New Hampshire APRN Bundle
Contact Hours: 30
Author(s):
- Cathleen Adams CNO, RN CENP
- Amy White, CNO, RN
- Maureen Sullivan-Tevault MS, BSN, RN, CEN, CDE
- Sarah Schulze MSN, NP
- Amanda Marten MSN, FNP-C
- Edith Fomuso MSN, CCM, RN
- Sadia A. MPH, MSN, WHNP-BC
- Abbie Schmitt MSN, RN
- Charmaine Robinson MSN-Ed, BSN, RN
- R.E. Hengsterman MSN, RN, MA
Course Highlights
- In this course we will learn about the commonly prescribed opioids for pain management and understand their side effects and indications of use.
- You’ll also learn about the drug classification of Tirzepatide.
- You’ll leave this course with a broader understanding of common side effects, including severe possible side effects, of oral medications used to manage sexually transmitted infections (STIs).
Controlled Substances
Introduction
Pain is complex and subjective. The experience of pain can significantly impact an individual’s quality of life. According to the National Institute of Health (NIH) (40), pain is the most common complaint in a primary care office, with 20% of all patients reporting pain. Chronic pain is the leading cause of disability, and effective pain management is crucial to health and well-being, particularly when it improves functional ability. Effective pain treatment starts with a comprehensive, empathic assessment and a desire to listen and understand. Nurse Practitioners are well-positioned to fill a vital role in providing comprehensive and empathic patient care, including pain management (23).
While the incidence of chronic pain has remained a significant problem, how clinicians manage pain has significantly changed in the last decade, primarily due to the opioid epidemic. This education aims to discuss pain and the assessment of pain, federal guidelines for prescribing, the opioid epidemic, addiction and diversion, and recommendations for managing pain.
Ask yourself...
- How would you define pain?
- How has the opioid epidemic impacted your practice?
- In what ways does chronic pain bring challenges to pain control?
- What does effective pain treatment start with?
- How do you currently assess and reassess pain?
Definition of Pain
Understanding the definition of pain, differentiating between various types of pain, and recognizing the descriptors patients use to communicate their pain experiences are essential for Nurse practitioners involved in pain management. By understanding the medical definition of pain and how individuals may communicate it, nurse practitioners can differentiate varying types of pain to target assessment.
According to the International Association for the Study of Pain (27), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or terms of described such in damage.” In July 2020, the IASP expanded its definition of pain to include context further.
Their expansion is summarized below:
- Pain is a personal experience influenced by biological, psychological, and social factors.
- Pain cannot be inferred solely from activity in sensory neurons.
- Individuals learn the concept of pain through their life experiences.
- A person’s report of an experience in pain should be respected.
- Pain usually serves an adaptive role but may adversely affect function and social and psychological well-being.
- The inability to communicate does not negate the possibility of the experience of pain.

Ask yourself...
- Analyze how changes to the definition of pain may affect your practice.
- Discuss how you manage appointment times, knowing that 20% of your scheduled patients may seek pain treatment.
- How does the approach to pain management change in the presence of a person with a disability?
- How does bias impact your ability to assess pain adequately?
- What factors impact pain?
Types of Pain
Pain originates from different mechanisms, causes, and areas of the body. As a nurse practitioner, understanding the type of pain a patient is experiencing is essential for several reasons (23).
- Determining an accurate diagnosis. This pain can provide valuable clues to the underlying cause or condition.
- Creating a treatment plan. Different types of pain respond better to specific treatments or interventions.
- Developing patient education. A nurse practitioner can educate patients about their condition, why they may experience the pain as they do, its causes, and treatment options. Improving the patient’s knowledge and control over their condition improves outcomes.
Acute Pain
Acute pain is typically short-lived and is a protective response to an injury or illness. Patients are usually able to identify the cause. This pain resolves as the underlying condition improves or heals (12).
