Course
Managing Pain and Fatigue in Rheumatoid Arthritis
Course Highlights
- In this Managing Pain and Fatigue in Rheumatoid Arthritis course, we will learn about the typical clinical presentation and symptoms of RA.
- You’ll also learn the immune-mediated pathophysiology of RA, including the role of genetic, environmental, and lifestyle factors in its development.
- You’ll leave this course with a broader understanding of current treatment modalities for RA, including the use of Disease-Modifying Antirheumatic Drugs (DMARDs), biologic agents, and lifestyle modifications.
About
Contact Hours Awarded: 1
Course By:
R.E. Hengsterman, MSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Medical history credits the dissertation of Augustin Jacob Landré-Beauvais’ in the year 1800 as the first recognized documentation of Rheumatoid Arthritis (RA) [1]. Historical experts reference the renowned Greek philosopher Hippocrates who provided a description that aligns with RA symptoms [1].
Hippocrates wrote:
“In arthritis which generally shows itself about the age of thirty-five, there is frequently no great interval between the affection of the hands and feet; both these becoming similar in nature, slender, with little flesh…For the most part their arthritis passeth from the feet to the hands, next the elbows and knees, after these the hip joint. It is incredible how fast the mischief spreads [42].”
Case Study: Managing Pain and Fatigue in Rheumatoid Arthritis
Name: Sarah Nicole Miller
Age: 37
Gender: Female
Occupation: Accountant
Medical History: No significant medical history except for occasional seasonal allergies.
Non-smoker, moderate alcohol use. No family history of autoimmune diseases.
Presenting Symptoms: Ms. Miller reports experiencing pain and stiffness in her hands, wrists, knees, and ankles for the past six months. The pain is more severe in the morning and after periods of inactivity and improves with movement throughout the day.
Ms. Miller struggles with opening jars, gripping small objects, and climbing stairs due to pain and stiffness in her hands and knees. And she reports debilitating fatigue that worsens with physical activity which often lingers despite adequate rest. The pain and fatigue impact her work and daily activities.
Physical Examination:
The patient presents with tenderness and swelling in multiple joints, including metacarpophalangeal joints, wrists, knees, and ankles with a reduced range of motion.
There is diminished grip strength in both hands. No deformities, rashes, or psoriatic lesions were observed.
Vital Signs: BP 130/85 mmHg, HR 78 bpm, Temp 98.6°F, RR 16/min
Laboratory Results:
- Elevated erythrocyte sedimentation rate (ESR): 45 mm/hr. (normal < 20 mm/hr.)
- C-reactive protein (CRP): 30 mg/L (normal < 5 mg/L)
- Rheumatoid factor (RF): Positive
- Anti-citrullinated peptide antibodies (ACPA): Positive
- CBC: Mild anemia (Hb: 11.2 g/dL)
- Rheumatoid Factor (RF): Positive
- Anti-Cyclic Citrullinated Peptide (Anti-CCP) Antibody: Positive
- X-rays of Hands and Knees: Signs of joint erosion in right knee
Diagnosis: Early-stage Rheumatoid arthritis (RA) based on clinical presentation, physical examination, and positive laboratory tests for inflammatory markers and autoantibodies.
Management Plan: Oral Methotrexate at 15 mg per week increasing it by 5 mg each month to a target range of 25–30 mg per week [5].

Self-Quiz
Ask Yourself...
- Considering the historical descriptions of RA symptoms by Hippocrates and the first recognized documentation by Augustin Jacob Landré-Beauvais in 1800, how might the understanding and diagnostic criteria for RA have evolved over the centuries?
- In the context of Sarah Nicole Miller’s case, which presents typical RA symptoms like pain, stiffness, and positive laboratory tests for inflammatory markers and autoantibodies, how do her symptoms align with the historical descriptions of RA by Hippocrates and Landré-Beauvais?
Definition
Rheumatoid arthritis (RA) is a chronic immune-mediated inflammatory disease (IMID) with a complex pathophysiology involving a combination of genetic, environmental, and immunological factors [18]. The immune system drives the inflammatory dysregulation that targets the synovium (the lining of the membranes surrounding the joints) causing pain, stiffness, and fatigue [3].
