Course
Kentucky Addiction Disorders
Course Highlights
- In this Kentucky Addiction Disorders course, we will learn about opioid use disorder (OUD).
- You’ll also learn the mechanism of action for methadone.
- You’ll leave this course with a broader understanding of how advanced practice registered nurses (APRNS) can engage in safe prescribing practices in the care of patients with opioid use disorders.
About
Pharmacology Contact Hours Awarded: 2.5
Course By:
Abbie Schmitt
RN, MSN-Ed
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
The United States is facing an epidemic of opioid-related mortality and morbidity that has an unparalleled impact. Drug overdoses are the leading cause of accidental deaths in the U.S. (13). Roughly two-thirds of drug overdose deaths were caused by opioids – both legal and illicit (18). There are two intertwined epidemics: the excessive use of opioids for both legal and illicit purposes, and unprecedented levels of consequent opioid use disorder (OUD).
Addiction remains one of the most critical public health and safety issues facing the Commonwealth of Kentucky (8). There is hope on the horizon for those in Kentucky who are impacted by addiction disorders. The data and statistics suggest that the interventions established are having a meaningful impact. Kentucky had a decrease of over 5% of overdose deaths in 2022 from 2021. Significant legislation has been implemented to battle this crisis within the state of Kentucky.
As we explore addiction disorders, it is meaningful to understand the pharmacology of the most common opioids and the pharmacokinetics of Fentanyl to fully understand how a common treatment, Methadone, is effectively used.

Self Quiz
Ask yourself...
- What ethical consideration should APRNs be aware of when prescribing opioids?
- How can APRNs balance pain management needs with the risk for addiction?
- What is the most commonly misused opioid in our country?
- Can you think of any commonly used medications that treat addiction disorders?
Etiology and Statistics on Addiction Disorders in Kentucky
Opioids are the primary culprit of drug overdose deaths. In 2000, opioid overdoses represented 48% of drug overdose deaths in the U.S.; by 2021, they represented 75% of these deaths (11).
The Office of the State Medical Examiner (OSME) and toxicology reports submitted by Kentucky coroners state that 90% of deaths in 2022 involved opioids (13).
It is important to look at trends in data. Recent statistics of addiction disorders in Kentucky:
- In 2020, there were 1,964 overdose deaths in KY
- In 2021, there were 2,250 overdose deaths in KY (14.5% increase from 2020)
- In 2022, there were 2,135 overdose deaths (5% decrease from 2021)
The Office of Drug Control Policy (ODCP) reports that 90% of deaths in 2022 involved opioids. The most prevalent drug contributing to overdose deaths is fentanyl, accounting for 72.5% nationwide in 2022 [See Figure 1]. In Kentucky, of the 2,135 overdose deaths in 2022, 1,548 (73%) were identified from toxicology testing (11). The age group with the greatest number of drug overdose deaths in Kentucky in 2022 included those between the ages of 35 and 44 [See Figure 2] (11).

Figure 1. Kentucky Fentanyl-Related Drug Overdose Deaths in 2022 (11)
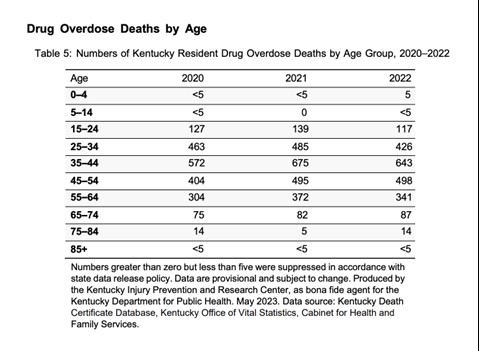
Figure 2. Kentucky Drug Overdose Deaths by Age 2020-2022 (11)
Terminology Related to Addiction and Misuse
These terms are similar, but providers and clinicians should be aware of the differences (6).
- Addiction - the constant need for a drug despite harmful consequences.
- Pseudoaddiction - constant fear of being in pain, hypervigilance; usually there is a resolution with pain resolution.
- Dependence - physical adaptation to a medication where it is necessary for normal function and withdrawal occurs with lack of the medication.
- Tolerance - lack of expected response to a medication resulting in an increase in dose to achieve the same pain relief, resulting from central nervous system (CNS) adaptation to the medication over time.