Chronic Pain
Chronic pain is diagnosed when it continues beyond the expected healing time. Pain is defined as chronic when it persists for longer than three months. It may result from an underlying disease or injury or develop without a clear cause. Chronic pain often significantly impacts a person’s physical and emotional well-being, requiring long-term management strategies. The prolonged experience of chronic pain usually indicates a central nervous system component of pain that may require additional treatment. Patients with centralized pain often experience allodynia or hyperalgesia (12).
Allodynia is pain evoked by a stimulus that usually does not cause pain, such as a light touch. Hyperalgesia is the effect of a heightened pain response to a stimulus that usually evokes pain (12).
Nociceptive Pain
Nociceptive pain arises from activating peripheral nociceptors, specialized nerve endings that respond to noxious stimuli. This type of pain is typically associated with tissue damage or inflammation and is further classified into somatic and visceral pain subtypes.
Somatic pain is most common and occurs in muscles, skin, or bones; patients may describe it as sharp, aching, stiffness, or throbbing.
Visceral pain occurs in the internal organs, such as indigestion or bowel spasms. It is more vague than somatic pain; patients may describe it as deep, gnawing, twisting, or dull (12).
Neuropathic pain
Neuropathic pain is a lesion or disease of the somatosensory nervous system. Examples include trigeminal neuralgia, painful polyneuropathy, postherpetic neuralgia, and central poststroke pain (10).
Neuropathic pain may be ongoing, intermittent, or spontaneous. Patients often describe it as burning, prickling, or squeezing. Neuropathic pain is a common chronic pain. Patients commonly describe allodynia and hyperalgesia as part of their chronic pain experience (10).
Affective pain
Affective descriptors reflect the emotional aspects of pain and include terms like distressing, unbearable, depressing, or frightening. These descriptors provide insights into the emotional impact of pain on an individual’s well-being (12).
Ask yourself...
- How can nurse practitioners effectively elicit patient descriptors to accurately assess the type of pain the patient is experiencing?
- Expand on how pain descriptors can guide interventions even if the cause is not yet determined.
- What strategies ensure patients feel comfortable describing their pain, particularly regarding subjective elements such as quality and location?
- How would you define acute pain?
- How can acute pain become chronic pain?
- What is the difference between nociceptive pain and neuropathic pain?
- What is somatic pain?
- How would you define visceral pain to a patient who was diagnosed with it?
- What is affective pain?
- How can pan impact a patient’s mental health?
Case Study
Mary Adams is a licensed practical nurse who has just relocated to town. Mary will be the utilization review nurse at a local long-term care facility. Mary was diagnosed with Postherpetic Neuralgia last year, and she is happy that her new job will have her mostly doing desk work and not providing direct patient care as she had been before the relocation. Mary was having difficulty at work at her previous employer due to pain. She called into work several times, and before leaving, Mary’s supervisor had counseled her because of her absences.
Mary wants to establish primary care immediately because she needs ongoing pain treatment. She is hopeful that, with her new job and pain under control, she will be able to continue a successful career in nursing. When Mary called the primary care office, she specifically requested a nurse practitioner as her primary care provider because she believes that nurse practitioners tend to spend more time with their patients.
Assessment
The assessment effectively determines the type of treatment needed, the options for treatment, and whether the patient may be at risk for opioid dependence. Since we know that chronic pain can lead to disability and pain has a high potential to negatively affect the patient’s ability to work or otherwise, be productive, perform self-care, and potentially impact family or caregivers, it is imperative to approach the assessment with curiosity and empathy. This approach will ensure a thorough review of pain and research on pain management options. Compassion and support alone can improve patient outcomes related to pain management (23).
Record Review
Regardless of familiarity with the patient, reviewing the patient’s treatment records is essential, as the ability to recall details is unreliable. Reviewing the records can help identify subtle changes in pain description and site, the patient’s story around pain, failed modalities, side effects, and the need for education, all impacting further treatment (23).
Research the patient’s current prescription and whether or not the patient has achieved the maximum dosage of the medication beforehand. Analyzing the patient’s past prescription could reveal a documented failed therapy even though the patient did not receive the maximum dose (23).
A review of documented allergens may indicate an allergy to pain medication. Discuss with the patient the specific response to the drug to determine if it is a true allergy, such as hives or anaphylaxis, or if the response may have been a side effect, such as nausea and vomiting.
Research whether the patient tried any non-medication modalities for pain, such as physical therapy (PT), occupational therapy (OT), or Cognitive Behavioral Therapy (CBT). Note that any non-medication modalities were documented as failed therapies. The presence of any failed therapies should prompt further discussion with the patient, family, or caregiver about the experience. The incompletion of therapy should not be considered failed therapy. Explore further if the patient abandoned appointments.
Case Study
You review the schedule for the week, and there are three new patient appointments. One is Mary Adams. The interdisciplinary team requested and received Mary’s treatment records from her previous primary care provider. You make 15 minutes available to review Mary’s records and the questionnaire Mary filled out for her upcoming appointment. You see that Mary has been diagnosed with Postherpetic Neuralgia and note her current treatment regimen, which she stated was ineffective. You write down questions you will want to ask Mary. You do not see evidence of non-medication modalities or allergies to pain medication.
Ask yourself...
- What potential risks or complications can arise from neglecting to conduct a thorough chart review before initiating a pain management assessment?
- In your experience, what evidence supports reviewing known patient records?
- What is an alternative to reviewing past treatment if records are not available?
- What barriers to assessment could you encounter? What could you do to overcome those barriers?
- What resources are available to help assess a patient’s medication record?
Pain Assessment
Several acronyms help explore all aspects of the patient’s experience when physically assessing pain. Acronyms commonly used to assess pain are SOCRATES, OLDCARTS, and COLDERAS. These pain assessment acronyms are also helpful in determining treatment since they include a character and duration of pain assessment (23).
| O-Onset | S-Site | C-Character |
| L-Location | O-Onset | O-Onset |
| D-Duration | C-Character | L-Location |
| C-Character | R-Radiate | D-Duration |
| A-Alleviating | A-Associated symptoms | E-Exacerbating symptoms |
| R-Radiating, relieving | T-Time/Duration | R-Relieving, radiating |
| T-Temporal patterns (frequency) | E-Exacerbating | A-Associated symptoms |
| S-Symptoms | S-Severity | S-Severity of illness |
Inquire where the patient is feeling pain. The patient may have multiple areas and types of pain. Each type and location must be explored and assessed. Unless the pain is from a localized injury, a body diagram map, as seen below, is helpful to document, inform, and communicate locations and types of pain. In cases of Fibromyalgia, rheumatoid arthritis, or other centralized or widespread pain, it is vital to inquire about radiating pain. The patient with chronic pain could be experiencing acute pain or a new pain site, such as osteoarthritis, that may need further evaluation and treatment (23).
Inquire with the patient how long their pain has been present and any associated or known causative factors. Pain experienced longer than three months defines chronic versus acute pain. Chronic pain means that the pain is centralized or a function of the Central Nervous System, which should guide treatment decisions.
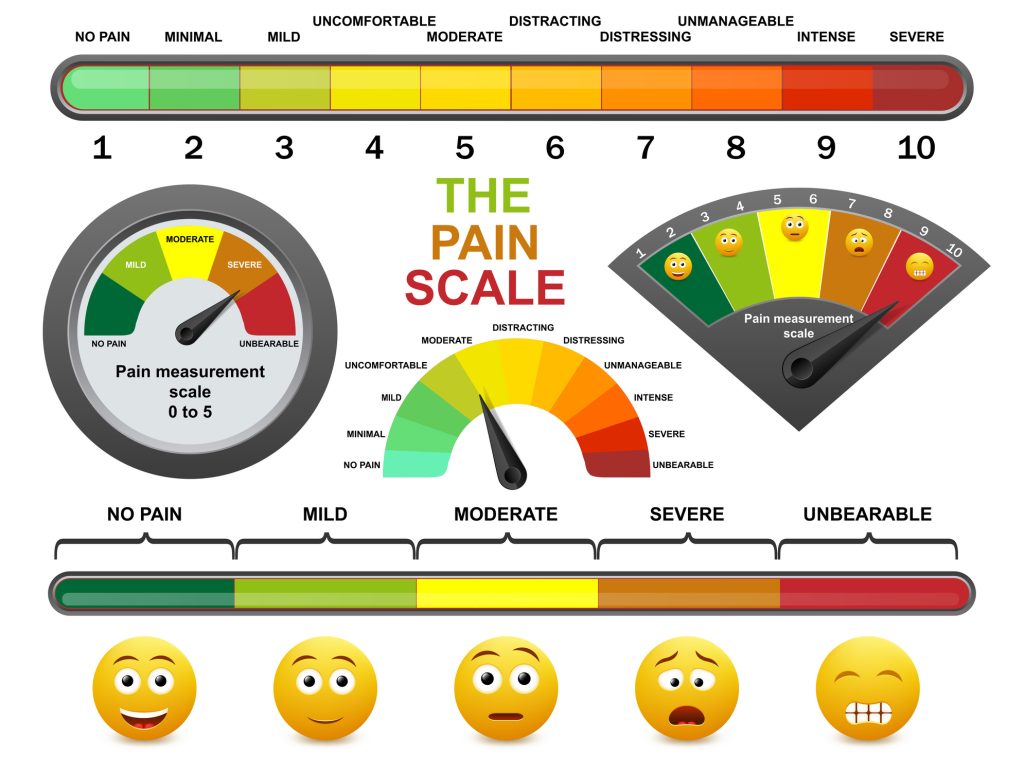
To help guide treatment, ask the patient to describe their pain. The description helps identify what type of pain the patient is experiencing: Allodynia and hyperalgesia indicate centralized pain; sharp, shooting pain could indicate neuropathic pain. Have the patient rate their pain. There are various tools, as shown below, for pain rating depending on the patient’s communication ability. Not using the pain rating number alone is imperative. Ask the patient to compare the severity of pain to a previous experience. For example, a 1/10 may be experienced as a bumped knee or bruise, whereas a 10/10 is experienced on the level of a kidney stone or childbirth (23).
Several pain rating scales are appropriate, in addition to the 0-10 rating scale, depending on the patient’s needs. They are listed below.
The 0-5 and Faces scales may be used for all adult patients and are especially effective for patients experiencing confusion.
The Defense and Veterans Pain Rating Scale (DVPRS) is a five-item tool that assesses the impact of pain on sleep, mood, stress, and activity levels (20).
The FLACC scale is practical for patients unable to self-report pain, such as those intubated in the ICU or with late-stage neurological diseases. It was initially created to assess pain in infants. Note: The patient need not cry to be rated 10/10.
| Behavior | 0 | 1 | 2 |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent or constant quivering chin, clenched jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn |
| Activity | Lying quietly, in a normal position, or relaxed | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry, wake or asleep | Moans or whimpers: occasional complaints | Crying steadily, screams, sobs, and frequent complaints |
| Consolability | Content, relaxed | Distractable, reassured by touching, hugging, or being talked to | Difficult to console or comfort |
(21).
Assess contributors to pain such as insomnia, stress, exercise, diet, and any comorbid conditions. Limited access to care, socioeconomic status, and local culture also contribute to the patient’s experience of pain (23). Most patients have limited opportunity to discuss these issues, and though challenging to bring up, it is compassionate and supportive care. A referral to social work or another agency may be helpful if you cannot explore it fully.
Assess for substance abuse disorders, especially among male, younger, less educated, or unemployed adults. Substance abuse disorders increase the likelihood of misuse disorder and include alcohol, tobacco, cannabis, cocaine, and heroin (29).
Inquire as to what changes in function the pain has caused. One question to ask is, “Were it not for pain, what would you be doing?” As seen below, a Pain, Enjoyment, and General Activity (PEG) three-question scale, which focuses on function and quality of life, may help determine the severity of pain and the effect of treatment over time.
| What number best describes your pain on average in the past week? 0-10 |
| What number best describes how, in the past week, pain has interfered with your enjoyment of life? 0-10 |
| What number determines how, in the past week, pain has interfered with your general activity? 0-10 |
(21).
Assess family history, mental health disorders, chronic pain, or substance abuse disorders. Each familial aspect puts patients at higher risk for developing chronic pain (23).
Evaluate for mental health disorders the patient may be experiencing, particularly anxiety and depression. The Patient Health Questionnaire (PHQ4) is a four-question tool for assessing depression and anxiety.
In some cases, functional MRI or imaging studies effectively determine the cause of pain and the treatment. If further assessment is needed to diagnose and treat pain, consult Neurology, Orthopedics, Palliative care, and pain specialists (23).
Case Study
You used OLDCARTS to evaluate Mary’s pain and completed a body diagram. Mary is experiencing allodynia in her back and shoulders, which is described as burning and tingling. It is exacerbated when she lifts, such as moving patients at the long-term care facility and, more recently, boxes from her move to the new house. Mary has also been experiencing anxiety due to fear of losing her job, the move, and her new role. She has moved closer to her family to help care for her children since she often experiences fatigue. Mary has experienced a tumultuous divorce in the last five years and feels she is still undergoing some trauma.
You saw in the chart that Mary had tried Gabapentin 300 mg BID for her pain and inquired about what happened. Mary explained that her pain improved from 8/10 to 7/10 and had no side effects. Her previous care provider discontinued the medication and documented it as a failed therapy. You reviewed the minimum and maximum dosages of Gabapentin and know Mary can take up to 1800mg/day.
During the assessment, Mary also described stiffness and aching in her left knee. She gets a sharp pain when she walks more than 500 steps, and her knee is throbbing by the end of the day. Mary rated the pain a 10/10, but when she compared 10/10 to childbirth, Mary said her pain was closer to 6/10. Her moderate knee pain has reduced Mary’s ability to exercise. She used to like to take walks. Mary stated she has had knee pain for six months and has been taking Ibuprofen 3 – 4 times daily.
Since Mary’s pain is moderate, you evaluate your options for drugs for moderate to severe pain.

Ask yourself...
- How do you assess and evaluate a patient’s pain level?
- What are the different types of pain and their management strategies?
- How do you determine the appropriate dosage of pain medications for a patient?
- How do you assess the effectiveness of pain medications in your patients?
- How do you adjust medication dosages for elderly patients with pain or addiction?
- How do you address the unique challenges in pain management for pediatric patients?
- What is the role of non-pharmacological interventions in pain management?
- How do you incorporate non-pharmacological interventions into your treatment plans?
- Which pain assessments do you find most effective? Why?
- Which pain assessments have you found to be the least effective? Why?
Opioid Classifications and Drug Schedules
A comprehensive understanding of drug schedules and opioid classifications is essential for nurse practitioners to ensure patient safety, prevent drug misuse, and adhere to legal and regulatory requirements. Nurse practitioners with a comprehensive understanding of drug schedules and opioid classifications can effectively communicate with colleagues, ensuring accurate medication reconciliation and facilitating interdisciplinary care. Nurse practitioners’ knowledge in facilitating discussions with pharmacists regarding opioid dosing, potential interactions, and patient education is essential (49).
Drug scheduling became mandated under the Controlled Substances Act. The Drug Enforcement Agency (DEA) Schedule of Controlled Drugs and the criteria and common drugs are listed below.
| Schedule | Criteria | Examples |
| I | No medical use; high addiction potential | Heroin, marijuana, PCP |
| II | Medical use; high addiction potential | Morphine, oxycodone, Methadone, Fentanyl, amphetamines |
| III | Medical use; high addiction potential | Hydrocodone, codeine, anabolic steroids |
| IV | Medical use, low abuse potential | Benzodiazepines, meprobamate, butorphanol, pentazocine, propoxyphene |
| V | Medical use; low abuse potential | Buprex, Phenergan with codeine |
(Pain Physician, 2008)
Listed below are drugs classified by their schedule and mechanism of action. “Agonist” indicates a drug that binds to the opioid receptor, causing pain relief and also euphoria. An agonist-antagonist indicates the drug binds to some opioid receptors but blocks others. Mixed antagonist-agonist drugs control pain but have a lower potential for abuse and dependence than agonists (7).
| Schedule I | Schedule II | Schedule III | Schedule IV | Schedule V | |
| Opioid agonists | Benzomorphine, Dihydromorphone, Ketobemidine, Levomoramide, Morphine-methylsulfate,
Nicocodeine, Nicomorphine, Racemoramide |
Codeine, Fentanyl, Sublimaze, Hydrocodone, Hydromorphone, Dilaudid,
Meperidine, Demerol, Methadone, Morphine, Oxycodone, Endocet, OxyContin, Percocet, Oxymorphone, Numorphan |
Buprenorphine Buprenex, Subutex,
Codeine compounds, Tylenol #3, Hydrocodone compounds, Lortab, Lorcet, Tussionex, Vicodin |
Propoxyphene, Darvon, Darvocet | Opium, Donnagel, Kapectolin |
| Mixed Agonist -Antagonist | BuprenorphineNaloxone, Suboxone | Pentazocine,
Naloxone, Talwin-Nx |
|||
| Stimulants | N-methylampheta-mine 3, 4-methylenedioxy amphetamine, MDMA, Ecstasy | Amphetamine, Adderall, Cocaine, Dextroamphetamine, Dexedrine, Methamphetamine, Desoxyn, Methylphenidate, Concerta, Metadate, Ritalin, Phenmetrazine, Fastin, Preludin | Benapheta-mine, Didrex, Pemolin, Cylert, Phendimetrazine, Plegine | Diethylpropion, Tenuate, Fenfluramine, Phentermine, Fastin | 1-dioxy-ephedrine-Vicks Inhaler |
| Hallucinogen-gens, other | Lysergic Acid Diamine LSD, marijuana, Mescaline, Peyote, Phencyclidine PCP, Psilocybin, Tetrahydrocannabinol | Dronabinol, Marinol | |||
| Sedative Hypnotics | Methylqualine, Quaalude, Gamma-hydroxybutyrate, GHB
|
Amobarbitol, Amytal, Glutethamide, Doriden, Pentobarbital, Nembutal, Secobarbital, Seconal | Butibarbital. Butisol, Butilbital, Florecet, Florinal,
Methylprylon, Noludar |
Alprazolam, Xanax, Chlordiazepoxide, Librium, Chloral betaine, Chloral hydrate, Noctec, Chlorazepam, Clonazepam, Klonopin, Clorazepate, Tranxene, Diazepam, Valium, Estazolam, Prosom, Ethchlorvynol, Placidyl, Ethinamate, Flurazepam, Dalmane, Halazepam, Paxipam, Lorazepam, Ativan, Mazindol, Sanorex, Mephobarbital, Mebaral, Meprobamate, Equanil, Methohexital, Brevital Sodium, Methyl-phenobarbital,
Midazolam, Versed, Oxazepam, Serax, Paraldehyde, Paral, Phenobarbital, Luminal, Prazepam, Centrax, Temazepam, Restoril, Triazolam, Halcion, Sonata, Zolpidem, Ambien |
Diphenoxylate preparations, Lomotil |
(41).
Ask yourself...
- What are the potential risks and benefits of using opioids for pain management?
- How can nurse practitioners effectively monitor patients on long-term opioid therapy?
- What are the potential risks and benefits of using long-acting opioids for chronic pain?
- How do you monitor patients on long-acting opioids for safety and efficacy?
- What is the difference between a Schedule I and a Schedule II Substance?
- What is the difference between a Schedule II and a Schedule III Substance?
- How would you explain a Schedule V Substance?
- How does a Schedule V and Schedule IV Substance?
- What Schedule Substance is marijuana?
- Which medications do you most frequently administer/prescribe?
Commonly Prescribed Opioids, Indications for Use, and Typical Side Effects
Opioid medications are widely used for managing moderate to severe pain. Referencing NIDA (2023), this section aims to give healthcare professionals an overview of the indications and typical side effects of commonly prescribed Schedule II opioid medications, including hydrocodone, oxycodone, morphine, Fentanyl, and hydromorphone.
Opioids are derived and manufactured in several ways. Naturally occurring opioids come directly from the opium poppy plant. Synthetic opioids are manufactured by chemically synthesizing compounds that mimic the effects of a natural opioid. Semi-synthetic is a mix of naturally occurring and man-made (35).
Understanding the variations in how an opioid is derived and manufactured is crucial in deciding the type of opioid prescribed, as potency and analgesic effects differ. Synthetic opioids are often more potent than naturally occurring opioids. Synthetic opioids have a longer half-life and slower elimination, affecting the duration of action and timing for dose adjustments. They are also associated with a higher risk of abuse and addiction (38).
Hydrocodone
Mechanism of Action and Metabolism
Hydrocodone is a Schedule II medication. It is an opioid agonist and works as an analgesic by activating mu and kappa opioid receptors located in the central nervous system and the enteric plexus of the bowel. Agonist stimulation of the opioid receptors inhibits the release of nociceptive neurotransmitters and reduces neuronal excitability (17).
- Produces analgesia.
- Suppresses the cough reflex at the medulla.
- Causes respiratory depression at higher doses.
Hydrocodone is indicated for treating severe pain after nonopioid therapy has failed. It is also indicated as an antitussive for nonproductive cough in adults over 18.
Available Forms
Hydrocodone immediate release (IR) reaches maximum serum concentrations in one hour with a half-life of 4 hours. Extended-release (ER) Hydrocodone reaches peak concentration at 14-16 hours and a half-life of 7 to 9 hours. Hydrocodone is metabolized to an inactive metabolite in the liver by cytochrome P450 enzymes CYP2D6 and CYP3A4. Hydrocodone is converted to hydromorphone and is excreted renally. Plasma concentrations of hydromorphone are correlated with analgesic effects rather than hydrocodone.
Hydrocodone is formulated for oral administration into tablets, capsules, and oral solutions. Capsules and tablets should never be crushed, chewed, or dissolved. These actions convert the extended-release dose into immediate release, resulting in uncontrolled and rapid release of opioids and possible overdose.
Dosing and Monitoring
Hydrocodone IR is combined with acetaminophen or ibuprofen. The dosage range is 2.5mg to 10mg every 4 to 6 hours. If formulated with acetaminophen, the dosage is limited to 4gm/day.
Hydrocodone ER is available as tablets and capsules. Depending on the product, the dose of hydrocodone ER formulations in opioid-naïve patients is 10 to 20 mg every 12 to 24 hours.
Nurse practitioners should ensure patients discontinue all other opioids when starting the extended-release formula.
Side Effects and Contraindications
Because mu and kappa opioid receptors are in the central nervous system and enteric plexus of the bowel, the most common side effects of hydrocodone are constipation and nausea (>10%).
Other adverse effects of hydrocodone include:
- Respiratory: severe respiratory depression, shortness of breath
- Cardiovascular: hypotension, bradycardia, peripheral edema
- Neurologic: Headache, chills, anxiety, sedation, insomnia, dizziness, drowsiness, fatigue
- Dermatologic: Pruritus, diaphoresis, rash
- Gastrointestinal: Vomiting, dyspepsia, gastroenteritis, abdominal pain
- Genitourinary: Urinary tract infection, urinary retention
- Otic: Tinnitus, sensorineural hearing loss
- Endocrine: Secondary adrenal insufficiency (17)
Hydrocodone, being an agonist, must not be taken with other central nervous system depressants as sedation and respiratory depression can result. In formulations combined with acetaminophen, hydrocodone can increase the international normalized ratio (INR) and cause bleeding. Medications that induce or inhibit cytochrome enzymes can lead to wide variations in absorption.