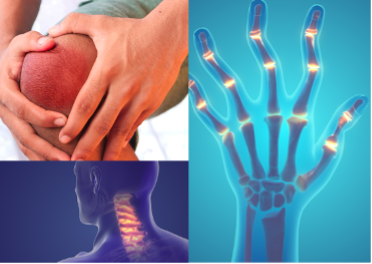
The inflammation caused by RA leads to joint damage, deformity, and disability and often begins in small joints of the hands, affecting the metacarpophalangeal joints (MCP), proximal interphalangeal joints (PIPJ), and wrists [3]. Rheumatoid arthritis can present as bilateral and symmetrical with impact to the knees, ankles, elbows, shoulders, metatarsophalangeal joints, cervical spine, and temporomandibular joints with eventual manifestation in the skin, eyes, heart, kidneys, and lungs [3].
RA fluctuates between periods of increased disease activity and worsening inflammation (known as flares) and periods of relative remission where symptoms diminish. The exact cause of RA is not known but the disease process involves a combination of genetic and environmental factors. The most significant correlations are being female, having a family history of RA, possessing the genetic marker known as the ‘shared epitope,’ and exposure to tobacco smoke [6].
While RA involves immune system dysregulation, other forms of arthritis have different etiologies. Osteoarthritis and rheumatoid arthritis are both joint conditions. Osteoarthritis, the most common type of arthritis, results from wear and tear and the breakdown of joint cartilage over time [7].
The crystallization of uric acid within the joints, leading to severe pain and swelling is the cause of Gout, a form of inflammatory arthritis [8]. Psoriatic arthritis is associated with the skin condition psoriasis and affects both the joints and the skin leading to joint pain, swelling, and stiffness in individuals with the chronic skin condition known as psoriasis [9].

Self-Quiz
Ask Yourself...
- Given that rheumatoid arthritis (RA) involves a complex interplay of genetic, environmental, and immunological factors, how might these diverse elements contribute to the pathogenesis of RA?
- Considering the differences in etiology between RA and other forms of arthritis such as osteoarthritis, gout, and psoriatic arthritis, what does this suggest about the importance of precise diagnosis and tailored treatment strategies?
- Reflecting on the fluctuating nature of RA, with periods of increased disease activity (flares) and periods of relative remission, how might this impact the long-term management and quality of life of patients with RA?
Epidemiology
Rheumatoid arthritis is the most common type of inflammatory musculoskeletal disorder, affecting about 1% of the global population, with two to three times as many women as men having the disease [10] [12]. The disease is more prevalent in Western and Northern Europe, North America, and countries with a significant population of European descent, such as Australia [19]. In contrast, RA is lower in Central and South America, and less common in East Asia and Africa [19].
As of 2019, an estimated 18 million people worldwide were living with RA [11]. The global burden of RA is increasing, with a projection of a 1.4-fold increase in incident cases, from approximately 1.07 million in 2019 to about 1.5 million by 2040 [14].
Industrialized countries report a higher prevalence of rheumatoid arthritis compared to low-and-middle-income countries (LMIC). This disparity may be due to a combination of an older average population age, increased exposure to environmental toxins, and lifestyle-related risk factors prevalent in industrialized regions [11]. The lower rates of RA occur in less affluent countries, influenced by under-diagnosis and less comprehensive healthcare reporting [11] [13].
Rheumatoid arthritis (RA) has a considerable economic impact including the direct costs related to medications, hospital admissions, medical office visits, and caregiver support [13]. Indirect costs stem from productivity losses due to absenteeism or early retirement. In addition, those with RA incur intangible costs, which affect quality of life.
In the United States, the direct cost for RA management per person is around US$ 13,500 per year, while indirect costs range from $1,000 to $33,000 per person each year [13]. Several unmodifiable factors influence the development of rheumatoid arthritis, such as genetic predisposition (HLA human leukocyte antigen) class II genotypes and gender, with females being at a higher risk [15].
In addition, changeable lifestyle and environmental aspects are contributing factors for RA. These modifiable risks include smoking, obesity, lack of physical activity, unhealthy diet, excessive consumption of alcohol, and exposure to air pollution, each playing a role in the likelihood of developing rheumatoid arthritis [11] [16].
Diet and nutrition are significant variables that can trigger rheumatoid arthritis (RA) [19]. A typical Western diet, characterized by high caloric intake, richness in fats, and low fiber content, leads to an increased risk of RA [32]. In contrast, consuming long-chain omega-3 polyunsaturated fatty acids are associated with a decreased risk of developing RA [28].
Obesity is a well-documented risk factor for rheumatoid arthritis (RA) [19]. The risk of RA rises by 30% in individuals with a body mass index (BMI) exceeding 30 kg/m^2, and there is a 15% heightened risk for those with a BMI ranging from 25 to 29.9 kg/m^2 [33].

Self-Quiz
Ask Yourself...
- Given the higher prevalence of rheumatoid arthritis (RA) in women and its greater occurrence in Western and Northern Europe, North America, and Australia, what might these patterns suggest about the potential roles of genetic and environmental factors in the development of RA?
- Reflecting on the higher incidence of RA in industrialized countries compared to low-and-middle-income countries, how might factors such as aging populations, environmental toxins, and lifestyle choices contribute to this disparity?
- Considering the significant direct and indirect costs associated with RA management, including medications, medical care, and loss of productivity, how do these financial burdens reflect the broader socio-economic impact of the disease?
- Given the association of lifestyle factors like smoking, obesity, and diet with the risk of developing RA, what does this imply about the potential for preventative measures in reducing the incidence of RA?
Pathophysiology & Etiology
The immune response in rheumatoid arthritis (RA) originates in areas away from the synovial joints, including the lungs, gums, and gastrointestinal tract [19]. In these sites, proteins undergo modifications through biochemical processes like citrullination. Environmental factors may trigger the onset of RA secondary to the recurrent activation of the body’s innate immune system [19].
Within the spectrum of Rheumatoid Arthritis (RA), a distinct subtype is seropositive rheumatoid arthritis [19]. Individuals with seropositive rheumatoid arthritis (RA) have specific antibodies that target and attack an individual’s tissues. This immune response leads to inflammation in the joints. The antibodies involved are known as anti-cyclic citrullinated peptides (anti-CCPs) or anti-citrullinated protein antibodies (ACPAs) [26] [43]. These are key indicators that healthcare professionals, including rheumatologists, look for when diagnosing and managing RA.
RA is a systemic autoimmune disease with extra-articular manifestations that can include complications within the skin, eyes, lungs, heart, and blood vessels [25]. In synovial fluid, polymorphonuclear leukocytes (white blood cells), constitute almost 60% of the total white blood cell count [17]. This process involves various immune cells that misidentify joint tissue, including T cells, B cells, macrophages, and dendritic cells, along with pro-inflammatory cytokines like tumor necrosis factor (TNF) and interleukins which facilitate joint degradation [23].
The overgrown synovial tissue, known as pannus, secretes various inflammatory substances. This chronic inflammation can lead to the destruction of cartilage and bone within the joint and erosive polyarthritis [26]. The substances produced, including matrix metalloproteinases (enzymes), degrade the extracellular matrix (ECM) [19] [20].
These mediators contribute to destructive polyarthritis and the erosion of cartilage, the bone beneath the cartilage (subchondral bone), the joint capsule, and ligaments [21]. In rheumatoid arthritis, proteolysis (the process that breaks down proteins into smaller amino acids or polypeptides) of the extracellular matrix (ECM) results in remnant epitopes that enhance and perpetuate autoimmune processes in susceptible hosts [20].
Specified genes increase the risk of developing RA. The most significant is the HLA-DR4 allele, part of the human leukocyte antigen (HLA) system, which plays a critical role in the immune system [22]. However, not everyone with the HLA-DR4 allele develops RA, indicating that other factors are involved.
These factors include exposure to silica dust and inhaled particulate air pollution along with certain infections that may trigger RA in individuals who have susceptible genetics [24]. Autoantibodies including rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) contribute to the pathogenesis and can serve as diagnostic markers [26]. Autoantibodies appear before the onset of clinical arthritis [19].
Smoking remains the most significant modifiable risk factor linked to rheumatoid arthritis [19] [27]. Research indicates that for individuals with a positive marker for anti-citrullinated protein antibodies (anti-CCP), there is a significant interaction between smoking and the shared alleles (SE) that elevates the risk of developing RA [35].

Self-Quiz
Ask Yourself...
- Considering that the immune response in rheumatoid arthritis (RA) originates in areas away from the synovial joints, such as the lungs, gums, and gastrointestinal tract, what does this suggest about the systemic nature of RA and the potential for early detection and intervention?
- Given that individuals with seropositive RA have specific autoantibodies like anti-CCPs and ACPAs, which target their own tissues leading to joint inflammation, how does this differentiate their condition and treatment approach from those with seronegative RA?
- Considering the significant role of the HLA-DR4 allele and environmental factors like smoking and air pollution in the development of RA, what does this interplay between genetics and environment suggest about the complexity of RA pathogenesis?
- Considering the research indicating a significant interaction between smoking and the presence of specific genetic markers in elevating the risk of developing RA, what does this reveal about the importance of lifestyle modifications in RA management and prevention? How might public health initiatives have focused on smoking cessation benefit individuals at risk for RA?
Clinical Signs and Symptoms
The primary symptoms of rheumatoid arthritis (RA) include joint pain and swelling developing over weeks to months [19]. A key characteristic of RA is morning stiffness [30]. While the onset of symptoms is often slow, some individuals may experience a pattern of episodic symptoms known as palindromic rheumatism [43].
Palindromic rheumatism has recurrent episodes of joint pain and swelling that can affect multiple joints [45]. The duration of these episodes can range from a few hours to several days and the occurrence of these attacks is unpredictable, with some individuals experiencing them daily, while others may have several episodes throughout the year [45].
Palindromic rheumatism does not lead to rheumatoid arthritis (RA) in all patients, the effective response of these individuals to hydroxychloroquine implies that palindromic rheumatism could represent a unique variant or phenotype within the spectrum of RA [31] [45].
During a physical examination, affected joints may be painful to touch or move, with or without visible swelling [19]. Palpation reveals synovial thickening with a ‘boggy’ texture [19]. Joint redness and warmth are not often present [19].
In cases with multiple joint involvements, reduced grip strength occurs [19]. Advanced chronic RA may show characteristic physical signs including ulnar deviation, metacarpophalangeal joint subluxation, swan neck deformity, Boutonniere deformity, and the “bowstring” sign [19].
Later stages of RA can also feature reduced motion range in shoulders, elbows, and knees, with common foot deformities including hallux valgus and other toe abnormalities [19]. Patients often experience a general feeling of malaise and fatigue, which can precede other symptoms [15] [19].
The affected joints can feel warm and appear red due to inflammation and as the RA progresses, it can lead to decreased mobility and range of motion in the affected joints [15][19]. Firm bumps of tissue, known as rheumatoid nodules, may form around pressure points, such as the elbows [19].
Constitutional symptoms of RA can include low-grade fever, loss of appetite, and weight loss [15][19]. There can be extra-articular manifestations of RA leading to anemia, dry eyes, and mouth (Sjögren’s syndrome), lung inflammation, and cardiovascular issues [15].

Self-Quiz
Ask Yourself...
- Considering that palindromic rheumatism, characterized by episodic joint pain and swelling, does not always lead to rheumatoid arthritis (RA), what does this suggest about the complexity and variability within the spectrum of rheumatic diseases?
- Given the range of symptoms in RA, from initial joint pain and morning stiffness to advanced signs like ulnar deviation and rheumatoid nodules, how do these varying manifestations reflect the progression of the disease? What implications does this progression have for the timing of intervention and the likelihood of preventing severe joint damage and disability?
- With RA leading to systemic symptoms such as anemia, dry eyes and mouth, and lung and cardiovascular issues, what does this suggest about the need for a multidisciplinary approach to managing RA? How might these extra-articular symptoms influence the overall treatment plan and quality of life for patients with RA?
Management and Treatment
The primary objective in treating rheumatoid arthritis (RA) is to ensure early diagnosis and prompt commencement of treatment, to prevent irreversible joint damage [19]. The International Task Force Guidelines published in 2014 make the following recommendations regarding the treatment of RA [32].
The primary goal of treatment is to achieve long-term clinical remission and optimize quality of life with the absence of signs and symptoms associated with inflammatory disease activity [32]. In the absence of clinical remission, low disease activity is an acceptable alternative [32].
Providers should assess ongoing disease monthly in patients with moderate to severe disease and in patients with low disease activity or clinical remission, assessments every 3 to 6 months [32].
Disease-modifying antirheumatic Drugs (DMARDs) represent a class of medications used in the treatment of rheumatoid and other forms of inflammatory arthritis [4]. Disease-modifying antirheumatic Drugs (DMARD) are immunosuppressive and immunomodulatory agents that suppress the immune system and slow RA progression [4].
The most common conventional DMARDs are methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide [4]. Methotrexate is the most common agent in the initial treatment of RA [4]. Biologic DMARDs are augments when conventional DMARD therapy does control disease activity or when evidence exists of ongoing clinical or radiographic progression of the disease [4].
Examples of biologic DMARDs include infliximab, adalimumab, etanercept, rituximab, abatacept, tocilizumab, tofacitinib, and others [4]. These biologic DMARDs target specific pathways in the immune system with high precision [33]. The composition of these drugs varies: including monoclonal antibodies, which can be chimeric or humanized, while others act via fusion proteins combining receptors with parts of human immunoglobulin [33].
Tumor Necrosis Factor (TNF) is a cytokine that promotes inflammation and contributes to joint inflammation and damage in rheumatoid arthritis (RA) [34]. Blocking TNF can lead to improvements in RA symptoms and slow down the progression seen in radiographic studies [33][34]. There are five biologic agents approved for RA treatment that target TNF: infliximab (INF), etanercept (ETN), adalimumab (ADA), certolizumab (CMZ), and golimumab (GLM) [33].
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as Ibuprofen may provide pain relief, and corticosteroids including Prednisone help manage acute inflammation and flare-ups [33]. Patient education plays a crucial role in understanding RA and the importance of adhering to medication regimens [33].
Physical therapy may help patients to maintain joint function and reduce stiffness through tailored exercises [33]. Occupational therapy can provide training on adaptive techniques for daily activities to minimize joint strain and conserve energy [33]. Dietary consultations can offer insights into anti-inflammatory diets [33].
For optimal health and well-being in RA patients, it is essential to focus on lifestyle modifications that include a balanced diet, regular exercise, sufficient sleep, effective stress management, and cessation of smoking [33].


Self-Quiz
Ask Yourself...
- Given that the primary goal in treating rheumatoid arthritis (RA) is to achieve long-term clinical remission and optimize quality of life, how might a provider assess the effectiveness of various treatment modalities like DMARDs and biologic agents in terms of achieving these objectives?
- Considering the precision targeting of immune system pathways by biologic DMARDs, how might a patient’s disease stage, severity, and response to initial treatments influence a provider’s decision to use these agents over conventional DMARDs?
- Reflecting on the importance of lifestyle modifications, patient education, and supportive therapies like physical and occupational therapy in the management of RA, how do these interventions complement pharmacological treatments? What role do they play in improving the overall quality of life and disease outcomes for patients with RA?
Research Findings
A 2023 study in Nature Reviews Rheumatology revealed that the amino acid L-arginine may play a significant role in mitigating arthritis and preventing inflammatory bone loss [38]. This occurs through a metabolic reprogramming of osteoclasts, shifting their energy metabolism from glycolysis to oxidative phosphorylation.
Key findings of the study include L-arginine’s ability to inhibit arthritis, block the formation of osteoclasts, and protect against bone erosion by altering purine metabolism within these cells [38]. L-arginine serves as a degradation substrate for enzymes like Arg-1, known for its anti-inflammatory effects, thus aiding in the management of inflammatory diseases [38].
The study further highlights L-arginine’s potential in enhancing fracture healing by improving local blood supply, supplementing growth factors, and boosting collagen synthesis [38].
In a novel approach, scientists have developed a hybrid therapy by fusing ceria nanoparticles with mesenchymal stem cell (MSC) nanovesicles [39]. This innovative treatment has shown promising results in reducing inflammation and fostering immunotolerance in a mouse model of collagen-induced arthritis (CIA) [39].
A study featured in Nature Reviews Rheumatology suggests that targeting both glutaminolysis and glycolysis in fibroblast-like synoviocytes could be a promising strategy for managing rheumatoid arthritis (RA), as demonstrated in a mouse model [40]. In RA, synovial fibroblasts are crucial in sustaining chronic inflammation due to their interactions with immune and inflammatory cells, and they also contribute to the invasion of cartilage and bone [40].
In a separate research investigation, a study in Nature Reviews Rheumatology presents a detailed single-cell analysis of synovial tissue from rheumatoid arthritis (RA) patients, examining over 314,000 cells [41]. This comprehensive analysis has identified six distinct inflammation patterns involving various cell types and disease pathways in RA [41]. These findings shed light on the disease’s heterogeneity and could guide the development of more targeted treatment strategies in the future.

Self-Quiz
Ask Yourself...
- Considering the findings that L-arginine may play a significant role in mitigating arthritis and preventing inflammatory bone loss, what are the potential implications of this discovery for the future treatment of rheumatoid arthritis (RA)? How might these findings change the current understanding of nutritional and metabolic interventions in managing inflammatory diseases?
- Given the recent studies exploring innovative approaches like hybrid therapies with ceria nanoparticles and mesenchymal stem cell (MSC) nanovesicles, and targeting metabolic pathways in synovial fibroblasts, how might these novel strategies enhance the current treatment landscape for RA?
- Given that rheumatoid arthritis (RA) affects about 1% of the global population with a higher prevalence in regions of European descent, how might genetic and environmental factors interact to influence the geographic distribution of RA?
- Considering the multifaceted nature of RA, which includes not only physical symptoms but also the risk of psychological comorbidities and increased potential for other chronic diseases, how should treatment strategies be adapted to address the comprehensive needs of RA patients? How important is the role of interdisciplinary care, including psychological support and lifestyle interventions, in improving outcomes for RA patients?
Conclusion
Rheumatoid arthritis (RA), first documented by Augustin Jacob Landré-Beauvais in 1800 and described earlier by Hippocrates, is a chronic immune-mediated inflammatory disease characterized by joint pain, stiffness, and fatigue, often beginning in small joints [15]. It affects about 1% of the global population, with higher prevalence in regions of European descent [19].
RA causes pain, disability, and joint deformities that may result in permanent loss of function and mobility [15]. Rheumatoid arthritis can increase the potential of premature death and elevate the risk of chronic diseases like heart disease and diabetes [37]. Factors contributing to RA include genetics, environmental triggers including smoking, and lifestyle factors such as diet and obesity [36].
Its pathophysiology involves immune response starting in areas like the lungs and gums, leading to synovitis and joint degradation [19]. Treatment of RA includes early diagnosis, DMARDs, biologic agents targeting TNF, NSAIDs, and corticosteroids, complemented by patient education, and physical and occupational therapy [4] [33]. Lifestyle modifications are essential for managing RA [33]. Complications include joint deformities, increased risk of cardiovascular diseases, and psychological challenges like depression and anxiety, with psychiatric comorbidity linked to poorer outcomes [15] [37].
References + Disclaimer
- Entezami, P., Fox, D. A., Clapham, P. J., & Chung, K. C. (2011). Historical perspective on the etiology of rheumatoid arthritis. Hand Clinics, 27(1), 1–10. https://doi.org/10.1016/j.hcl.2010.09.006
- Copeman WSC. A Short History of Gout. Berkeley and Los Angeles: University of California Press; 1964
- Mohammed, R. H. (2023, August 17). Hand and wrist rheumatoid arthritis. Stat Pearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560890/
- Benjamin, O. (2023, July 3). Disease-Modifying Antirheumatic Drugs (DMARD). Stat Pearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK507863/
- Visser, K., & Van Der Heijde, D. (2008). Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Annals of Rheumatic Diseases, 68(7), 1094–1099. https://doi.org/10.1136/ard.2008.092668
- Deane, K. D., Demoruelle, M. K., Kelmenson, L. B., Kuhn, K. A., Norris, J. M., & Holers, V. M. (2017). Genetic and environmental risk factors for rheumatoid arthritis. Best Practice & Research Clinical Rheumatology, 31(1), 3–18. https://doi.org/10.1016/j.berh.2017.08.003
- Osteoarthritis (OA) | Arthritis | CDC. (2023). https://www.cdc.gov/arthritis/basics/osteoarthritis.htm
- Branch, N. S. C. a. O. (2023, December 21). Gout. National Institute of Arthritis and Musculoskeletal and Skin Diseases. https://www.niams.nih.gov/health-topics/gou\
- Patient education: Psoriatic arthritis (Beyond the Basics) – UpToDate. (2023). UpToDate. https://www.uptodate.com/contents/psoriatic-arthritis-beyond-the-basics/print
- Branch, N. S. C. a. O. (2023, July 27). Rheumatoid arthritis. National Institute of Arthritis and Musculoskeletal and Skin Diseases. https://www.niams.nih.gov/health-topics/rheumatoid-arthritis
- World Health Organization: WHO & World Health Organization: WHO. (2023, June 28). Rheumatoid arthritis. https://www.who.int/news-room/fact-sheets/detail/rheumatoid-arthritis
- Badley EM, Tennant A. Disablement associated with rheumatic disorders in a British population: problems with activities of daily living and level of support. Br J Rheumatol. 1993; 32:601–8. doi: 10.1093/rheumatology/32.7.601.