Self Quiz
Ask yourself...
- Can you discuss the demographics that have the highest number of overdose deaths?
- How would you describe the statistics on Kentucky addiction disorders relating to opioids?
- Can you summarize the terms addiction, psuedoaddiction, dependence, and tolerance?
- Have overdose deaths relating to opioids increased or decreased over the past 20 years?
Opioid Use Disorder (OUD)
An opioid use disorder (OUD) is defined as a problematic pattern of opioid use that leads to serious impairment or distress (5).
In the late 1990s, prescription opioid use increased in all regions of the U.S. Unregulated prescription opioid use was promoted, in large part by the pharmaceutical industry (5). Misuse and diversion of these medications became widespread; by 2017, an estimated 1.7 million people in the U.S. suffered from substance use disorders related to prescription opioid pain medications (5).
The DSM-5 Criteria is an excellent guide for diagnosing OUD. To be eligible for methadone treatment, patients must meet DSM-5 criteria for opioid use disorder. According to the DSM-5, the presence of at least two of the following symptoms indicates OUD (1).
- Opioids are often taken in larger amounts or over a longer period than was intended
- There is persistent desire or unsuccessful efforts to cut down or control opioid use
- A great deal of time is spent in activities necessary to obtain the opioid, use the opioid, or recover from its effects
- Craving or a strong desire to use opioids
- Recurrent opioid use resulting in a failure to fulfill major role obligations at work, school, or home
- Continued opioid use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of opioids
- Important social, occupational, or recreational activities are given up or reduced because of opioid use
- Recurrent opioid use in situations in which it is physically hazardous
- Continued use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by opioids
- Tolerance as defined by either of the following:
- Need for markedly increased amounts of opioids to achieve intoxication or desired effect
- Markedly diminished effect with continued use of the same amount of opioid
- Withdrawal as manifested by either of the following:
- Characteristic opioid withdrawal syndrome.
- Same (or a closely related) substance is taken to relieve or avoid withdrawal
The severity of OUD is defined as (1):
MILD: The presence of 2 to 3 symptoms
MODERATE: The presence of 4 to 5 symptoms
SEVERE: The presence of 6 or more symptoms

Self Quiz
Ask yourself...
- How is Opioid Use Disorder (OUD) defined?
- What is the DSM-5 criteria for this diagnosis?
- How can clinicians determine if opioids are having an impact on a patient’s functional level?
- Can you think of reasons it is important to appropriately diagnose the disorder prior to prescribing medications for the treatment?
Opiates and Opioids
Opiates are chemical compounds that are extracted or refined from natural plant matter (poppy sap and fibers).
Examples of opiates:
- Opium
- Morphine
- Codeine
- Heroin
Opioids are chemical compounds that generally are not derived from natural plant matter. Most opioids are synthesized.
Though a few opioid molecules — hydrocodone (e.g., Vicodin), hydromorphone (e.g., Dilaudid), oxycodone (e.g., Oxycontin, Percocet) — may be partially synthesized from chemical components of opium, other popularly-used opioid molecules are designed and manufactured in laboratories.
The pharmaceutical industry has created more than 500 different opioid molecules.
Common opioids used in the U.S. for treatment of pain:
- Fentanyl/fentanyl (e.g., Ultiva, Sublimaze, Duragesic patch)
- Dextropropoxyphene (e.g., Darvocet-N, Darvon)
- Hydrocodone (e.g., Vicodin)
- Oxycodone (e.g., Oxycontin, Percocet)
- Oxymorphone (e.g., Opana)
- Meperidine (e.g., Demerol)
Pharmacokinetics of Fentanyl
Fentanyl is a synthetic opioid agonist that is 80-100 times stronger than morphine and is often added to heroin to increase its potency (6). It can cause severe respiratory depression and death, particularly when mixed with other drugs or alcohol. It has high addiction potential.
Drug Class
Opioid, narcotic agonist (Schedule II).
Uses
Pain relief, preop medication; adjunct to general or regional anesthesia. Management of chronic pain (transdermal).
Mechanism of Action
Opioids can be classified according to their effect on opioid receptors and can be considered as agonists, partial agonists, antagonists, and agonist-antagonists.
- Agonists - interact with an opioid receptor to produce a maximal response from that receptor.
- Antagonists - bind to receptors but produce no functional response, while at the same time preventing an agonist from binding to that receptor (naloxone).
- Partial agonists - bind to receptors but elicit only a partial functional response regardless of the amount of drug administered
- Agonist-antagonists - act as agonist to a certain opioid receptor, but have antagonist activity to another opioid receptor
Fentanyl is an μ-opioid agonist that binds to μ-opioid G-protein-coupled receptors, which prevents the release of pain neurotransmitters by decreasing the cellular calcium level. These receptors bind opioids, so they are also commonly referred to as mu-opioid receptors (MORs).
The connection between the receptor and the first stage of signal transduction becomes established through the G proteins (alpha, beta, and gamma subunits). The main targets of G proteins include the adenyl cyclase, which is the enzyme responsible for the formation of the second messenger, and cyclic adenosine monophosphate (cAMP) (10).
The phospholipase C is the enzyme responsible for the formation of several ion channels such as the calcium and potassium channels (10). Essentially, GPCRs can directly control the activity of ion channels through mechanisms that do not involve the second messengers. Opioids reduce neuronal excitability by opening the G protein-dependent and rectifying potassium (irk) channels (GIRK) and subsequent cell membrane hyperpolarization (10).
The opening of the channel occurs by the direct interaction between the subunits of the G protein and the potassium ion channel.
Endogenous and exogenous opioids operate through both inhibitory and excitatory action at the presynaptic and postsynaptic sites. In particular, the MORs interact with a G protein of the inhibitory type (10).
In the resting state, G-alpha-beta-gamma complex and the subunit α causes the bind, guanosine diphosphate (GDP). The binding of the opioid agonist (endogenous or exogenous) to the extracellular N-terminus domain of the MOR induces dissociation of GDP from the G-alpha subunit, which is replaced by guanosine triphosphate (GTP).
Because the enzymatic GTP turnover lasts approximately two to five minutes, a new signal may find the receptor still not ready to respond, so the regulator of G-protein signaling (RGS) protein speeds up the GTP hydrolysis up to 100-fold; this protein binds the G-alpha subunit and removes the active G-alpha-GTP and beta-gamma species (10).
MORs are present in the CNS and are the most highly expressed of all opioid receptors. These receptors are expressed in neurons throughout the dorsal horn of the spinal cord and in different regions or the brain (10). Within the spinal cord, MORs are localized (presynaptic and postsynaptic) and receive sensory information from primary afferent nerve fibers innervating the skin and deeper tissues of the body (10).

Self Quiz
Ask yourself...
- How are the mechanisms of action different between agonists, partial agonists, antagonists, and agonist-antagonists?
- Can you describe how fentanyl prevents the release of pain neurotransmitters?
- Where are MORs located in the body?
- How much more potent is fentanyl than morphine?
Fentanyl Pharmacodynamics/Kinetics
- Onset of action for adults:
- IM: 7 to 8 minutes
- IV: Almost immediate (maximal analgesic and respiratory depressant effects may not be seen for several minutes)
- Transdermal patch (initial placement): 6 hours
- Transmucosal: 5 to 15 minutes
- Duration:
- IM: 1 to 2 hours
- IV: 0.5 to 1 hour
- Distribution: Highly lipophilic, redistributes into muscle and fat
- Note: IV fentanyl exhibits a 3-compartment distribution model
- Changes in blood pH may alter ionization of fentanyl and affect its distribution between plasma and CNS
- Vdss: Adults: 4 to 6 L/kg
- Protein binding: 79% to 87%, primarily to alpha-1 acid glycoprotein; also binds to albumin and erythrocytes.
- Metabolism: Hepatic, primarily via CYP3A4 by N-dealkylation and hydroxylation to other inactive metabolites.
- Half-life elimination:
- IV: Adults: 2 to 4 hours; when administered as a continuous infusion, the half-life prolongs with infusion duration due to the large volume of distribution (Sessler 2008)
- Sub-Q bolus injection: 10 hours
- Transdermal device: Terminal: ~16 hours
- Transdermal patch: 20 to 27 hours
- Transmucosal products: 3 to 14 hours (dose dependent)
- Intranasal: 15 to 25 hours (based on a multiple-dose pharmacokinetic study when doses are administered in the same nostril and separated by a 1-, 2-, or 4-hour time lapse)
- Buccal film: ~14 hours
- Buccal tablet: 100-200 mcg: 3 to 4 hours; 400 to 800 mcg: 11 to 12 hours
- Time to peak:
- Buccal film: 0.75 to 4 hours (median: 1 hour)
- Buccal tablet: 20 to 240 minutes (median: 47 minutes)
- Lozenge: 20 to 480 minutes (median: 20 to 40 minutes)
- Intranasal: Median: 15 to 21 minutes
- SubQ bolus injection: 10 to 30 minutes (median: 15 minutes)
- Sublingual spray: 10 to 120 minutes (median: 90 minutes)
- Sublingual tablet: 15 to 240 minutes (median: 30 to 60 minutes)
- Transdermal patch: 20 to 72 hours; steady state serum concentrations are reached after two sequential 72-hour applications
- Excretion: Urine 75%; feces ~9%

Self Quiz
Ask yourself...
- What is the time of onset for the various forms of fentanyl?
- How is knowing the half-life meaningful when prescribing fentanyl?
Fentanyl Side Effects
- IV: Postop drowsiness, nausea, vomiting.
- Transdermal (10%– 3%): Headache, pruritus, nausea, vomiting, diaphoresis, dyspnea, confusion, dizziness, drowsiness, diarrhea, constipation, decreased appetite (12)
Occasional:
- IV: Postop confusion, blurred vision, chills, orthostatic hypotension, constipation, difficulty urinating.
- Transdermal (3%–1%): Chest pain, arrhythmias, erythema, pruritus, syncope, agitation, skin irritations (12)

Self Quiz
Ask yourself...
- What are some common side effects of fentanyl?
- Can you name some management strategies for these side effects?
Fentanyl Adverse Effects
Overdose or too-rapid IV administration may produce severe respiratory depression, skeletal/thoracic muscle rigidity. This muscle rigidity may lead to apnea, laryngospasm, bronchospasm, cold/clammy skin, cyanosis, coma. Tolerance to analgesic effect may occur with repeated use (12).
Transdermal Fentanyl
Mechanism of Action
As mentioned, Fentanyl is nearly 100 times more potent than morphine, resulting in an estimated conversion ratio of 1 to 100 to provide an equal degree of analgesia. It has low molecular weight, high potency, and lipid solubility, which makes it ideal for use with the transdermal route.
Fentanyl is a 4-anilidopiperidine compound, it exerts its effect by acting as a high-affinity agonist on selective Mu-opioid receptors in the brain (17). It also has effects on delta and kappa receptors. The activation of Mu-opioid receptors causes analgesia and stimulates areas of the brain responsible for addictive potential.
The transdermal route eliminates the first-pass metabolism of fentanyl by the liver, increasing bioavailability to 90%, making it possible to use lower doses of the drug, thus reducing the incidence of adverse effects.
Fentanyl can be detected in serum after about one to two hours after first application but does not reach the therapeutic index until approximately 12 to 16 hours due to the need for fentanyl to saturate the epidermis before more efficient absorption (17). The patches are designed to deliver fentanyl at a constant rate.
Fentanyl is available in various doses: 12, 25, 50, 75, and 100 mcg/hour; requiring replacement every 72 hours.
Fentanyl metabolism occurs via cytochrome P450 (CYP34A) enzymes into inactive metabolites; hence drugs that enhance or inhibit cytochrome P450 will affect its metabolism (17).
The elimination half-life after patch removal is 13 to 22 hours; this is due to the slow release of fentanyl from the skin (16).
Several studies have shown that when compared to sustained-release oral morphine (SROM), transdermal fentanyl has a 30% lower incidence of adverse effects such as constipation and sedation (p<0.05). (16)
Administration
The patch has an adhesive side that contains an active ingredient that must be applied directly flat on the skin, and it should be applied to intact, clean, and healthy skin. Skin with scars, rashes, or open wounds should be avoided. Areas with excess hair require clipping if applying the patch in that location.
The ideal areas to apply the adhesive patch are the chest, back, and arms. Patches should not be applied consecutively in the same location (17). The transdermal fentanyl patch must be removed after 72 hours. Proper disposal is important to avoid intentional abuse and misuse of the discarded patch.
Adverse Effects
The most common adverse drug reactions of transdermal fentanyl are nausea, vomiting, and constipation (17). Adverse side effects are manageable with stool softeners and antiemetics. There is a higher incidence of respiratory depression in patients who have not previously been exposed to opioid analgesics. Additional adverse effects include rash and erythema at the application site of the patch that abates after removal or with antihistamine therapy (17). Hypoventilation has been noted as an adverse effect.
Withdrawal symptoms of transdermal fentanyl may cause adverse side effects such as nausea, vomiting, diarrhea, and shivering. These symptoms may occur when decreasing dosage, abrupt cessation of use, or changing to an alternative opioid medication (17).
Contraindications
Contraindications of transdermal fentanyl include patients who experience hypoventilation, respiratory compromise (including acute or severe asthma) or respiratory depression should not take transdermal fentanyl. Also, fentanyl transdermal should be avoided during the acute postoperative pain period for short term pain control, intermittent pain control, or mild pain (17).
Pediatric patients under 12 or children under 50 kgs and under 18 should not use this drug. Avoid use in patients with a history of sensitivity or reactions to adhesives.
Prescriber Monitoring
Fentanyl is an extremely potent opioid and requires prescriber monitoring to maintain a safe therapeutic concentration. The gold standard method of assessing fentanyl concentration is Liquid chromatography-mass spectrometry. A blood concentration of 0.6 ng/ml to 3.0 ng/ml is appropriate for analgesia.
Monitoring Fentanyl is increasingly important when the patient is taking multiple medications. Drugs that are important review carefully are those which inhibit CYP3A4 metabolism, which causes an increase in Fentanyl concentration and can lead to toxicity. Examples of these drugs include azole class antifungals and macrolide antibiotics (17). CYP3A4 inducers, such as rifampin, phenytoin, and carbamazepine, may also reduce the level of fentanyl to a non-therapeutic level (17).
Antidote for Fentanyl: Naloxone
Nursing Considerations (12):
- Prepare: Resuscitative equipment and opiate antagonist (naloxone 0.5 mcg/kg) should be available for initial use.
- Establish baseline blood pressure, pulse rate, and respirations.
- Assess type, location, intensity, duration of pain.
- Assess fall risk and implement appropriate precautions.
- Assist with ambulation and encourage patient to turn, cough, deep breathe every two hours.
- Monitor respiratory rate, B/P, heart rate, oxygen saturation.
- Assess for relief of pain.
- For patients with prolonged high-dose use, continuous infusions (critical care, ventilated patients), clinicians should consider weaning the drip gradually or transitioning to a fentanyl patch to decrease symptoms of opiate withdrawal.

Self Quiz
Ask yourself...
- Can you describe how increasing knowledge among prescribers can help to battle the opioid crisis?
- What are some contraindications when prescribing fentanyl?
- Why is it important to document the reason for prescribing this drug?
- How can knowledge of the mechanisms of action of opioids help to guide understanding of treatments for opioid abuse disorders?
Medication-Assisted Treatment (MAT) for OUD
Medication-assisted treatment (MAT) is the use of medications, in combination with counseling and behavioral therapies, in the treatment of opioid use disorders (OUD). The goal is sustained recovery. Often, individuals have actual pain and became dependent on prescription narcotic drugs, then switch to illicit opioids or the opiate heroin when the medically supplied narcotics run out.
The U.S. Food and Drug Administration (FDA) has only approved three medication assisted treatments (MATs) for opioid use disorder (OUD): methadone, buprenorphine, and naltrexone.
We will discuss the pharmacokinetics of Methadone in this course.
FDA-approved methadone products approved for the treatment of OUD include:
- Dolophine (methadone hydrochloride) tablets
- Methadose (methadone hydrochloride) oral concentrate
Buprenorphine and methadone have been shown to decrease mortality among those with OUD. A recent study reported that buprenorphine was associated with a lower risk of overdose during active treatment compared to post-discontinuation.

Self Quiz
Ask yourself...
- Can you name the drugs that are approved by the FDA for the treatment of OUD?
- How can partnerships between policy makers and healthcare providers enhance MATs?
- How would you describe your experience in addiction treatment programs?
- Have you ever administered methadone in your nursing practice?
Methadone
Methadone is a medication approved by the Food and Drug Administration (FDA) to treat OUD, as well as for management of chronic pain. Methadone is safe and effective when taken as prescribed. Methadone is a component of a comprehensive treatment plan, which includes counseling and other behavioral health therapies to provide patient-centered care.
Definition
Methadone, a long-acting opioid agonist, it can help relieve cravings and withdrawal, while also blocking the effects of opioids (15). It is available in liquid, powder, and diskettes forms. Patients taking methadone to treat OUD must receive the medication under the supervision of a medical provider, but after a period of stability and consistent compliance, patients may be allowed to take methadone at home between program visits (15).
Drug Class
Opioid agonist (Schedule II). CLINICAL: Opioid analgesic. Opioid dependency management. (12)
Uses
Methadone is a first-line Opioid Addiction Treatment (OAT) option, along with buprenorphine. Methadone may be preferable to buprenorphine for patients who are at high risk of treatment cessation and subsequent fentanyl overdose. It also alters processes affecting analgesia, emotional responses to pain, and reduces withdrawal symptoms from other opioid drugs
Mechanism of action
Methadone hydrochloride is a mu-agonist, which is a synthetic opioid analgesic with multiple actions that are similar to those of morphine (7). The most prominent actions impact the central nervous system and organs composed of smooth muscle.
Methadone binds to opiate receptors in the CNS, causing inhibition of ascending pain pathways and altering the perception of and response to pain (12). It also produces generalized CNS depression (12). Methadone has also been shown to have N-methyl-D-aspartate (NMDA) receptor antagonism. The contribution of NMDA receptor antagonism to methadone’s efficacy is unknown.
Methadone binds to plasma proteins in circulation, most predominantly α1-acid glycoprotein (9). This is an important consideration as certain conditions or medications may alter plasma protein levels. There is considerable tissue distribution of methadone, and it is possible for tissue levels to exceed plasma levels.
The lipophilic nature of methadone allows for rapid absorption, long duration of action, and slow release from tissues into the bloodstream. This accounts for the wide variation in half-life, recorded as a range from two to 65 hours.
Methadone is metabolized into inactive molecules by the liver CYP450 enzyme and the intestinal CYP3A4/CYP2D6 enzymes before elimination in the feces or urine.

Self Quiz
Ask yourself...
- Can you name the uses of methadone?
- How does the mechanism of action of methadone help to alleviate withdrawal symptoms from opioids?
- Is the half-life of this drug considered long or short?
- Why is it important to recognize that methadone binds to plasma proteins in circulation?
Methadone Pharmacokinetics

Figure 3. Pharmacokinetics of Methadone (12)
- Well absorbed after IM injection.
- Protein binding: 85%–90%.
- Metabolized in liver. Primarily excreted in urine.
- Not removed by hemodialysis.
- Half-life: 7– 59 hrs.
- Crosses placenta and found in breast milk.
Respiratory issues may occur in neonates if mother received opiates during labor.
Elderly patients are more susceptible to respiratory depressant effects.
Age-related renal impairment may increase the risk of urinary retention.
Caution: Renal/ hepatic impairment, elderly/debilitated patients, risk for QT prolongation, medications that prolong QT interval, conduction abnormalities, severe volume depletion, hypokalemia, hypomagnesemia, cardiovascular disease, depression, suicidal tendencies, history of drug abuse, respiratory disease, and biliary tract dysfunction.
Drug Interactions
Alcohol, other CNS depressants (e.g., Lorazepam, morphine, zolpidem) may increase CNS effects, respiratory depression, and hypotension.
CYP3A4 inducers (e.g., carbamazepine, phenobarbital) may decrease concentration/effects; CYP3A4 inhibitors (e.g., rifampin, clarithromycin) (12).

Self Quiz
Ask yourself...
- Can you name drugs that should be carefully monitored when prescribed along with methadone?
- What are examples of additional precautions for elderly patients?
Methadone Side Effects
As with other opioid medications, general side effects of methadone are related to excessive opioid receptor activity, including but not limited to:
- Diaphoresis/flushing
- Pruritis
- Nausea
- Dry mouth
- Constipation
- Sedation
- Lethargy
- Respiratory depression
Adverse Effects
Cardiovascular: Bigeminy, bradycardia, cardiac arrhythmia, cardiac failure, cardiomyopathy, ECG changes, edema, extrasystoles, flushing, hypotension, inversion T wave on ECG, palpitations, phlebitis, prolonged QT interval on ECG, shock, syncope, tachycardia, torsades de pointes, ventricular fibrillation, ventricular tachycardia
Central nervous system: Agitation, confusion, disorientation, dizziness, drug dependence (physical dependence), dysphoria, euphoria, hallucination, headache, insomnia, sedation, seizure
Dermatologic: Diaphoresis, hemorrhagic urticaria (rare), pruritus, skin rash, urticaria
Endocrine & metabolic: Adrenocortical insufficiency, altered hormone level (androgen deficiency; chronic opioid use), amenorrhea, antidiuretic effect, decreased libido, decreased plasma testosterone, hypokalemia, hypomagnesemia, weight gain
Gastrointestinal: Abdominal pain, anorexia, biliary tract spasm, constipation, glossitis, nausea, vomiting, xerostomia
Genitourinary: Asthenospermia, decreased ejaculate volume, male genital disease (reduced seminal vesicle secretions), prostatic disease (reduced prostate secretions), spermatozoa disorder (morphologic abnormalities), urinary hesitancy, urinary retention
Hematologic: Thrombocytopenia (reversible, reported in patients with chronic hepatitis)
Neuromuscular & skeletal: Amyotrophy, bone fracture, osteoporosis, weakness
Ophthalmic: Visual disturbance
Respiratory: Pulmonary edema, respiratory depression
Warnings
- May prolong QT interval, which may cause serious arrhythmias.
- May cause serious, life-threatening, or fatal respiratory depression.
- Monitor for signs of misuse, abuse, addiction.
- Prolonged maternal use may cause neonatal withdrawal syndrome.
Do not confuse methadone with Mephyton, Metadate CD, Metadate ER, methylphenidate, or morphine
Serious adverse effects: pancreatitis, hypothyroidism, Addison’s disease, head injury, increased intracranial pressure.
Methadone Dosing and Titration for APRNs
The following are tips for nurse clinicians in dosing and titrating Methadone (3):
- The clinician should attempt to reach an optimal dose of methadone safely and quickly (3).
- Starting methadone at 30mg is recommended.
- The starting dose of methadone can be increased by 10–15mg every three to five days.
- Slower titration is recommended for patients at higher risk of toxicity (e.g., older age, sedating medications or alcohol, patients new to methadone).
- Patients who have recently been on methadone dosing at higher doses (i.e., in the previous week) can be considered for more rapid dose increases based on their tolerance.
- Once a dose of 75–80mg is reached, the dose can then be increased by 10mg every five to seven days.
- If four consecutive missed doses, the dose of methadone should be reduced by 50% or to 30mg, whichever is higher. If five or more consecutive doses are missed, methadone should be restarted at a maximum of 30mg and titrated according to patient need.
- Sustained-release oral morphine (SROM), at a maximum starting dose of 200mg, can be added on the day of a restart as long as the patient has not become completely opioid-abstinent.
- For patients who use fentanyl regularly, methadone doses of 100mg or higher are often appropriate.
- Use prescription practices that promote treatment retention, including phone visits, check-ins, extending prescriptions, or leaving longer duration methadone prescriptions for 30mg at the pharmacy so patients can restart treatment.
- Be aware of the limitations of urine drug testing.
- Provide treatment for concurrent psychiatric illnesses and substance use disorders.

