Course
Kentucky Pharmacology of Medical Cannabis
Course Highlights
- In this Kentucky Pharmacology of Medical Cannabiscourse, we will learn about the prescribing laws and regulations of medical cannabis at both the federal and state levels, with a focus on regulations in the state of Kentucky.
- You’ll also learn the therapeutic uses of medical cannabis for conditions such as chronic pain, epilepsy, multiple sclerosis, and PTSD.
- You’ll leave this course with a broader understanding of how to develop treatment plans incorporating medical cannabis, considering factors such as dosage, method of administration, and patient-specific conditions.
About
Pharmacology Contact Hours Awarded: 3
Course By:
Abbie Schmitt
MSN-Ed, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
With the recent legalization of medical cannabis in Kentucky, healthcare professionals are at the forefront of new treatment possibilities for patients. This course offers a deeper understanding of the pharmacology, clinical applications, and legal landscape surrounding medical cannabis in the Commonwealth of Kentucky.
As the demand for alternative treatments grows, medical cannabis has shown potential in managing chronic pain, epilepsy, and a variety of other conditions. However, understanding how to responsibly prescribe, monitor, and educate patients on its use is crucial.
Prepare to explore the science behind cannabinoids, the legal requirements specific to Kentucky, and the ethical considerations that accompany this emerging treatment option. Whether you are new to cannabis medicine or looking to expand your knowledge, this course will empower you to provide your patients with safe and informed access to medical cannabis.

Kentucky Prescribing Laws
Kentucky is faced with an overwhelming crisis of addiction and deaths related to opioids. Medical cannabis can be a powerful tool to fight against this crisis. Governor Andy Beshear signed Senate Bill 47 on March 31, 2023, which legalizes medical cannabis effective Jan. 1, 2025 (6). The Office of Medical Cannabis in the Cabinet for Health and Family Services is responsible for implementing and administering Kentucky's Medical Cannabis Program (6).
There are several FDA-approved medical cannabis products are used for specific medical conditions. Beyond these FDA-approved products, certain states within the U.S., now including Kentucky, have legalized the use of medical cannabis products (containing THC, CBD, or both) for specific medical conditions. These products are not FDA-approved but are regulated by state laws.
Medical providers may choose to prescribe FDA-approved medications and/or give qualifying patients a written certification for medical cannabis and guide them in purchasing at dispensaries based on their condition and unique medical needs.
Key aspects of the law regarding prescribing medical cannabis in Kentucky can be organized by (1) prescribing guidelines, (2) qualifying conditions, (3) regulation, (4) prescribing limits, and (5) business licensing regulations.
Prescribing Process
To obtain medical cannabis, patients must visit a Registered Medical Cannabis Practitioner to receive a written certification. This certification is then submitted to the state, and if approved, the patient will be issued a medical cannabis card allowing them to legally purchase and use cannabis.
To become a Registered Medical Cannabis Practitioner in Kentucky, healthcare providers must follow specific steps per Senate Bill 47 and guidelines established by the Kentucky Office of Medical Cannabis.
These criteria and steps (6):
- Hold a Valid Medical License
- Applicants must be licensed medical providers, such as a physician (MD/DO) or advanced practice registered nurse (APRN), in good standing with the Kentucky Board of Medical Licensure or the Kentucky Board of Nursing.
- Complete Required Training
- Kentucky requires practitioners to complete specialized training or continuing education on medical cannabis, including its therapeutic uses, legal requirements, and potential risks.
- The Cabinet for Health and Family Services has developed the program’s regulations.
- Register with the Kentucky Medical Cannabis Program
- Practitioners must register with the Kentucky Office of Medical Cannabis to become authorized to recommend medical cannabis to patients.
- The application process verifies your licensure and completion of any required training.
- Comply with Medical Cannabis Certification Requirements
- Once registered, practitioners can issue medical cannabis certifications to patients with qualifying conditions.
- Practitioners must follow state laws, including appropriate documentation, patient education, and ensuring compliance with the specific qualifying conditions outlined by Kentucky law.
- Maintain Compliance with State Regulations
- Providers must comply with ongoing legal and ethical standards.
- The Cabinet for Health and Family Services will monitor compliance, and practitioners may be required to submit records or documentation for review.
Practitioners can stay updated on regulatory changes by visiting the official website: Kentucky Medical Cannabis Program.
Qualifying Conditions
Medical cannabis can be prescribed to patients diagnosed with conditions such as cancer, multiple sclerosis, chronic pain, epilepsy, PTSD, and other serious ailments. The Cabinet for Health and Family Services will regulate the full list of qualifying conditions.
The conditions allowed for medical cannabis prescription in Kentucky include (6):
- Cancer
- Chronic pain
- Epilepsy
- Multiple sclerosis (MS)
- Post-Traumatic Stress Disorder (PTSD)
- Chronic nausea
- Muscle spasms
- For other medical conditions, the Kentucky Center for Cannabis will grant allowance if sufficient scientific data and evidence are presented supporting that an individual diagnosed with that condition is likely to receive medical, therapeutic, or palliative benefits from the use of medicinal cannabis.
These conditions are deemed severe enough to warrant medical cannabis as a treatment option to help manage symptoms such as pain, nausea, seizures, and muscle spasms. The state may update or expand this list based on future regulations and medical findings (6).
Patients must obtain a certification from a registered medical provider, after which they will be issued a medical cannabis card to legally purchase and use cannabis within the prescribed limits (6).
Regulation
The Cabinet for Health and Family Services will oversee the implementation of the medical cannabis program, ensuring that products are contaminant-free, accurately labeled, and that dispensaries and other businesses operate safely. Regulations will emphasize precautions to keep cannabis away from minors and unauthorized users.
Prescribing Limits
There is a limit on the amount of medical cannabis an individual can possess. For example, within 30 days, a patient may hold up to 4 ounces of raw cannabis, or 28 grams of concentrate.
Business Licensing
Medical cannabis will be dispensed through licensed and regulated dispensaries. There will be a limited number of cultivation and dispensary licenses issued throughout the state to ensure a controlled distribution of cannabis products. Regulations for cannabis dispensary businesses outline how cultivators, processors, producers, safety compliance facilities, and dispensaries can apply, become licensed, and operate in Kentucky. (6)
Steps for Patients to Obtain Medical Cannabis in Kentucky
- Obtain a Medical Cannabis Card: After receiving a written certification from a registered healthcare provider, patients must apply to the Kentucky Office of Medical Cannabis to get a medical cannabis card.
