpearls – NCBI bookshelf. StatPearls . https://www.ncbi.nlm.nih.gov/books/NBK553030/
- Federal Controlled Substance Act 21 USC 844 (1970).
- James, A., & Williams, J. (2020). Basic opioid pharmacology — an update. British Journal of Pain, 14(2), 115–121. https://doi.org/10.1177/2049463720911986
- Loeser, J. D., & Melzack, R. (1999). Pain: An overview. The Lancet, 353(9164), 1607–1609. https://doi.org/10.1016/s0140-6736(99)01311-2
- Mayo Clinic Staff. (2022, April 12). Am I vulnerable to opioid addiction?. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/in-depth/how-opioid-addiction-occurs/art-20360372?p=1
- Medical Board of California. (2023). Guideline for Prescribing Controlled Substances for Pain. Medical Board of California. July, 2023. https://www.mbc.ca.gov/Download/Publications/pain-guidelines.pdf
- Newson, G. G. (2004, December 4). Criteria for furnishing number utilization by Nurse Practitioners . State of California Department of Consumer Affairs. https://www.rn.ca.gov/pdfs/regulations/npr-i-16.pdf
- PDR Search. PDR.Net. (n.d.). https://www.pdr.net/
- Smith, H. J. (2020). Ethics, public health, and addressing the opioid crisis. AMA Journal of Ethics, 22(8). https://doi.org/10.1001/amajethics.2020.647
- Spencer, M., Miniño, A., & Warner, M. (2022). Drug overdose deaths in the United States, 2001–2021. NCHS Data Brief, (457). https://doi.org/10.15620/cdc:122556.
- Tighe, P., Buckenmaier, C. C., Boezaart, A. P., Carr, D. B., Clark, L. L., Herring, A. A., Kent, M., Mackey, S., Mariano, E. R., Polomano, R. C., & Reisfield, G. M. (2015). Acute pain medicine in the United States: A status report. Pain Medicine, 16(9), 1806–1826. https://doi.org/10.1111/pme.12760
- Trachsel, L. A., Munakomi, S., & Cascella, M. (2023, April 17). Pain theory: Treatment & management: Point of care. StatPearls. https://www.statpearls.com/point-of-care/26535
- U.S. Department of Justice Drug Enforcement Administration, Diversion Control Division. (2023, July). Controlled Substance Schedules. Controlled substance schedules. https://www.deadiversion.usdoj.gov/schedules/
Suggested Readings:
Benzon, H. T., Sun, E. C., & Chou, R. (2022). The opioid crisis, Centers for Disease Control Opioid guideline, and naloxone coprescription for patients at risk for opioid overdose. Anesthesia & Analgesia, 135(1), 21–25. https://doi.org/10.1213/ane.0000000000006029
Aubry, L., & Carr, B. T. (2022). Overdose, opioid treatment admissions and prescription opioid pain reliever relationships: United States, 2010–2019. Frontiers in Pain Research, 3. https://doi.org/10.3389/fpain.2022.884674
Dydyk AM, Sizemore DC, Fariba KA, Sanghavi DK & Porter BR. (2022, October 26). Florida controlled substance prescribing. National Center for Biotechnology Information. https://pubmed.ncbi.nlm.nih.gov/33428370/
Dydyk, A. M., Jain, N. K., & Gupta, M. (2023, April 29). Opioid use disorder – statpearls – NCBI bookshelf. Opioid Use Disorder. . https://www.ncbi.nlm.nih.gov/books/NBK553166/
NG;, M. M. M. (2022, June 6). The role of informatics in implementing guidelines for chronic opioid therapy risk assessment in primary care: A narrative review informed by the socio-technical model. Studies in health technology and informatics. https://pubmed.ncbi.nlm.nih.gov/35673054/
Pergolizzi, J. V., Raffa, R. B., & Rosenblatt, M. H. (2020). Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. Journal of Clinical Pharmacy and Therapeutics, 45(5), 892–903. https://doi.org/10.1111/jcpt.13114
Reynolds, A. M., Reynolds, C. J., & Craig-Rodriguez, A. (2021). Aprns’ controlled substance prescribing and readiness following Florida Legislative changes. The Nurse Practitioner, 46(6), 48–55. https://doi.org/10.1097/01.npr.0000751796.01625.17
Saloner, B., & Karthikeyan, S. (2015). Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004-2013. JAMA, 314(14), 1515–1517. https://doi.org/10.1001/jama.2015.10345
- American Psychiatric Association. (2020). What is schizophrenia? Retrieved from https://www.psychiatry.org/patients-families/schizophrenia/what-is-schizophrenia/
- Bahta, M., Berhe, T., Russom, M., Tesfamariam, E. H., & Ogbaghebriel, A. (2020). Magnitude, nature, and risk factors of adverse drug reactions associated with first generation antipsychotics in outpatients with schizophrenia: a cross-sectional study. Integrated pharmacy research & practice, 9, 205–217. https://doi.org/10.2147/IPRP.S271814
- Cavallaro, R., & Colombo, C. (Eds.). (2022). Fundamentals of psychiatry for health care professionals. Springer. https://doi.org/10.1007/978-3-031-07715-9
- Chokhawala K, Stevens L. Antipsychotic Medications. [Updated 2023 Feb 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519503/
- Grinchii D, Dremencov E. (2020). Mechanism of action of atypical antipsychotic drugs in mood disorders. International Journal of Molecular Sciences. 21(24):9532. https://doi.org/10.3390/ijms21249532
- Katzung B.G., Kruidering-Hall M, Tuan R, Vanderah T.W., & Trevor A.J.(Eds.). (2021) Antipsychotic & bipolar disorder agents. Katzung & Trevor’s Pharmacology: Examination & Board Review, 13e. McGraw-Hill Education. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3058§ionid=255305897
- Kidron A, Nguyen H. Phenothiazine. [Updated 2023 May 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556113/
- National Alliance on Mental Illnesses (NAMI). (2023). Psychosis. Retrieved from https://www.nami.org/About-Mental-Illness/Mental-Health-Conditions/Psychosis?gad_source=1&gclid=Cj0KCQiAw6yuBhDrARIsACf94RUVJ9RgZl6u71jk32H1dD9N67XFuivUjDnIwTqS7TRiyowwk_90kt0aAlwBEALw_wcB
- Rocca, P., & Bellino, S. (Eds.). (2022). Psychosis and personality disorders: unmet needs in early diagnosis and treatment. Springer. https://doi.org/10.1007/978-3-031-09058-5
- Skidmore-Roth, Linda. Mosby’s 2020 Nursing Drug Reference E-Book : Mosby’s 2020 Nursing Drug Reference E-Book, Mosby, 2019. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/liberty/detail.action?docID=5978994.
- Vasan S, Padhy RK. Tardive Dyskinesia. [Updated 2023 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448207/
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Aripiprazole. McGraw Hill
Retrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426473#monoNumber=426473§ionID=243193191&tab=tab0 - Wolters Kluwer Clinical Drug Information, Inc. (2024). Thiothixene. McGraw Hill
Retrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426942#monoNumber=426942§ionID=243275754&tab=tab0 - Wolters Kluwer Clinical Drug Information, Inc. (2024). Loxapine. McGraw Hill; Access Pharmacy.
- Yang S, Boudier-Revéret M, Choo YJ, Chang MC. Association between Chronic Pain, and Alterations in the Mesolimbic Dopaminergic System. Brain Sciences. 2020; 10(10):701. https://doi.org/10.3390/brainsci10100701
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate