Course
Living with COPD: Management and Treatment
Course Highlights
- In this Living with COPD: Management and Treatment course, we will learn about causes of COPD.
- You’ll also learn the progressive nature of COPD.
- You’ll leave this course with a broader understanding of management strategies for COPD.
About
Contact Hours Awarded: 1
Course By:
R.E. Hengsterman MSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Chronic Obstructive Pulmonary Disease (COPD), the most prevalent chronic respiratory disease, is a long-term inflammatory lung condition that is both preventable and manageable [1]. COPD occurs from reduced airflow resulting from multiple factors: decreased elasticity in the airways and air sacs, destruction of the walls of air sacs, airway wall thickening and inflammation, and excessive mucus production, which can block the airways [3].
Individuals with COPD experience increased breathing difficulty due to a reduced airflow, often leading to symptoms like shortness of breath and fatigue [1]. As COPD progresses, the difficulty in breathing may be apparent during physical activities but can escalate to challenges in both exhaling and inhaling even during rest [2].
Chronic obstructive pulmonary disease (COPD) leads to irreversible lung damage and airway constriction (bronchi), resulting in breathing difficulties in advanced stages [3]. Engaging in daily activities such as ascending stairs, gardening, or going for a walk can become strenuous and cause breathlessness [3]. COPD develops over several years and does not appear without warning [4]. Early signs include the most common, continuous early-morning paroxysm of hacking cough with sputum or “smoker’s cough,” often identified as asthma [4]. Additional symptoms include shortness of breath during physical activities, wheezing, chest tightness, and a chronic cough that may produce mucus (sputum). Individuals with COPD may experience frequent respiratory infections, fatigue, and unintended weight loss in the later stages of the disease, and swelling in the ankles, feet, or legs [1][4].
Chronic obstructive pulmonary disease, or COPD, encompasses a group of diseases, including emphysema and chronic bronchitis, leading to airflow obstruction, and breathing difficulties [5]. In the United States, chronic obstructive pulmonary disease (COPD) impacts more than 16 million adults. A considerable number remain undiagnosed. Among those diagnosed, over 50% are women [6].
While asthma also involves airway narrowing and breathing complications, it is distinct from COPD, though some individuals may experience a combination of both conditions [4]. COPD is a consequence of prolonged exposure to irritating gases or particulate matter including cigarette smoke, and the disease processes increases the risk of heart disease, lung cancer, and other comorbid conditions [1].
Chronic bronchitis involves the inflammation of the bronchial tube lining, responsible for air transport to and from the lungs’ air sacs (alveoli) and defined by daily cough and mucus production [1]. Emphysema is the destruction of alveoli at the ends of the smallest lung air passages (bronchioles), due to damage from cigarette smoke and other irritants [1].


Self-Quiz
Ask Yourself...
- What factors might contribute to the delayed diagnosis or misidentification of COPD as asthma?
- How do lifestyle choices and environmental factors contribute to the development and progression of COPD?
- What are the key differences in the pathophysiology and clinical presentation of COPD and asthma?
- How can these differences inform treatment and management strategies for patients presenting with respiratory symptoms?
Case Study: Management of Chronic Obstructive Pulmonary Disease (COPD)
Name: Jane Doe
Age: 62
Gender: Female
Occupation: Retired schoolteacher
Medical History
Long-term smoker (40 years), diagnosed with hypertension and type 2 diabetes. Family history of COPD.
Presenting Complaint
Jane Doe presented to the clinic with increasing shortness of breath, during light physical activities, a persistent cough producing white sputum, and frequent episodes of wheezing. She reports these symptoms have worsened over the past 5 years.
Differential Diagnosis Considerations
- Asthma
- Asthma-COPD Overlap Syndrome
- Interstitial Lung Disease
- Bronchiolitis Obliterans
- Diffuse Panbronchiolitis (DPB)
- Congestive Heart Failure
- Thromboembolic Disease
- Lymphangioleiomyomatosis (LAM)
- Tuberculosis
- Cystic Fibrosis
- Bronchiectasis
- Lung Cancer (Malignancy)
Clinical Examination
Vital Signs: Blood pressure 140/90 mmHg, pulse 88 bpm, respiratory rate 22 breaths/min, oxygen saturation 92% on room air.
Physical Exam: Prolonged expiratory phase, wheezes on auscultation, barrel chest appearance.
Investigations
Spirometry: Revealed a reduced FEV1/FVC ratio (60% predicted), indicating airflow obstruction.
Chest X-ray: Showed hyperinflated lungs and flattened diaphragm, suggestive of emphysema.
Blood Tests: Normal complete blood count (CBC) elevated fasting blood glucose.
Diagnosis: Chronic Obstructive Pulmonary Disease (COPD), emphysema dominant, complicated by long-term smoking and a family history of COPD.
Management Plan
Pharmacological Treatment:
- Prescribed a combination inhaler containing a long-acting beta-agonist (LABA) and inhaled corticosteroid (ICS).
- Short-acting bronchodilator (SABA) for acute symptom relief.
- Continued management of hypertension and diabetes.
Non-Pharmacological Interventions:
- Initiation of a pulmonary rehabilitation program, focusing on exercise training and breathing techniques.
- Smoking cessation counseling and support.
- Dietary consultation to manage weight and diabetes.
Preventive Care
- Annual influenza vaccine and pneumococcal vaccines as per age-related guidelines.
- Regular follow-up appointments for COPD and comorbidity management.
Patient Education
- Educated about COPD, its progression, the importance of medication adherence, and recognizing signs of exacerbation.
- Instructed on the correct use of inhalers and the importance of lifestyle modifications, including diet and exercise.
Follow-Up and Monitoring
- Scheduled for a follow-up visit in 6 weeks to assess response to treatment.
- Plan for regular spirometry tests to monitor lung function.
Discussion
Jane Doe’s case is typical of a patient with a history of long-term smoking leading to the development of COPD. The management of her condition involves a combination of pharmacological and non-pharmacological approaches, tailored to her individual needs and comorbid conditions. Smoking cessation is a key component of her treatment plan, alongside pharmacotherapy and lifestyle modifications. The aim is to control her symptoms, improve her quality of life, and prevent disease progression. Regular monitoring and patient education are crucial for effective disease management.

Self-Quiz
Ask Yourself...
- How might Jane Doe’s diagnosis of COPD and her medical history of long-term smoking, hypertension, and type 2 diabetes influence the choice and effectiveness of her COPD management plan?
- Considering Jane’s lifestyle as a retired schoolteacher and a long-term smoker, what specific challenges might she face in adhering to the non-pharmacological aspects of her treatment plan, such as smoking cessation and pulmonary rehabilitation?
- In educating Jane about her condition and the importance of medication adherence, what are the key aspects to ensure she recognizes and responds to signs of exacerbation?
Definition / Etiology
In defining COPD, numerous factors lead to narrowed airways, including destruction of lung parts, mucus blockage, inflammation, and swelling of the airway lining [5]. The development of COPD is gradual, often due to a combination of risk factors such as tobacco exposure (both active smoking and passive second-hand smoke), occupational exposure to dust, fumes, or chemicals, and indoor air pollution from biomass fuels or coal in low- and middle-income countries [7].
Early life factors like poor growth in utero, prematurity, and severe childhood respiratory infections, as well as childhood asthma, contribute to its development [1]. In addition, a rare genetic condition, alpha-1 antitrypsin deficiency, can lead to early-onset COPD [8]. Alpha-1 antitrypsin (AAT) deficiency is a genetic condition that increases the risk of developing lung and liver diseases. AAT, a protein produced in the liver, plays a crucial role in protecting the lungs [8]. Insufficient AAT production leaves the lungs more vulnerable to damage from smoking, environmental pollution, or dust, leading to chronic obstructive pulmonary disease (COPD) or bronchiectasis, another type of lung disease [8]. Lung disease often manifests in individuals over 30 [8].