- Visit a Licensed Dispensary: Once a medical cannabis card is obtained, patients will be able to purchase cannabis products from one of the licensed dispensaries operating throughout Kentucky.
- Follow Purchase and Use Guidelines: Patients can purchase medical cannabis products within the legal limits set by the program (e.g., certain amounts of raw plant material, concentrates, or THC-infused products).

Self Quiz
Ask yourself...
- What date will the legalization of medical cannabis (with significant regulations) become legalized in the state of Kentucky?
- Can you describe the qualifications and process of becoming a Registered Medical Cannabis Practitioner?
- What are the steps for patients with a qualifying medical condition should take to legally purchase medical cannabis in Kentucky?
- Can you list the conditions allowed for medical cannabis prescriptions?
Overview and Definitions
Cannabis is one of the most commonly used substances worldwide. It has been used for centuries for recreational and medical use. Cannabis comes in a variety of strains with different concentrations of phytocannabinoids.
Cannabis is becoming more popular due to research supporting therapeutic effects on medical conditions with fewer safety issues. For example, it is not associated with fatal overdoses (1). The human lethal dose is estimated at over 15 g of THC, which is well above the recommended dose. The lethal dose is 750 times greater than a typical intoxicating dose of 20 mg (1). Additionally, unlike opioids, cannabis does not cause respiratory depression due to low cannabinoid receptor expression in the brainstem.
Cannabis has been known by many names, including marijuana, weed, pot, ganja, and Mary Jane. The primary product is the dried flowers of the Cannabis Sativa plant.
Cannabis has been used in various forms, the most common being (13):
- Pulmonary Route
- Smoking and vaping
- Gastrointestinal Route
- Edibles, tea, and other food products
- Dermal Route
- Creams and ointments
The Cannabis Plant and its Components
The cannabis plant is a complex organism that has been used for medicinal, recreational, and industrial purposes for centuries. It belongs to the Cannabaceae family and contains a variety of chemical compounds that contribute to its effects on the human body (11).
Medicinal cannabis encompasses a diversity of products. Cannabis contains approximately 500 molecules that create about 100 plant-derived cannabis compounds (phytocannabinoids), terpenes, and flavonoids. Widely used phytocannabinoids include A9-tetrahydrocannabinol (THC) and cannabidiol (CBD).
THC is responsible for the intoxicating effects of recreational cannabis, whereas CBD is not intoxicating (1). There are unique therapeutic properties, which are attributed to its chemical components, including cannabinoids, terpenes, and flavonoids. Below is a description of the key components of the cannabis plant:
Cannabinoids
- THC (Tetrahydrocannabinol): This is the primary psychoactive compound in cannabis, responsible for the “high” associated with its use. THC affects mood, perception, and cognitive functions. It also has therapeutic uses, such as pain relief and appetite stimulation.
- CBD (Cannabidiol): Unlike THC, CBD is non-psychoactive and has other therapeutic properties, including anti-inflammatory, analgesic, anti-anxiety, and anti-seizure effects.
- Other Cannabinoids: CBG (Cannabigerol), CBN (Cannabinol), and THCV (Tetrahydrocannabivarin), each with unique effects and potential therapeutic benefits.
Terpenes
- Terpenes are aromatic compounds found in cannabis and many other plants. They contribute to the plant’s distinct scent and flavor profiles. Beyond aroma, terpenes also have therapeutic effects and work synergistically with cannabinoids in what is known as the “entourage effect.”
- Some of the key terpenes found in cannabis include:
- Limonene: This terpene has a citrus-like aroma, and it’s shown to often have anti-anxiety and mood-enhancing properties.
- Myrcene: Has a musky, earthy scent and is believed to help with sleep.
- Pinene: Found in pine trees, it may improve focus and memory and has anti-inflammatory properties.
- Linalool: Has a lavender-like scent, this terpene is believed to reduce anxiety and stress.
Flavonoids
Flavonoids are not a well-known group of compounds found in cannabis. They are responsible for the color and pigmentation of the plant, while they also have antioxidant and anti-inflammatory effects (13).
Plant Structure
- Leaves: Cannabis leaves are uniquely shaped and used to identify the plant. They contain trichomes (small glands) that produce and store cannabinoids and terpenes.
- Flowers (Buds): The flowers or buds of the female cannabis plant contain the highest concentration of cannabinoids and terpenes. This is the part of the plant that is typically harvested for consumption.
- Seeds: Cannabis seeds do not contain cannabinoids but are used for industrial purposes, such as producing hemp oil and protein.
- Stalks and Fibers: In industrial applications, the fibers of the cannabis plant are used to make textiles, paper, and building materials. Hemp, a variety of cannabis, is particularly known for its durable fibers.
Types of Cannabis Plants
There are several types of cannabis plants, and they are typically classified based on their species, characteristics, and effects. Dispensaries may offer products based on types of cannabis plants.
The most common types of cannabis plants include (11):
- Cannabis Sativa
- Native to equatorial regions such as Central and South America, Southeast Asia, and Africa.
- Sativa plants are tall, often growing over 10 feet, with narrow, light green leaves.
- Known for providing an energizing and uplifting high, Sativa strains are often used to enhance focus, creativity, and productivity.
- Uses: Commonly recommended for treating conditions like depression and chronic fatigue.
- Cannabis Indica
- Commonly found in colder, mountainous regions such as Afghanistan, Pakistan, and India.
- Indica plants are shorter, bushier, and have broad, dark green leaves. They tend to grow faster and have shorter flowering periods.
- Indica strains are known for their sedative and relaxing effects.
- Uses: Managing chronic pain, insomnia, anxiety, and muscle spasms.
- Cannabis Ruderalis
- Native to Central and Eastern Europe and Russia.
- It is generally used for medicinal use, as its low THC content makes it less psychoactive but still useful in treating certain medical conditions.
- Hybrid Strains of Cannabis
- Hybrids are made by crossbreeding Sativa, Indica, and sometimes Ruderalis to combine the best effects of both Sativa and Indica for specific therapeutic or recreational needs.
- Sativa-dominant hybrids: Uplifting and energizing effects.
- Indica-dominant hybrids: Calming, relaxing effects.
- Balanced hybrids: A mix of mental and physical effects.
- Hybrids are tailored to meet individual needs, often used for managing conditions like pain, anxiety, insomnia, and lack of appetite.

Self Quiz
Ask yourself...
- Can you explain the different effects between THC and CBD?
- Why is knowledge of the differences important in prescribing and teaching patients about medical cannabis and its use for certain medical conditions?
- Can you name the different types of cannabis plants and their suggested effects on the body?
- Consider the different routes that medical cannabis can be consumed. Why should the route be different for each patient?
Pathophysiology
Cannabinoid receptors are G protein-coupled receptors that regulate the activity of neurotransmitters that maintain homeostasis. Activation of cannabinoid receptors occurs when the endocannabinoids produced by the body, or compounds derived from the cannabis plant or synthetic cannabinoids, bind to them. Activation of cannabinoid receptors inhibits the action of adenylyl cyclases and blocks specific calcium voltage-gated channels while stimulating selected mitogen-activated protein kinases and potassium channels (7). The cannabinoid receptors are modulated by fatty acid neurotransmitters called endocannabinoids or endogenous cannabinoids.
G-protein coupled receptors (GPCRs) are a large family of receptors on the surface of a cell that play a crucial role in transmitting signals from the outside of a cell to the inside. These receptors respond to a variety of external stimuli, such as hormones, neurotransmitters, and sensory signals (like light or odor molecules). Once activated by these signals, GPCRs interact with G-proteins inside the cell, which then trigger various cellular responses, including changes in enzyme activity, ion channel function, or gene expression.
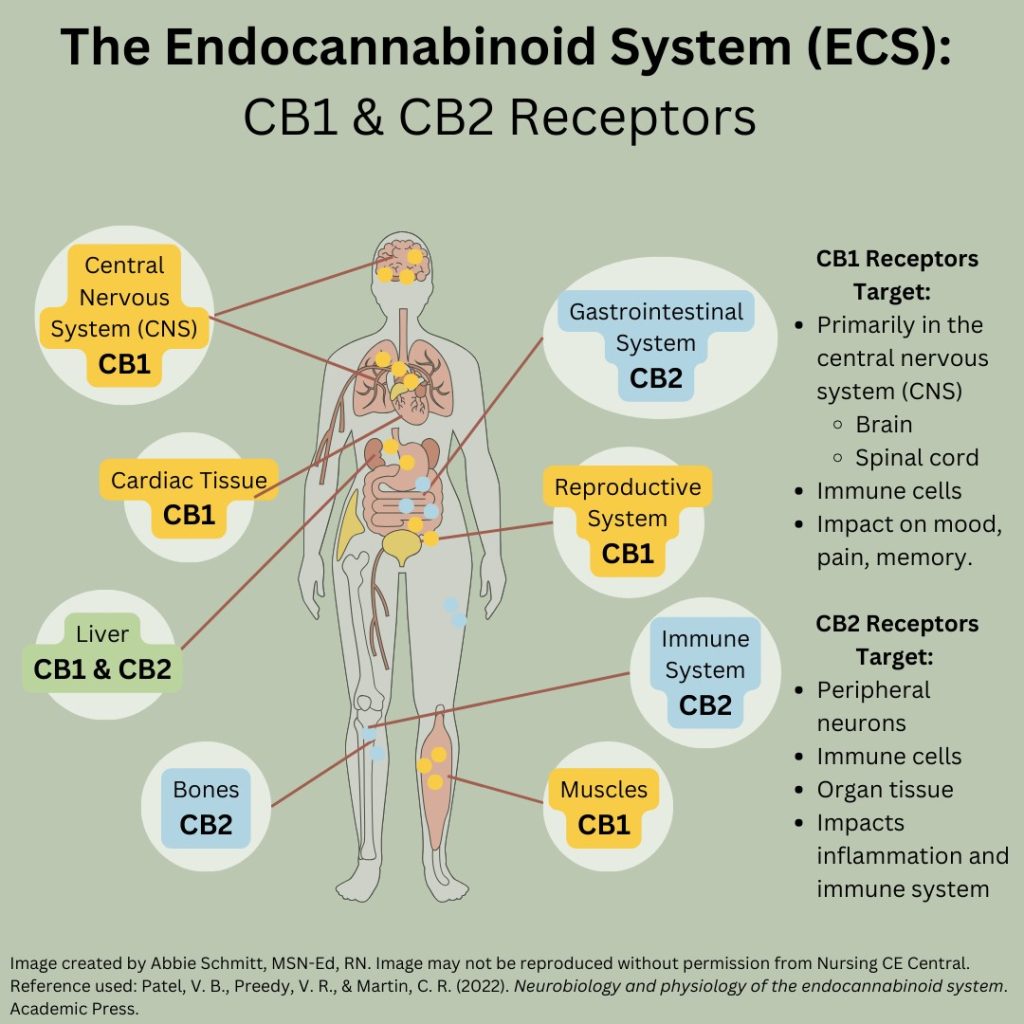
There is widespread distribution of 2 G-protein-coupled receptors (GPCRs) – cannabinoid (CB) CB1 and CB2 – throughout the human body (12). CB1 receptors are primarily concentrated in the central nervous system, but CB1 receptors have also been identified in other areas, including in cardiac tissue, reproductive organs, and the gastrointestinal tract (12). CB2 receptors are concentrated in the immune system and other peripheral regions and organs (12).
CB1 receptors are found throughout the nervous system.
- Effects of this receptor include:
- Euphoric effects
- Hypotension
- Anti-inflammatory response
- Immunosuppression
- Analgesia
- Appetite stimulant
CB2 receptors are found in tissues of the immune system, liver, and some neurons.
- Associated with anti-inflammatory effects and immune modulation.
The Endocannabinoid System
The endocannabinoid system (ECS) comprises a large neuromodulator network throughout the central nervous system (CNS) and body. Essentially, this system is a mediator to regulate a harmonious balance between the brain and the body. The ECS is an extensive network of receptors, their endogenous ligands, and enzymes that synthesize and degrade those ligands (12). Major problems arise when the nervous system and body are out of synch – imagine the ECS is a language translator between two individuals, relaying either positive or negative messages.
The ECS affects biological functions, including sleep, memory, mood, learning, hunger and feeding, and pain (9).
The endogenous or exogenous activation of CB1 and CB2 receptors from the cannabis plant has a significant influence on the regulation of diverse systems throughout the body, which points toward numerous possible clinical implications for the therapeutic use of cannabis in managing chronic conditions.

Self Quiz
Ask yourself...
- How would you describe the endocannabinoid system (ECS) and its impact on biological functions?
- Which receptors are found primarily in the CNS and impact mood, pain sensation, and memory?
- Why is it important to recognize that activation of cannabinoid receptors blocks specific calcium voltage-gated channels?
- Can you name neurological conditions that would likely be affected by CB1 receptors?
Clinical Application and Use
Cannabis for medical conditions is being actively researched. Cannabis does not cause respiratory depression like opioids, which would be an ideal alternative or adjunct to opioids.
Pharmaceutical products may be obtained with a valid prescription at pharmacies in the United States. However, whole-plant and artisanal formulations are available from stores only in states that have legalized cannabis for medical or recreational use since cannabis is largely still illegal at the federal level at this time. Kentucky is legalizing it only for appropriate medical purposes with guidelines and restrictions.
Qualifying conditions include any type or form of cancer, chronic pain, epilepsy or other seizure disorder, spasticity, multiple sclerosis, chronic nausea or vomiting, and post-traumatic stress disorder. There are other medical conditions or diseases for which the Kentucky Center for Cannabis may find medical cannabis appropriate if research supports its effectiveness.
Patients under 18 cannot purchase cannabis, their parents or legal would need to pick up and administer their medical cannabis.
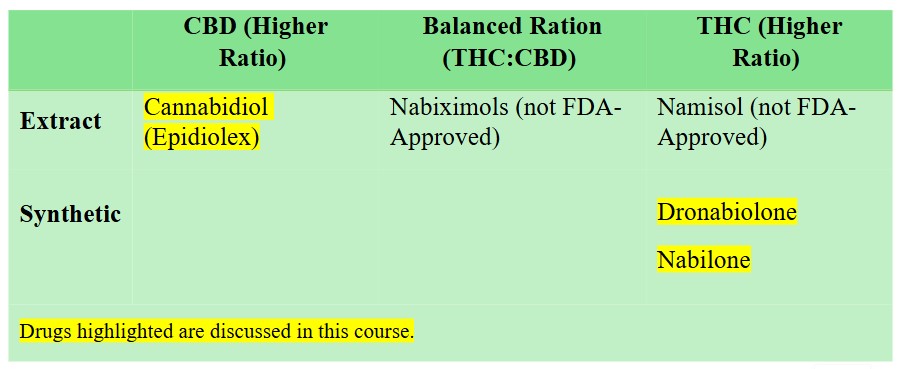
Remember, prescribers must understand the varying ratios of THC and CBD in medical cannabis and that different ratios are more effective for certain medical conditions.
Cannabis cultivators classify the products under the following chemotypes:
- Chemotype I – THC: CBD ratio is ≥10
- Chemotype II – THC: CBD ratio is between 0.2 and 10
- Chemotype II – THC: CBD <0.2
- Chemotype IV is predominantly Cannabigerol
THC is responsible for most of the pharmacological outcomes of cannabis, including analgesic, antioxidant, anti-inflammatory, bronchodilator, antipruritic, and anti-spastic activities (4). However, THC has a psychoactive effect and can promote dependency among chronic users as it interacts with the dopaminergic system (4). CBD is reported to reduce inflammation, muscle spasms, seizures, and anxiety, which can also be helpful in therapeutic cancer management.
Cancer
Pain is very common among those with cancer. Roughly 30% to 50% of people with cancer will experience moderate‐to‐severe pain. Pain can be a consequence of the tumor itself or treatment interventions, including surgery, chemotherapy, and radiotherapy (4). This can have a major negative impact on their quality of life. However, pain is not sufficiently relieved by opioid medications in 10% to 15% of those with cancer, so alternatives are needed to effectively and safely supplement or replace opioids. Currently, an increasing number of patients with chronic cancer pain are seeking alternative treatment options.
Nausea and vomiting can also have a significant impact on cancer patients, as they are common side effects of chemotherapeutic agents.
Poor appetite and nutrition can result from cancer treatments. Tumors can also release molecules that mimic natural hormones involved in satiety.
Research on medicinal cannabis in simulating appetite has been tried in cancer patients with positive outcomes (13).
Chronic Noncancer Pain
The ECS is intricately involved in pain perception and pain modulation, with endocannabinoids and CB receptors found in peripheral tissues, the spinal cord, and areas of the brain associated with nociception. Endocannabinoids and phytocannabinoids have demonstrated antinociceptive effects for acute pain, inflammatory pain, and neuropathic pain (12).
A systematic review published in 2022 reviewed placebo groups and various THC: CBD ratios (high, comparable, or low); the studies found that synthetic products with high THC: CBD ratios were associated with a moderately beneficial effect on pain and an increased risk for sedation (12).
Given the increasing consequences of the use of opioids for the treatment of chronic pain and the risk for opioid abuse and diversion, there is considerable interest in whether medical cannabis could replace or reduce opioids in these patients. A recent systematic review found evidence from observational studies suggesting that cannabis-based medicines are associated with a reduction in opioid use. However, additional longitudinal studies are needed.
Epilepsy and other Seizure Disorders
The ECS regulates neuronal excitability and is used for the treatment of epilepsy. There is high-quality evidence that medical cannabis has been shown to decrease the frequency of seizures compared to placebo (13).
The only approved purified CBD formulation in the U.S. is Epidiolex, which is a form of purified CBD (~99%) with minimal <1% THC (13). Epidiolex is the first and only FDA-approved drug derived from the cannabis plant and is used to treat seizures associated with three rare and severe forms of epilepsy: Lennox-Gastaut syndrome (LGS), Dravet syndrome, and Tuberous Sclerosis Complex (TSC).
Multiple Sclerosis
Multiple sclerosis (MS) is a chronic disease of the CNS and is characterized by demyelinating plaques in grey and white matter that leads to progressive neuroaxonal loss.
The most distressing and challenging to treat symptoms of multiple sclerosis are spasticity, painful spasms, and neuropathic pain.
Nabiximol was developed to treat MS symptoms, including neuropathic pain and spasticity.
Post-Traumatic Stress Disorder
The cannabinoid-1 receptor (CB1) and endocannabinoid anandamide have been implicated in the etiology and pathophysiology of PTSD (10). However, research studies have found varying results on the effectiveness of this treatment. One study found an increase in suicidal ideation among veterans diagnosed with PTSD following the use of medical cannabis (10). Essentially, further research and close monitoring are needed.

Self Quiz
Ask yourself...
- Can you discuss the complications of cancer treatment and how medical cannabis could be a treatment option?
- Which of these drugs is approved for the treatment of rare and severe forms of epilepsy?
- Higher ratios of THC: CBD has correlated with greater levels of pain control, but also has increased levels of what?
- True or False: Individuals under the age of 18 can legally purchase medical cannabis in Kentucky, as long as they have their written certification.
Pharmacokinetics
As of 2024, several FDA-approved medical cannabis products are used for specific medical conditions. The drugs are derived from cannabis or synthetic cannabinoids and have been approved for use in treating certain conditions.
Medical providers can prescribe these medications or guide patients in purchasing medical cannabis at dispensaries based on their condition and unique medical needs. Prescribers need to carefully consider the A9-tetrahydrocannabinol (THC) and/or cannabidiol (CBD) content of the products, essentially the ratios of each compound and the use for specific medical conditions. Essentially, medical cannabis is not a “one size fits all”.
CBD is not intoxicating and has fewer adverse effects than THC. However, THC has increased analgesia effects. Meta-analysis of studies found that synthetic products with high THC: CBD ratios (e.g., dronabinol, nabilone) were associated with a moderately beneficial effect on pain severity but had an increased risk for sedation (12).
It is recommended to prescribe relatively low doses and slowly increase the dosage to minimize dose-related toxicities and the potential for drug-drug interactions with concomitant medications (1).
FDA-Approved Medical Cannabis Products:
- Epidiolex (Cannabidiol)
- Marinol (Dronabinol)
- Cesamet (Nabilone)

Self Quiz
Ask yourself...
- Can you name the FDA-approved cannabis drugs?
- Does CBD or THC have more intoxicating and sedating effects?
- How would you describe the difference between a synthetic product and an extract?
- Does Epidiolex have a higher ratio of CBD or THC?
Cannabidiol (Epidiolex)
Cannabidiol is an oral cannabinoid indicated for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis complex (3).
Cannabidiol is a derivative; however, it lacks the psychoactive properties commonly associated with delta-9-tetrahydrocannabinol (THC). Adjunctive cannabidiol treatment significantly reduced seizure frequency in this population with a reduction in seizures observed within 4 weeks of initiation (3).
Drug Class
- Anticonvulsant
- Cannabinoid
Uses
Cannabidiol is an oral cannabinoid indicated for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis complex (3, 14).
Mechanism of Action
The precise mechanisms of action of this drug's anticonvulsant effect are unknown. Epidiolex does not appear to bind with cannabinoid receptors (3). It is suspected to be effective in epilepsy by modulating the endocannabinoid system by preventing the degradation of anandamide, which is an endocannabinoid that may have a role in seizure inhibition. Epidiolex is also believed to regulate T-type calcium channels and nuclear peroxisome proliferator-activated receptor gamma, which are both involved in seizure activity (3).
Pharmacokinetics
Pharmacokinetics (3):
- Cannabidiol is administered orally.
- Protein binding of cannabidiol and its metabolites is more than 94%.
- Cannabidiol is metabolized by the liver (primarily) and the gut.
- The active metabolite, 7-OH-CBD, is further converted to an inactive metabolite, 7-COOH-CBD.
- Half-life ranges from 56 to 61 hours after twice-daily dosing for 7 days.
- Plasma clearance after a single 1,500 mg dose is 1,111 L/hour.
- Excretion occurs in the feces with minor renal clearance.
Adverse Reactions
Adverse reactions (14):
- Elevated hepatic enzymes, hyperammonemia
- Serum creatinine elevations of approximately 10% were observed within 2 weeks of starting cannabidiol.
- Cannabidiol causes elevated hepatic enzymes (8% to 25%). Promptly measure serum transaminases and total bilirubin and discontinue if the patient develops clinical signs or symptoms suggestive of liver injury (e.g., unexplained nausea, vomiting, right upper quadrant abdominal pain, fatigue, anorexia, jaundice, and/or dark urine).
- Anemia, eosinophilia, thrombocytopenia
- Cannabidiol can cause decreases in hemoglobin and hematocrit. Anemia (7%), decreased platelet count/thrombocytopenia (5%), and increased eosinophil count/eosinophilia (5%) were reported in cannabidiol-treated patients during tuberous sclerosis complex (TSC) trials.
- Agitation and irritability
- Insomnia and sleep disturbance
- Drowsiness and lethargy
- Hypersalivation
- Angioedema, erythema, pruritus, rash
- Suicidal ideation and behavior
- Monitor all patients beginning treatment with AEDs or currently receiving such treatment closely for emerging or worsening suicidal thoughts/behavior or depression. Patients and caregivers should be informed of the increased risk of suicidal thoughts and behaviors and should be advised to immediately report the emergence or worsening of depression, the emergence of suicidal thoughts or behavior, thoughts of self-harm, or other unusual changes in mood or behavior.
- Anorexia (16% to 22%), diarrhea (9% to 31%), vomiting (17%), nausea (9%), weight loss (3% to 7%), gastroenteritis (0% to 8%), and abdominal pain/discomfort (3%) were reported during clinical trials.
Warnings / Contraindications
THC found in medicinal cannabis may acutely impair cognitive function. It should not be prescribed for children or adolescents unless the benefits outweigh the risks. THC-containing cannabis products should not be prescribed to individuals with angina or a history of myocardial infarction, or to those who have a personal or family history of psychosis, depression, or suicidal ideation. (3)
Cannabidiol is contraindicated in patients with a history of cannabidiol hypersensitivity, sesame oil hypersensitivity, or hypersensitivity to any of the ingredients in the product.
Cannabidiol dosage adjustment is recommended in patients with moderate or severe hepatic disease. Obtain serum transaminase (ALT and AST) and total bilirubin concentrations before starting cannabidiol treatment, at 1 month, 3 months, and 6 months after initiation, and periodically thereafter or as clinically indicated (3). Patients taking valproate or clobazam are at greater risk, so consider more frequent monitoring of serum transaminases and bilirubin in patients who are taking valproate or who have elevated baseline hepatic enzymes.
Patients should avoid abrupt discontinuation, cannabidiol should be discontinued gradually. As with all antiepileptic drugs, abrupt discontinuation of cannabidiol can increase the risk of seizure frequency and status epilepticus (14).