Self Quiz
Ask yourself...
- Can you explain the major side effects and adverse effects of methadone?
- Can you describe the recommendations on missed doses of methadone?
- What is the recommended starting dose of this drug?
- What are some ways to manage gastrointestinal effects of the drug?
Pregnancy and Methadone
Opioid withdrawal is associated with a high risk for spontaneous abortion and preterm labor, so pregnant patients with OUD should be started as soon as possible and titrated to avoid withdrawal symptoms (3). Hospital admission for rapid up-titration of methadone with augmenting opioids is recommended if possible. When caring for a pregnant patient using fentanyl, it is vital to contact an obstetrical team early. Use of opiates during pregnancy produces withdrawal symptoms in neonate, including irritability, excessive crying, tremors, hyperactive reflexes, fever, vomiting, diarrhea, yawning, sneezing, seizures (12).
Guidance for Kentucky APRNs
Prescribing Opioids
Before prescribing opioids, complete a detailed patient history and assessment that includes:
- Indication of pain relief request
- Location, nature, and intensity of pain
- Prior pain treatments and response
- Diagnostic testing
- Comorbid conditions
- Potential physical and psychologic pain impact on function
- Family support, employment, and housing
- Leisure activities, mood, sleep, and substance use
- Signs of emotional, physical, or sexual abuse
When considering opioids, weigh the benefits with the risks of abuse or addiction, adverse drug reactions, overdose, and physical dependence.
Assessment Tools
Screening tools can assist in determining risk level and the degree of monitoring and structuring required for a treatment plan. Examples include:
- Brief Intervention Tool
- 26-item "yes-no" questionnaire used to identify signs of opioid addiction or abuse.
- Current Opioid Misuse Measure (COMM)
- The Current Opioid Misuse Measure is a 17-item patient self-report assessment.
- Diagnosis, Intractability, Risk, and Efficacy (DIRE) Tool
- The Diagnosis, Intractability, Risk, and Efficacy is a clinician-rated questionnaire used to predict patient compliance with long-term opioid therapy.
- Opioid Risk Tool
- The Opioid Risk Tool is a five-item assessment to evaluate for aberrant drug-related behavior.
- Pain Assessment and Documentation Tool (PADT)
- The U.S. Centers for Disease Control and Prevention (CDC), the Federation of State Medical Boards, and Joint Commission stress documentation from both a quality and diagnostic perspective.
- Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R)
- The Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is a screening with questions addressing the history of alcohol or substance use, cravings, mood/psychological status, and stress.
- Urine Drug Tests (UDT)
- The CDC recommends drug testing before starting opioid therapy and routinely to detect unanticipated drug use.
Checklists For Prescribers and Dispensers
- Dose, frequency, and length prescribed consistent with the indication - avoid long-acting opioids for acute pain
- Age, weight, height, and sex considered
- Evaluate for potential drug interactions
- Evaluate for potential allergic reactions
- Patient informed and verbalized understanding of the risks and benefits
- Warnings about addiction, abruptly halting, use of power equipment, side-effects, respiratory depression, avoid sharing or using not as prescribed.
- Medication use agreement in place.
- Instructions for storage and disposal of unused opioids.
Potential Signs of Drug Misuse
The following are red flags of misuse:
- Only drugs prescribed are controlled substances
- Early refills
- Pays cash, although insurance is present.
- Lost prescriptions
- Remote address
- Multiple prescribers
- Concerning PDMP or KASPAR (Kentucky All Schedule Prescription Reporting) results


Self Quiz
Ask yourself...
- Can you name a few tools available to clinicals when addiction disorders are suspected?
- How can these assessment tools be integrated into screening at primary care clinics?
Prescription Drug Monitoring Programs (PDMPs)
Kentucky has mandatory PDMP Use Laws for clinicians who prescribe certain medications. APRNs are required to use and apply the information gained from this database into practice.
Prescription drug monitor programs (PDMPs) are in place in various methods and degrees in all states. These databases assist prescribers and dispensers in working together to decrease drug misuse.
The key benefits include (6):
- Assists in monitoring opioid prescriptions.
- Identify if multiple providers are providing prescriptions for the same individual (avoids "doctor shopping").
- Assists regulatory boards, Medicaid, medical examiners, law enforcement, and research organizations in gathering data on the effectiveness and enforcement of regulations.
- PMP Interconnect allows the sharing of prescription information across state lines.
KASPAR (Kentucky All Schedule Prescription Reporting)
KASPER is a controlled substance prescription monitoring system designed to assist practitioners and pharmacists. KASPER also provides an investigative tool for law enforcement and regulatory agencies to assist with authorized reviews and investigations. The report shows all Schedule II through V controlled substance prescriptions a patient has received and a list of prescribers who prescribed them (6).
Practitioners can request this report to review data on controlled substances administered or dispensed to their patient prior to prescribing. This is also a tool to assess the prescriptions obtained by a birth mother of an infant being treated for neonatal abstinence syndrome or prenatal drug exposure.
To obtain the report, go to the Kentucky Online Gateway website or call KOG Help Desk at 502-564-0104)
Requirements for an initial prescribing of a controlled substance for pain or associated with the same primary complaint (201 KAR 9:260 Section 3): (6)
- Appropriate medical history and physical exam.
- Obtain KASPER report for the previous 12-month period.
- Do not prescribe or dispense long-acting or controlled-release controlled substances for acute pain not associated with recent surgery.
- Explain that the medication is intended to treat acute pain for a time-limited use, and to discontinue when resolved.
- If Schedule II Controlled Substance or Schedule III Controlled Substances with hydrocodone:
- Make a written plan stating the objectives of the treatment and further diagnostic examinations required
- Discuss the risks and benefits of controlled substance use.
- Obtain written consent for treatment