- Rudan, I., Sidhu, S., Papana, A., Meng, S., Yu, X., Wang, W., Campbell-Page, R. M., Demaio, A., Nair, H., Sridhar, D., Τheodoratou, E., Dowman, B., Adeloye, D., Majeed, A., Car, J., Campbell, H., & Chan, K. Y. (2015). Prevalence of rheumatoid arthritis in low- and middle-income countries: A systematic review and analysis. DOAJ (DOAJ: Directory of Open Access Journals), 5(1), 010409. https://doi.org/10.7189/jogh.05.010409
- Shi, G., Liao, X., Zhang, L., Liu, W., Luo, X., Zhan, H., & Cai, X. (2023). Estimation of the global prevalence, incidence, years lived with disability of rheumatoid arthritis in 2019 and forecasted incidence in 2040: results from the Global Burden of Disease Study 2019. Clinical Rheumatology, 42(9), 2297–2309. https://doi.org/10.1007/s10067-023-06628-2
- Rheumatoid arthritis (RA) | Arthritis | CDC. (2023). https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html
- Schäfer, C., & Keyßer, G. (2022). Lifestyle factors and their Influence on rheumatoid arthritis: A Narrative review. Journal of Clinical Medicine, 11(23), 7179. https://doi.org/10.3390/jcm11237179
- Yaseen, K. (2023, December 8). Rheumatoid arthritis (RA). Merck Manuals Professional Edition. https://www.merckmanuals.com/professional/musculoskeletal-and-connective-tissue-disorders/joint-disorders/rheumatoid-arthritis-ra
- Johns Hopkins Arthritis Center. (2019, March 27). RA Pathophysiology • Johns Hopkins Arthritis Center. https://www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-pathophysiology-2/
- Chauhan, K. (2023, May 25). Rheumatoid arthritis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK441999/
- Grillet, B., Pereira, R. V. S., Van Damme, J., El‐Asrar, A. M. A., Proost, P., & Opdenakker, G. (2023). Matrix metalloproteinases in arthritis: towards precision medicine. Nature Reviews Rheumatology, 19(6), 363–377. https://doi.org/10.1038/s41584-023-00966-w
- Ostrowska, M., Maśliński, W., Prochorec‐Sobieszek, M., Nieciecki, M., & Sudoł‐Szopińska, I. (2018). Cartilage and bone damage in rheumatoid arthritis. Reumatologia, 56(2), 111–120. https://doi.org/10.5114/reum.2018.75523
- Van Drongelen, V., & Holoshitz, J. (2017). Human leukocyte Antigen–Disease associations in rheumatoid arthritis. Rheumatic Diseases Clinics of North America, 43(3), 363–376. https://doi.org/10.1016/j.rdc.2017.04.003
- Deane, K. D., Demoruelle, M. K., Kelmenson, L. B., Kuhn, K. A., Norris, J. M., & Holers, V. M. (2017). Genetic and environmental risk factors for rheumatoid arthritis. Best Practice & Research Clinical Rheumatology, 31(1), 3–18. https://doi.org/10.1016/j.berh.2017.08.003
- Yap, H. Y., Tee, S. Z. Y., Wong, M. M. T., Chow, S. K., Peh, S., & Teow, S. (2018). Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells, 7(10), 161. https://doi.org/10.3390/cells7100161
- Cojocaru, M. (2010, December 1). Extra-articular manifestations in rheumatoid arthritis. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3152850/
- Song, Y., & Kang, E. H. (2009). Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM: An International Journal of Medicine, 103(3), 139–146. https://doi.org/10.1093/qjmed/hcp165
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010 Jan;69(1):70-81
- Philippou E, Nikiphorou E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun Rev. 2018 Nov;17(11):1074-1077.
- Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, Hu Z, Liang Y, Yang Z, Zhong R. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015 Mar 29;17(1):86.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016 Oct 22;388(10055):2023-2038.
- Koskinen E, Hannonen P, Sokka T. Palindromic rheumatism: long-term outcomes of 60 patients diagnosed in 1967-84. J Rheumatol. 2009 Sep;36(9):1873-5.
- Smolen, J. S., Breedveld, F. C., Burmester, G. R., Bykerk, V. P., Dougados, M., Emery, P., Kvien, T. K., Navarro-Compán, M. V., Oliver, S., Schoels, M., Scholte-Voshaar, M., Stamm, T., Stoffer, M., Takeuchi, T., Aletaha, D., Andreu, J. L., Aringer, M., Bergman, M., Betteridge, N., . . . Van Der Heijde, D. (2015). Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Annals of the Rheumatic Diseases, 75(1), 3–15. https://doi.org/10.1136/annrheumdis-2015-207524
- Mysler, E., Caubet, M., & Lizarraga, A. (2021). Current and emerging DMARDs for the treatment of rheumatoid arthritis. Open Access Rheumatology: Research and Reviews, Volume 13, 139–152. https://doi.org/10.2147/oarrr.s282627
- Jang, D., Lee, A., Shin, H. K., Song, H., Park, J., Kang, T., Lee, S., & Yang, S. (2021). The role of tumor necrosis factor alpha (TNF-Α) in autoimmune disease and current TNF-Α inhibitors in therapeutics. International Journal of Molecular Sciences, 22(5), 2719. https://doi.org/10.3390/ijms22052719
- Linn-Rasker, S. P., Van Der Helm-Van Mil, A. H. M., Van Gaalen, F. A., Kloppenburg, M., De Vries, R. R. P., Cessie, S. L., Breedveld, F. C., Toes, R. E. M., & Huizinga, T. W. J. (2006). Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Annals of Rheumatic Diseases, 65(3), 366–371. https://doi.org/10.1136/ard.2005.041079
- Patient education: Rheumatoid arthritis treatment (Beyond the Basics) – UpToDate. (2023). UpToDate. https://www.uptodate.com/contents/rheumatoid-arthritis-treatment-beyond-the-basics
- Amaowei, E. E. J., Anwar, S., Sridhar, K. K., Shabbir, K., Mohammed, E. H., Bahar, A. R., Talpur, A. S., Bhat, S., Zafar, S., & Qadar, L. T. (2022). Correlation of depression and anxiety with rheumatoid arthritis. Cureus. https://doi.org/10.7759/cureus.23137
- Onuora, S. L-arginine inhibits arthritis and bone loss by reprogramming osteoclast metabolism. Nat Rev Rheumatol 19, 760 (2023). https://doi.org/10.1038/s41584-023-01055-8
- Phillips, R. Nanohybrid therapy hits multiple arthritis targets. Nat Rev Rheumatol 20, 4 (2024). https://doi.org/10.1038/s41584-023-01064-7
- McHugh, J. Dual inhibitor targets fibroblast metabolism in RA. Nat Rev Rheumatol 19, 760 (2023). https://doi.org/10.1038/s41584-023-01051-y
- McHugh, J. Single-cell atlas unveils the diversity of RA synovial tissue. Nat Rev Rheumatol 20, 4 (2024). https://doi.org/10.1038/s41584-023-01059-4
- Entezami, P., Fox, D. A., Clapham, P. J., & Chung, K. C. (2011). Historical perspective on the etiology of rheumatoid arthritis. Hand Clinics, 27(1), 1–10. https://doi.org/10.1016/j.hcl.2010.09.006
- Liu, J., Gao, J., Wu, Z., Mi, L., Li, N., Wang, Y., Peng, X., Xu, K., Wu, F., & Zhang, L. (2022). Anti-citrullinated protein Antibody generation, pathogenesis, clinical application, and prospects. Frontiers in Medicine, 8. https://doi.org/10.3389/fmed.2021.802934
- Palindromic rheumatism | Arthritis Foundation. (2024). https://www.arthritis.org/diseases/palindromic-rheumatism
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