Pregnancy and breast-feeding: There is not enough data on the developmental risks associated with cannabidiol use during pregnancy or lactation. This drug is highly lipophilic, and it is expected to be secreted in human milk. Consider the developmental and health benefits of breastfeeding along with the mother's clinical need for cannabidiol and any potential adverse effects on the breast-fed infant from cannabidiol or the underlying maternal condition. (14)
Oral Dosage
- Adults: 2.5 mg/kg/dose PO twice daily; increase in weekly increments of 2.5 mg/kg/dose twice daily as tolerated. The recommended maintenance dosage is 5 mg/kg/dose PO twice daily. Max: 10 mg/kg/dose PO twice daily.
- Children and Adolescents (1 to 17 years): 2.5 mg/kg/dose PO twice daily; increase in weekly increments of 2.5 mg/kg/dose twice daily as tolerated. Continue to the recommended maintenance dosage of 5 mg/kg/dose PO twice daily. Max: 10 mg/kg/dose PO twice daily.
- If rapid titration is necessary, increase the dosage no more frequently than every other day.
Alternatives
Antiepileptic drugs (AEDs) would be an appropriate alternative, as well as sodium channel blockers, calcium channel blockers, and GABA drugs.

Self Quiz
Ask yourself...
- What are the contraindications of this drug?
- Should Epidiolex be recommended to women who are pregnant or breast-feeding?
- Can you discuss the appropriate lab work to obtain before this drug is initiated?
- How can prescribers alter the dosage for patients with hepatic disease?
Dronabinol
Dronabinol is a synthetic oral preparation of delta-9-tetrahydrocannabinol (delta-9-THC) and is a cannabinoid (3).
Brand Names: Marinol, SYNDROS
Drug Class
Cannabinoid
Uses
Dronabinol is FDA-approved for:
- Nausea and vomiting secondary to cancer chemotherapy or HIV/AIDS
- Appetite stimulant
Mechanism of Action
Dronabinol is an orally active cannabinoid (a synthetic form of THC). As mentioned earlier, the majority of effects of cannabinoids and endocannabinoids are completed by the two inhibitory G-protein coupled receptors (GPCRs): CB1 (present in high levels in several brain regions, including the prefrontal cortex, hippocampus, amygdala, basal ganglia, and the cerebellum) and CB2 (present in a minimal distribution in the brain stem and periphery including immune cells and neurons) (8). Dronabinol has effects on the CNS, including central sympathomimetic activity.
Dronabinol, like THC, is a partial agonist to the CB1 receptor. Cannabinoid signaling impacts pain modulation, cognition, appetite stimulation, nausea, and others (8). The anti-emetic and appetite stimulation effects of the CB1 receptor appear to mediate the therapeutic effects of dronabinol.
Cannabinoids exert a wide range of CNS effects including short-term memory deficits, sense of time-lapse, increased sensation, and cognitive reasoning. Cannabinoids also produce both euphoria and dysphoria, which depends on prior use and the dose administered. The brain distribution of CB1 receptors and receptor-activated G-proteins correlates with exhibited behavior (3).
Cannabinoids also exhibit CNS-mediated effects on thermoregulation, feeding behavior, nausea, and reward mechanisms. It is now hypothesized that the antiemetic effects are mediated by cannabinoid receptors in the vomiting center of the medulla (3). Cannabinoids may act in the vomiting center to combat the effects of serotonin to block the release of neurotransmitters from vagal afferent terminals, which help to produce emesis (3). Essentially, the neurological impact of the drug can impede messages of nausea and vomiting response.
Appetite stimulation effects are believed to be mediated by cannabinoid receptors in the lateral hypothalamus (3). Cannabinoids impact body temperature directly at brain regulatory centers by inhibiting noradrenergic activity in the hypothalamus.
Pharmacokinetics
Metabolism: Liver via extensive first-pass, cytochrome P450 2C9, and cytochrome P450 3A4.
Onset of Action: Typically, 0.5 to 1 hour
Half-life: 4 hours
Bioavailability: 10 to 20%
Adverse Effects
Adverse effects (8):
- Hypersensitivity to Dronabinol or Sesame Oil; reported signs commonly include lip swelling, hive, rash, oral lesions, skin burning, and throat tightness.
- Abdominal pain
- Dizziness
- Euphoria
- Paranoia
- Somnolence
- Psychiatric and cognitive changes.
- Possible mental and physical impairment and delay in reaction. Patients should not operate heavy machinery or motor vehicles until it is ruled out that the drug impairs their ability to operate.
- Hemodynamic Changes
- Dronabinol can cause hemodynamic instability in patients with existing cardiac disorders.
- Decreased or increased blood pressure, syncope, or rapid heart rate.
- Monitor for these changes after initiating treatment and modifying doses.
- Seizures in patients with a prior medical history of seizures.
- Drug misuse and abuse risk (especially in those with a substance abuse history).
- Paradoxical Nausea and Vomiting
Warnings
This drug is contraindicated in patients who have a history of hypersensitivity reactions to sesame oil or THC derivatives (8).
Signs and symptoms of toxicity include (8):
- Mild Intoxication: sleepiness, feelings of joy, heightened sensory vigilance, time perception difficulties, conjunctival injection, dry oral cavity, and elevated heart rate.
- Moderate Intoxication: Memory difficulty, feelings of detachment, mood changes, retention of urine, and decreased bowel motility.
- Severe: Decreased coordination of motor function, lethargy, slurring of speech, and orthostatic hypotension.
Panic attacks in those with anxiety and seizure activity in those with a history of seizures can also occur.


Self Quiz
Ask yourself...
- Can you list the uses for this drug?
- What are the signs and symptoms of toxic levels?
- Can you discuss the adverse effects and how to assess them?
- What patient education topics should be included for drugs that commonly cause drowsiness or dizziness?
Nabilone (Cesamet)
Nabilone is a synthetic cannabinoid that mimics the effects of THC (8).
Drug Class
Antiemetic
Uses
The treatment or prophylaxis of nausea and vomiting associated with chemotherapy in patients who have not responded adequately to other treatments.
Mechanism of Action
Nabilone acts as an agonist at endogenous cannabinoid receptors, CB1 and CB2, which decreases the excitability of neurons (13). The antiemetic effects are thought to be mediated by cannabinoid (CB1) receptors in the “vomiting center” of the medulla. Cannabinoids may act in the vomiting center to oppose the effects of serotonin (5-HT3) and block the release of neurotransmitters from vagal afferent terminals that produce emesis.