Alpha-1 antitrypsin deficiency is hereditary and often found in Caucasian of Northern-European backgrounds/populations and affects the lungs and liver [10]. In the lungs, it often leads to early-onset emphysema (patients in their 30s and 40s), most often panacinar emphysema at the lung bases [9]. However, there are cases of diffuse or upper lobe emphysema and bronchiectasis [11]. Common respiratory symptoms of this deficiency include shortness of breath, wheezing, and coughing [11]. Pulmonary function tests can reveal patterns consistent with chronic obstructive pulmonary disease (COPD) [12].

Self-Quiz
Ask Yourself...
- How do environmental factors like tobacco exposure and occupational hazards interplay with genetic predispositions such as alpha-1 antitrypsin deficiency in the development and progression of COPD?
- Considering the role of alpha-1 antitrypsin deficiency in leading to early-onset COPD, how might this condition’s diagnosis and management differ from COPD cases primarily caused by lifestyle or environmental factors?
Epidemiology
COPD is present in smokers and those greater than age 40 [13]. Prevalence increases with age, and it is the third most common cause of morbidity and mortality worldwide [1] [3].
Chronic obstructive pulmonary disease (COPD) ranks as the third leading cause of death across the globe, responsible for 3.23 million deaths in 2019 [1]. This condition affects low- and middle-income countries (LMICs) at a disproportionate rate, where 90% of COPD-related deaths under 70 years occur [1]. COPD also stands as the seventh leading cause of disability worldwide, as measured by disability-adjusted life years [1]. In high-income countries, over 70% of COPD cases have a connection to tobacco smoking, while in LMICs, tobacco accounts for 30-40% of cases, with household air pollution also being a significant risk factor [1].
The primary risk factors for chronic respiratory diseases (CRDs) include tobacco use, exposure to indoor and outdoor pollutants, allergens, occupational hazards, unhealthy diet, obesity, and physical inactivity [8]. The prevalence of COPD in adults over 40 ranges between 15% and 20% [14]. A comprehensive analysis spanning 30 countries over 14 years highlighted a higher COPD occurrence in individuals with a smoking history [14]. The incidence rate of COPD differs between genders; a study found it to be higher in men than in women, with a notable increase from the age of 45 in both sexes and higher rates among current and former smokers compared to non-smokers [15]. The risk of death from COPD is related to the severity of the disease, with both severe and moderate stages showing higher hazard ratios, indicating a greater likelihood of mortality [15]. The mortality rate from chronic obstructive pulmonary disease (COPD) is higher in men compared to women and tends to increase in individuals over the age of 45 [14].

Self-Quiz
Ask Yourself...
- Given that the prevalence of COPD increases with age and is high in individuals with a history of smoking, what might this suggest about the cumulative effects of smoking over time on lung health?
- Considering that COPD is a leading cause of death with a higher impact in low- and middle-income countries, how do socioeconomic factors and access to healthcare contribute to this disparity?
- What factors might contribute to the higher incidence and severity of COPD in men compared to women, as indicated by the study findings?
Pathophysiology
COPD encompasses two primary pathologies: emphysema, defined by alveolar wall destruction and irreversible enlargement of air spaces distal to the terminal bronchioles, and chronic bronchitis [19]. The structural impacts affect lung parenchyma, and pulmonary vasculature, often resulting from oxidative stress and imbalances between proteases and antiproteases and between oxidants and antioxidants [16]. This airflow obstruction is progressive and defined by an abnormal inflammatory response in the lungs, stemming from both innate and adaptive immune responses to prolonged exposure to harmful particles and gases including cigarette smoke [17]. These cells release inflammatory mediators, oxidants, and excess proteases, leading to the breakdown of air sacs and causing the destruction of elastin, an essential component for maintaining the lungs’ elasticity, results in airway collapse during exhalation [20].
While all cigarette smokers experience some level of lung inflammation, those who develop COPD exhibit an enhanced or abnormal response when exposed to these toxic agents [18]. This heightened inflammatory response can lead to mucous hypersecretion, which characterizes chronic bronchitis, tissue destruction indicative of emphysema, and disruption of normal lung repair and defense mechanisms [17]. This disruption often results in inflammation and fibrosis in the small airways, a condition known as bronchiolitis.
The inflammatory response in COPD leads to a decrease in forced expiratory volume (FEV1) and tissue destruction, causing airflow limitation and impaired gas exchange [3]. Lung hyperinflation, often observed in imaging studies, results from air trapping due to airway collapse during exhalation, which also elevates carbon dioxide (CO2) levels. As COPD progresses, gas exchange becomes more impaired, leading to CO2 retention and pulmonary hypertension, which results from diffuse vasoconstriction due to hypoxemia [21].
Alpha-1 antitrypsin deficiency (AATD) is a less common cause of emphysema [8]. This genetic condition involves a lack of antiproteases, leaving lung tissue vulnerable to protease-mediated damage [8]. AATD is the misfolding of the mutated protein, leading to its accumulation in the liver. In COPD patients with liver damage AATD requires consideration [3]. Unlike smoking-related emphysema, which affects the upper lobes of the lungs, AATD impacts the lower lobes [3].
Exacerbations in chronic obstructive pulmonary disease (COPD) result from intricate interactions among the patient’s own body, bacteria, viruses, and environmental pollutants [22]. These elements increase the inflammatory load in the lower airways, surpassing the body’s anti-inflammatory defenses and causing tissue damage. Frequent exacerbations lead to higher rates of illness and death, a more rapid deterioration of lung function, and a decline in overall health status [22].

Self-Quiz
Ask Yourself...
- How do the pathologies of emphysema and chronic bronchitis, as primary components of COPD affect lung function and patient symptoms?
- Given that COPD involves an abnormal inflammatory response to irritants like cigarette smoke, how does this prolonged inflammation contribute to the progression of the disease?
- How does alpha-1 antitrypsin deficiency lead to a specific form of emphysema, and what are the challenges in managing COPD in patients with this genetic condition?
- How do exacerbations in COPD, resulting from interactions between the body and external factors like bacteria and pollutants, affect the disease’s progression and patient outcomes? What strategies can be employed to reduce the frequency and severity of these exacerbations?
Treatment / Management
The primary aim of COPD treatment is to slow or halt the progression of the disease. Treatment goals seek to control symptoms, improve quality of life, and reduce exacerbations and mortality. Providers can manage acute exacerbations of COPD in outpatient or inpatient settings based on severity and treatment can include bronchodilators, corticosteroids, and antibiotics, with oxygen therapy ranging from nasal cannula to mechanical ventilation [3]. Patients can utilize pulmonary rehabilitation at all stages of COPD. Rehabilitation includes a comprehensive plan involving exercise training, education, and behavioral changes, aimed at improving the patient’s physical function and psychological condition [31]. Non-pharmacological approaches include smoking cessation and pulmonary rehabilitation [28].
Medication classes for COPD treatment include bronchodilators (beta2-agonists, antimuscarinics, methylxanthines), inhaled corticosteroids (ICS), systemic glucocorticoids, phosphodiesterase-4 (PDE4) inhibitors, and antibiotics [3].
Beta-2 agonists function by activating beta-2 receptors located in the muscles surrounding the airways. This activation leads to the relaxation of these muscles, resulting in the widening (dilation) of the airways [25]. Methylxanthines, used as an adjunct to LABA (long-acting beta-agonists) or LAMA (long-acting muscarinic antagonists) therapy, induce mild bronchodilation [26]. Inhaled corticosteroids, often used in combination with LABAs and LAMAs, decrease inflammation [27]. For acute exacerbations, oral glucocorticoids are used. The existing evidence endorses the use of systemic corticosteroids for patients either hospitalized or pending discharged from emergency departments [34]. However, it does not offer adequate information to support their use in outpatient settings [34].