Self Quiz
Ask yourself...
- Can you name specific laws and regulations for prescribers in Kentucky?
- How can KASPER reports be obtained?
- What are some benefits of Prescription Drug Monitoring Programs (PDMPs)?
- What schedule of controlled substance prescriptions are included in the KASPER report?
Conclusion
When designing a treatment strategy to battle this addiction and abuse crisis, it is important to look at common addiction disorders, the pharmacokinetics of opioids, prescribing guidance and laws, and pharmacological interventions that clinicians can use to help improve this crisis. Kentucky is facing poor outcomes in the battle against opioid use disorders (OUD), but with broader research and education, healthcare policy initiatives, and support of those impacted, there is significant hope!
References + Disclaimer
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5.™ 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.
- Brady, K., Levin, F. R., Galanter, M., & Kleber, H. D. (Eds.). (2021). The American Psychiatric Association Publishing textbook of substance use disorder treatment (Sixth edition.). American Psychiatric Association Publishing.
- Bromley L, Kahan M, Regenstreif L, Srivastava A, Wyman J. Methadone treatment for people who use fentanyl: Recommendations. Toronto, ON: META:PHI; 2021. www.metaphi.ca.
- Centers for Disease Control and Prevention (CDC). (2023). Provisional drug overdose death counts. Retrieved from https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- Deyo-Svendsen, M., Cabrera Svendsen, M., Walker, J., Hodges, A., Oldfather, R., & Mansukhani, M. P. (2020). Medication-Assisted Treatment for Opioid Use Disorder in a Rural Family Medicine Practice. Journal of primary care & community health, 11, 2150132720931720. https://doi.org/10.1177/2150132720931720
- Dydyk AM, Sizemore DC, Smock W, et al. Kentucky KASPER and Controlled Substance Prescribing. [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567726/
- Federal Drug Administration (FDA). (2019). Methadone hydrochloride injection. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021624s006lbl.pdf
- Freeman, P.R., McAninch, J., Dasgupta, N. et al. Drugs involved in Kentucky drug poisoning deaths and relation with antecedent controlled substance prescription dispensing. Subst Abuse Treat Prev Policy 18, 53 (2023). https://doi.org/10.1186/s13011-023-00561-y
- Hanna V, Senderovich H. Methadone in Pain Management: A Systematic Review. J Pain. 2021 Mar;22(3):233-245. doi: 10.1016/j.jpain.2020.04.004. Epub 2020 Jun 26. PMID: 32599153.
- Herman TF, Cascella M, Muzio MR. Mu Receptors. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551554/
- Kentucky Office of Drug Control Policy Commonwealth of Kentucky Justice & Public Safety Cabinet. (2023). 2022 Overdose fatality report. Team Kentucky. Retrieved from https://odcp.ky.gov/Reports/2022%20Overdose%20Fatality%20Report.pdf
- Kizior, Robert, and Keith Hodgson. Saunders Nursing Drug Handbook 2020, Elsevier – Health Sciences Division, 2019. ProQuest Ebook Central, https://ebookcentral.proquest.com/lib/liberty/detail.action?docID=5978998.
- National, Academies of Sciences, Engineering, and Medicine, et al. , edited by Michelle Mancher, and Alan I. Leshner. (2019). Medications for opioid use disorder save lives National Academies Press, 2019. ProQuest Ebook Central, https://ebookcentral.proquest.com/lib/liberty/detail.action?docID=5774508.
- Strickler, G. K., Zhang, K., Halpin, J. F., Bohnert, A. S. B., Baldwin, G. T., & Kreiner, P. W. (2019). Effects of mandatory prescription drug monitoring program (PDMP) use laws on prescriber registration and use and on risky prescribing. Drug and Alcohol Dependence, 199, 1-9. https://doi.org/10.1016/j.drugalcdep.2019.02.010
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2023). Methadone. Retrieved from https://www.samhsa.gov/medications-substance-use-disorders/medications-counseling-related-conditions/methadone
- Wolters Kluwer Clinical Drug Information, Inc. (2024). FentaNYL. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx#monoNumber=426639§ionID=243243556&tab=tab0
- Taylor KP, Singh K, Goyal A. Fentanyl Transdermal. [Updated 2023 Jul 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555968/
- Centers for Disease Control and Prevention. (2023). Drug overdose deaths. https://www.cdc.gov/drugoverdose/deaths/index.html
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