Pharmacokinetics
Absorption: Rapid and complete
Distribution: ~12.5 L/kg
Metabolism: Extensively metabolized to several active metabolites by oxidation and stereospecific enzyme reduction; CYP450 enzymes may also be involved
Half-life elimination: Parent compound: ~2 hours; Metabolites: ~35 hours
Peak, serum: Within 2 hours
Excretion: Feces (~60%); renal (~24%)
Adverse Effects
- Central nervous system (similar to other cannabinoids)
- Dizziness (52% to 59%)
- Drowsiness
- Ataxia
- Euphoria
- Headache
- Difficulty concentration
- Sleep disturbance
- Hypotension
- Anorexia
- Cardiac arrhythmias
- Syncope
- Tachycardia
- Flushing
- Cough and/or wheezing
Warnings
Warnings (15):
Nabilone is a schedule II-controlled substance, which is considered to have a high potential for abuse. Prescribers should limit prescriptions to the amount necessary for a single chemotherapy cycle. Monitor patients for signs of excessive use, abuse, and misuse. Use with caution in patients with a history of substance abuse.
Use with caution in patients with cardiovascular disease, it may cause tachycardia and/or orthostatic hypotension.
It may impair physical or mental abilities; patients must be cautioned about performing tasks that require mental alertness (e.g., operating machinery or driving).
Use with caution in patients with mania, depression, or schizophrenia; cannabinoid use may enhance symptoms of psychiatric disorders.
Use with caution in the elderly, it can cause postural hypotension.
Alternatives to Medical Cannabis
Alternative treatments for patients whose pain is partially responsive to opioids include antidepressants, anticonvulsants, local analgesics, and corticosteroids (4).

Self Quiz
Ask yourself...
- Why is it important to avoid use in patients with a history of substance abuse?
- Can you discuss the adverse effects of this drug?
- Why should caution be used in prescribing the drug to elderly patients?
- Should this drug be the first line of treatment for patients experiencing nausea and vomiting from chemotherapy treatment?
Cannabis Use Disorder
Cannabis Use Disorder (CUD) is a medical condition that is broadly defined as the inability to stop consuming cannabis even when it is causing physical or psychological harm (2). This disorder is estimated to affect roughly 10% of cannabis users worldwide (2).
CUD is in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as a substance use disorder, highlighting how some individuals develop a dependency or addiction to cannabis.
Endocannabinoids and the Reward System
Building on our knowledge learned earlier on the ECS, it is important to recognize that CB1 receptor stimulation can also indirectly activate the dopaminergic system that mediates the rewarding effects of many drugs (2). It is most likely that THC indirectly increases dopaminergic activity by encouraging the firing of dopaminergic neurons in the midbrain. (2).
Essentially, this release of dopamine can lead to addiction to this sensation and continued use for this purpose rather than the initial purpose intended (e.g. pain or anxiety).
Risk Factors
- Using cannabis at a younger age increases the likelihood of developing CUD.
- Daily or frequent cannabis use heightens the risk of dependency.
- Using cannabis in conjunction with other substances, like alcohol, illicit drugs, or nicotine, can increase the likelihood of developing CUD.
Manifestations of Cannabis Use Disorder
Individuals with CUD may use cannabis in larger amounts or for longer than prescribed. They may express a desire to stop but are unable to do so. Significant time is usually spent obtaining, using, and recovering from cannabis use, often interfering with responsibilities and social obligations. Tolerance and withdrawal are also major characteristics of CUD. Over time, the individual may need more cannabis to achieve the same effects due to reduced sensitivity (2).
Treatment
The optimal treatments for most substance use disorders) combine psychosocial and pharmacological interventions.
Psychosocial-based interventions:
- Cognitive behavioral therapy (CBT)
- Motivational enhancement therapy (MET)
- Abstinence-based contingency management combined with CBT and MET

Self Quiz
Ask yourself...
- Can you discuss the risk factors and manifestations of CUD?
- What are examples of psychosocial-based treatment options for those with CUD?
- How would you describe the impact of endocannabinoids on dopamine and the “reward system”?
- What percentage of the users of cannabis worldwide is estimated to have CUD?
Nursing Considerations and Follow-Up
When teaching patients to safely use medical cannabis, nurses and healthcare providers should emphasize several key points, ensuring patients understand how to use the medication effectively while minimizing potential risks.
Important Considerations
Dosage and Titration
- Start Low and Go Slow
- Educate patients on beginning with the lowest effective dose and gradually increase as needed. This minimizes side effects such as dizziness, fatigue, or psychoactive effects from THC.
- THC vs. CBD Ratio
- Providers should explain the difference between THC (which can be psychoactive) and CBD (which is non-psychoactive) to help patients choose a product with the appropriate balance of cannabinoids based on their condition and tolerance.
Administration Methods
- Inhalation (e.g., vaping, smoking)
- Although inhalation provides rapid onset, smoking should generally be discouraged due to the health risks associated with inhaling combusted material.
- Edibles and Oral Forms
- Edibles and tinctures take longer to take effect (30 minutes to 2 hours), but the effects last longer.
- Patients should be advised to avoid consuming too much too quickly due to the delayed onset.
- Sublingual tinctures are absorbed faster than edibles and allow for more precise dosing.
- Topicals
- Topicals can be helpful for localized pain (e.g., arthritis) without causing systemic psychoactive effects.
Potential Side Effects and Safety
Patients using products with THC should be informed about potential psychoactive effects like euphoria, drowsiness, delayed physical or mental abilities, altered judgment, or anxiety. Prescribers should discourage activities such as driving or operating heavy machinery while taking medical cannabis. Medical cannabis can interact with other medications, so it is important to review the patient’s medications and adjust as necessary.
Although cannabis has a low risk of addiction compared to opioids, patients should still be given education on the potential risk of abuse, particularly with high-THC products.
Legal Use and Safe Storage
Providers should ensure that patients understand the legal status of medical cannabis in their state and the need for a valid medical cannabis card to purchase products from licensed dispensaries. Patients should be instructed to store cannabis in a secure, childproof container and out of reach of children and pets to avoid accidental ingestion.
Patient Monitoring and Follow-Up Providers should follow up regularly to assess pain levels, side effects, and the effectiveness of the treatment. Adjustments to dosage or formulation may be needed. Providers should help patients choose the right strain or product (indica, sativa, hybrid). Prescribers should encourage patients to keep a journal of daily symptoms and to set realistic goals.