In severe cases, PDE4 inhibitors including Roflumilast, can reduce inflammation and exacerbation [28]. PDE4 inhibitors suppress the release of inflammatory cytokines and mediators from inflammatory cells [28].
Azithromycin can reduce exacerbation frequency in COPD patients [29]. The usual dosage is 250mg daily or 500mg three times a week for a duration of one year [29]. Providers should exercise caution with this treatment approach due to the risk of promoting bacterial resistance. Azithromycin use requires monitoring for bacterial resistance, QTc prolongation, and hearing impairment [29].
Research recommends that patients 65 years and older receive the 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23), spacing the two vaccinations at least one year apart [24]. For individuals aged 64 and younger who have significant comorbidities, such as diabetes mellitus, chronic heart disease, or chronic lung disease, protocols recommends the PPSV23 vaccine [24].
Long-term therapy choice depends on the disease severity and symptoms, as per the GOLD (Global Initiate for Chronic Obstructive Lung Disease) guidelines [30].
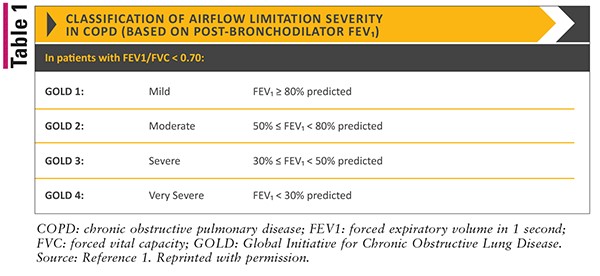
Table 1. 2022 GOLD Reports – Global Initiative for Chronic Obstructive Lung Disease – GOLD, 2021

Self-Quiz
Ask Yourself...
- How do the different classes of medications for COPD treatment, such as bronchodilators, inhaled corticosteroids, and PDE4 inhibitors contribute to achieving the primary goals of slowing disease progression, controlling symptoms, and improving quality of life?
- Considering the importance of non-pharmacological approaches like pulmonary rehabilitation and smoking cessation in COPD management, how do these interventions complement pharmacological treatments in terms of enhancing patient outcomes and reducing exacerbations?
- Given the potential benefits of azithromycin in reducing exacerbation frequency in COPD patients, what are the implications of long-term antibiotic use in terms of bacterial resistance and side effects like QTc prolongation and hearing impairment?
- Given the limitations of relying solely on FEV1 measurements for COPD diagnosis, how might integrating a broader range of pulmonary function tests, such as measuring airway resistance and conducting cardiopulmonary exercise testing, provide a more nuanced understanding of the disease and influence individualized treatment plans?
- Considering the ongoing research in genetics and COPD in areas like biomarker identification and alpha-1 antitrypsin deficiency, how might these advancements in understanding the biochemical, cellular, and molecular mechanisms of COPD transform future clinical trials and treatment strategies?
Conclusion
Managing chronic obstructive pulmonary disease (COPD) is challenging due to its diverse clinical symptoms and complex underlying pathophysiology. While airflow limitation, identified through spirometry, is central to diagnosing COPD, relying on the forced expiratory volume in the first second (FEV1) has limitations in understanding the disease’s complexity [12].
Integrating a variety of pulmonary function tests (PFTs) into the daily clinical assessment of COPD patients are key to providing a more comprehensive understanding of the condition. This includes measuring resting volume, capacity, airway resistance, diffusion capacity, applying the forced oscillation technique, conducting field and cardiopulmonary exercise testing, and evaluating muscle strength [12]. These tests are critical in customizing medical management for individual patients with COPD.
Current research in the field of genetics and COPD is advancing our understanding and treatment of the disease. However, the complete biochemical, cellular, and molecular mechanisms underlying COPD lack full understanding, highlighting the need for ongoing research. Researchers funded by the National Heart, Lung, and Blood Institute (NHLBI) are analyzing lung tissue samples to identify proteins that could serve as biomarkers, signaling the potential development of COPD [33]. This genetic analysis aims to unravel the biological mechanisms underlying COPD, which could be crucial for designing future clinical trials and enhancing treatment strategies.
The SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS II) is utilizing data from lung tests, biological samples, imaging, and genetic testing to better comprehend the risk factors for COPD and its development [33]. A significant focus is also on developing genetic therapies for alpha-1 antitrypsin deficiency (AAT), a genetic condition linked to increased COPD risk, with NHLBI-funded researchers pioneering new treatment approaches [33].
The primary aim of COPD treatment is to decelerate the disease’s progression, control symptoms, enhance quality of life, and reduce exacerbations and mortality, utilizing a blend of pharmacological approaches including bronchodilators, corticosteroids, and antibiotics, alongside non-pharmacological strategies including pulmonary rehabilitation and lifestyle modifications.
References + Disclaimer
- World Health Organization: WHO & World Health Organization: WHO. (2023, March 16). Chronic obstructive pulmonary disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- Institute for Quality and Efficiency in Health Care (IQWiG). (2019, March 14). Chronic obstructive pulmonary disease (COPD): Overview. InformedHealth.org – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK315789/
- Agarwal, A. K. (2023, August 7). Chronic obstructive pulmonary disease. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK559281/
- American Lung Association. (2024). Do you know the early warning signs of COPD? https://www.lung.org/lung-health-diseases/lung-disease-lookup/copd/symptoms-diagnosis/early-warning-signs
- COPD – Symptoms and causes – Mayo Clinic. (2020, April 15). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/copd/symptoms-causes/syc-20353679
- What is COPD? | NHLBI, NIH. (2023, October 25). NHLBI, NIH. https://www.nhlbi.nih.gov/health/copd
- Pando‐Sandoval, A., Ruano-Raviña, A., Candal-Pedreira, C., Rodríguez‐García, C., Represas-Represas, C., Golpe, R., Fernández-Villar, A., & Pérez-Ríos, M. (2022). Risk factors for chronic obstructive pulmonary disease in never‐smokers: A systematic review. Clinical Respiratory Journal, 16(4), 261–275. https://doi.org/10.1111/crj.13479
- Alpha-1 Antitrypsin deficiency | NHLBI, NIH. (2023, October 25). NHLBI, NIH. https://www.nhlbi.nih.gov/health/alpha-1-antitrypsin-deficiency
- Panacinar emphysema (Concept Id: C0264393) – MedGen – NCBI. (2024). https://www.ncbi.nlm.nih.gov/medgen/78106
- American Lung Association. (2024). Learn about Alpha-1 antitrypsin deficiency. https://www.lung.org/lung-health-diseases/lung-disease-lookup/alpha-1-antitrypsin-deficiency/learn-about-alpha-1-antitrypsin-defiency
- Brode, S. K., Ling, S. C., & Chapman, K. R. (2012). Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease: Figure 1: Canadian Medical Association Journal, 184(12), 1365–1371. https://doi.org/10.1503/cmaj.111749
- Kakavas, S., Kotsiou, O. S., Perlikos, F., Mermiri, M., Mavrovounis, G., Gourgoulianis, K., & Pantazopoulos, I. (2021). Pulmonary function testing in COPD: looking beyond the curtain of FEV1. Npj Primary Care Respiratory Medicine, 31(1). https://doi.org/10.1038/s41533-021-00236-w
- National Library of Medicine. (2024). COPD. Chronic Obstructive Pulmonary Disease | MedlinePlus. https://medlineplus.gov/copd.html
- Terzikhan, N., Verhamme, K., Hofman, A., Stricker, B. H. C., Brusselle, G., & Lahousse, L. (2016). Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. European Journal of Epidemiology, 31(8), 785–792. https://doi.org/10.1007/s10654-016-0132-z