Self Quiz
Ask yourself...
- What are creative ways to provide information to visual learners on the side effects of drugs?
- Should driving or operating heavy equipment be advised against while taking medical cannabis?
- Why should providers discuss the risk of addiction to cannabis?
- Have you provided education on cannabis to others before? If so, did you notice any knowledge deficits or stigmas regarding cannabis?
Upcoming Research and Legislation
There are challenges to using cannabis for medical purposes as well as developing clinical trials. This often stems from the legal issues as well as the stigma associated with cannabis use. Some of the challenges in building the evidence for cannabis use include (13):
- Difficulty in designing the clinical trials (randomization, source of cannabis, security measures, etc.).
- Approval to use cannabis for research (e.g., in the US, it would require FDA approval, registration with the Drug Enforcement Agency, and approval from the research ethics board).
- Finding agencies that will fund the study.
Researchers are overcoming these barriers and widening the span of cannabis for medical therapy. For example, there is significant research on the use of medical cannabis for the treatment of Alzheimer’s Disease and dementia, as well as other cognitive-related disorders (5).
Sativex is not yet FDA-approved in the U.S., but it’s used in other countries (e.g., Canada and the UK) for the treatment of multiple sclerosis-related spasticity and neuropathic pain (13). Sativex is under clinical trials in the U.S., particularly for treating multiple sclerosis and certain types of cancer pain, but it is still pending FDA approval. Clinical trials are ongoing to evaluate its safety and efficacy under U.S. regulatory standards (4).


Self Quiz
Ask yourself...
- Are you familiar with the process for gaining FDA approval?
- Can you name some challenges faced by medical cannabis researchers?
- Do you think there is hesitancy among medical providers to prescribe medical cannabis?
- How can medical professionals raise awareness of the benefits and precautions surrounding medical cannabis to the community?
Conclusion
The cannabis plant is a multifaceted organism with various chemical compounds contributing to its medicinal uses. Its cannabinoids, terpenes, and flavonoids work together to provide therapeutic effects, making it an increasingly popular option in modern medicine, especially as research continues to uncover its potential benefits. By providing education and ongoing follow-up, healthcare providers can help ensure that medical cannabis is used safely and effectively for medical conditions while minimizing risks and side effects.

Self Quiz
Ask yourself...
- Which FDA-approved drug is an oral cannabinoid indicated for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, and tuberous sclerosis complex?
- Can you discuss why it is important for prescribers to understand the different actions and effects between CBD and THC?
- Can you explain the process of becoming a Registered Medical Cannabis Practitioner in the Commonwealth of Kentucky?
- True or False: CBD does not have the intoxicating effects that THC commonly has.
- What are the classifications and uses of Dronabinol?
- How would you describe the mechanism of action of synthetic cannabinoids?
- Did the Kentucky government legalize all forms of cannabis for the consumption of all KY residents?
- Can you discuss how medical cannabis could have a positive impact on the opioid crisis faced across the U.S.?
- What government department/ body is responsible for the oversight and regulation of medical cannabis production and distribution?
- Are you likely to utilize these options for appropriate patients in your own practice? Why or why not?
References + Disclaimer
- Arnold, J. C. (2021). A primer on medicinal cannabis safety and potential adverse effects. Australian Journal of General Practice, 50(6), 345-350.
- Connor, J. P., Stjepanović, D., Le, F. B., Hoch, E., Budney, A. J., & Hall, W. D. (2021). Cannabis use and cannabis use disorder (Primer). Nature Reviews: Disease Primers, 7(1). https://doi.org/10.1038/s41572-021-00247-4
- Elsevier. (2024). Drug information. https://www.merckmanuals.com/professional/drug-names-generic-and-brand
- Gorzo, A., Havași, A., Spînu, Ș., Oprea, A., Burz, C., & Sur, D. (2022). Practical considerations for the use of cannabis in cancer pain management: What a medical oncologist should know. Journal of Clinical Medicine, 11(17), 5036. https://doi.org/10.3390/jcm11175036
- Kim, S. H., Yang, J. W., Kim, K. H., Kim, J. U., & Yook, T. H. (2019). A Review on Studies of Marijuana for Alzheimer’s Disease – Focusing on CBD, THC. Journal of Pharmaco-puncture, 22(4), 225–230. https://doi.org/10.3831/KPI.2019.22.030
- Kentucky Office of Medical Cannabis. (2023). Overview of Laws & Regulations. Retrieved from https://kymedcan.ky.gov/laws-and-regulations/Pages/default.aspx?
- Lu, H.C., & Mackie, K. (2021). Review of the endocannabinoid system. Biological Psychiatry., 6(6), 607–615. https://doi.org/10.1016/j.bpsc.2020.07.016
- O’Donnell B, Meissner H, Gupta V. Dronabinol. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557531/
- Patel, V. B., Preedy, V. R., & Martin, C. R. (2022). Neurobiology and physiology of the endocannabinoid system. Academic Press.
- Petersen, M., Koller, K., Straley, C., & Reed, E. (2021). Effect of cannabis use on PTSD treatment outcomes in veterans. The mental health clinician, 11(4), 238–242. https://doi.org/10.9740/mhc.2021.07.238
- Sayed, F., Eisenreich, W. (2024). Bioengineering of Cannabis Plants from Lab to the Field: Challenges and Opportunities. In: Kole, C., Chaurasia, A., Hefferon, K.L., Panigrahi, J. (eds) Applications of Plant Molecular Farming. Concepts and Strategies in Plant Sciences. Springer, Singapore. https://doi.org/10.1007/978-981-97-0176-6_24
- Sera L. & Hempel-Sanderoff, C. (2024). Cannabis Science and Therapeutics: An Overview for Clinicians. J Clin Pharm. 2024: 00-00. https://doi.org/10.1002/jcph.2400
- Valani, R. (2022). Cannabis use in medicine. Springer. https://doi.org/10.1007/978-3-031-12722-9
- Wolters Kluwer Clinical Drug Information (2024). Cannabidiol (Epidiolex). Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx#monoNumber=428027§ionID=00&tab=tab0
- Wolters Kluwer Clinical Drug Information (2024). Nabilone. Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426792#monoNumber=426792§ionID=243259862&tab=tab0
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