- Szalontai, K., Gémes, N., Furák, J., Varga, T., Neuperger, P., Balog, J., Puskás, L. G., & Szebeni, G. J. (2021). Chronic Obstructive pulmonary Disease: epidemiology, biomarkers, and paving the way to lung cancer. Journal of Clinical Medicine, 10(13), 2889. https://doi.org/10.3390/jcm10132889
- Pandey, K. C., De, S., & Mishra, P. K. (2017). Role of proteases in chronic obstructive pulmonary disease. Frontiers in Pharmacology, 8. https://doi.org/10.3389/fphar.2017.00512
- Kansal, H., Chopra, V., Garg, K., & Sharma, S. (2023). Role of thioredoxin in chronic obstructive pulmonary disease (COPD): a promising future target. Respiratory Research, 24(1). https://doi.org/10.1186/s12931-023-02574-4
- MacNee, W. (2006, May 5). ABC of chronic obstructive pulmonary disease: Pathology, pathogenesis, and pathophysiology. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1463976/
- Pahal, P. (2023, January 26). Emphysema. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482217/
- Mecham, R. P. (2018). Elastin in lung development and disease pathogenesis. Matrix Biology, 73, 6–20. https://doi.org/10.1016/j.matbio.2018.01.005
- Pulmonology Advisor. (2023, November 21). Chronic Obstructive Pulmonary Disease (COPD) – Pulmonology Advisor. https://www.pulmonologyadvisor.com/ddi/chronic-obstructive-pulmonary-disease-copd/
- Sapey, E. (2006). COPD exacerbations {middle dot} 2: Aetiology. Thorax, 61(3), 250–258. https://doi.org/10.1136/thx.2005.041822
- Chong, J., Leung, B., & Poole, P. (2017). Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. The Cochrane Library. https://doi.org/10.1002/14651858.cd002309.pub5
- Simon, S., Joean, O., Welte, T., & Rademacher, J. (2023). The role of vaccination in COPD: influenza, SARS-CoV-2, pneumococcus, pertussis, RSV, and varicella zoster virus. European Respiratory Review, 32(169), 230034. https://doi.org/10.1183/16000617.0034-2023
- NHS (2023, November 2). Bronchodilators. nhs.uk. https://www.nhs.uk/conditions/bronchodilators/
- Stable COPD: Initial pharmacologic management – UpToDate. (2024). UpToDate. https://www.uptodate.com/contents/stable-copd-initial-pharmacologic-management
- Lea, S., Higham, A., Beech, A., & Singh, D. (2023). How inhaled corticosteroids target inflammation in COPD. European Respiratory Review, 32(170), 230084. https://doi.org/10.1183/16000617.0084-2023
- Safka, K. A. (2015, January 1). Non-Pharmacological management of chronic obstructive pulmonary disease. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4330800/
- Herath, S., Normansell, R., Maisey, S., & Poole, P. (2018). Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). The Cochrane Library, 2018(10). https://doi.org/10.1002/14651858.cd009764.pub3
- Terry, P., & Dhand, R. (2020). Inhalation therapy for stable COPD: 20 years of GOLD reports. Advances in Therapy, 37(5), 1812–1828. https://doi.org/10.1007/s12325-020-01289-y
- Shenoy, M. A. (2023, July 25). Pulmonary rehabilitation. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK563166/
- 2022 GOLD Reports – Global Initiative for Chronic Obstructive Lung Disease – GOLD. (2021, November 23). Global Initiative for Chronic Obstructive Lung Disease – GOLD. https://goldcopd.org/2022-gold-reports/
- COPD Research | NHLBI, NIH. (2023, October 25). NHLBI, NIH. https://www.nhlbi.nih.gov/research/copd
- Thébault, J., Roche, N., Abdoul, H., Lorenzo, A., Similowski, T., & Ghasarossian, C. (2023). Efficacy and safety of oral corticosteroids to treat outpatients with acute exacerbations of COPD in primary care: a multicentre pragmatic randomised controlled study. ERJ Open Research, 9(5), 00057–02023. https://doi.org/10.1183/23120541.00057-2023
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