The most common drug interactions are listed below:
- Alcohol
- Benzodiazepines
- Barbiturates
- other opioids
- rifampin
- phenytoin
- carbamazepine
- cimetidine,
- fluoxetine
- ritonavir
- erythromycin
- diltiazem
- ketoconazole
- verapamil
- Phenytoin
- John’s Wort
- Glucocorticoids
Considerations
Use with caution in the following:
- Patients with Hepatic Impairment: Initiate 50% of the usual dose
- Patients with Renal Impairment: Initiate 50% of the usual dose
- Pregnancy: While not contraindicated, the FDA issued a black-boxed warning since opioids cross the placenta, and prolonged use during pregnancy may cause neonatal opioid withdrawal syndrome (NOWS).
- Breastfeeding: Infants are susceptible to low dosages of opioids. Non-opioid analgesics are preferred.
Pharmacogenomic: Genetic variants in hydrocodone metabolism include ultra-rapid, extensive, and poor metabolizer phenotypes. After administration of hydrocodone, hydromorphone levels in rapid metabolizers are significantly higher than in poor metabolizers.
Oxycodone
Mechanism of Action and Metabolism
Oxycodone has been in use since 1917 and is derived from Thebaine. It is a semi-synthetic opioid analgesic that works by binding to mu-opioid receptors in the central nervous system. It primarily acts as an agonist, producing analgesic effects by inhibiting the transmission of pain signals (Altman, Clark, Huddart, & Klein, 2018).
Oxycodone is primarily metabolized in the liver by CYP3A4/5. It is metabolized in the liver to noroxycodone and oxymorphone. The metabolite oxymorphone also has an analgesic effect and does not inhibit CYP3A4/5. Because of this metabolite, oxycodone is more potent than morphine, with fewer side effects and less drug interactions. Approximately 72% of oxycodone is excreted in urine (Altman, Clark, Huddart, & Klein, 2018).
Available Forms
Oxycodone can be administered orally, rectally, intravenously, and as an epidural. For this sake, we will focus on immediate-release and extended-release oral formulations.
- Immediate-release (IR) tablets
- IR capsules
- IR oral solutions
- Extended-release (ER) tablets
Dosing and Monitoring
The dosing of oxycodone should be individualized based on the patient’s pain severity, previous opioid exposure, and response. Initial dosages for opioid naïve patients range from 5-15 mg for immediate-release formulations, while extended-release formulations are usually initiated at 10-20 mg. Dosage adjustments may be necessary based on the patient’s response, but caution should be exercised. IR and ER formulations reach a steady state at 24 hours and titrating before 24 hours may lead to overdose.
Regular monitoring is essential to assess the patient’s response to treatment, including pain relief, side effects, and signs of opioid misuse or addiction. Monitoring should include periodic reassessment of pain intensity, functional status, and adverse effects (Altman, Clark, Huddart, & Klein, 2018).
Side Effects and Contraindications
Common side effects of oxycodone include:
- constipation
- nausea
- sedation
- dizziness
- respiratory depression
- respiratory arrest
- hypotension
- fatal overdose
Oxycodone is contraindicated in patients with known hypersensitivity to opioids, severe respiratory depression, paralytic ileus, or acute or severe bronchial asthma. It should be used cautiously in patients with a history of substance abuse, respiratory conditions, liver or kidney impairment, and those taking other medications that may interact with opioids, such as alcohol (4).
It is also contraindicated with the following medications and classes:
- Antifungal agents
- Antibiotics
- Rifampin
- Carbamazepine
- Fluoxetine
- Paroxetine
Considerations
- Nurse practitioners should consider the variations in the mechanism of action for the following:
- Metabolism differs between males and females: females have been shown to have less concentration of oxymorphone and more CYP3A4/5 metabolites.
- Infants have reduced clearance of oxycodone, increasing side effects.
- Pediatrics have 20-40% increased clearance over adults.
- Reduced clearance with age increases the half-life of oxycodone.
- Pregnant women have a greater clearance and reduced half-life.
- Impairment of the liver reduces clearance.
- Cancer patients with cachexia have increased exposure to oxycodone and its metabolite.
- Maternal and neonate concentrations are similar, indicating placenta crossing (4)
Morphine

Mechanism of Action and Metabolism
Morphine is a naturally occurring opioid alkaloid extracted from the opium poppy. It was isolated in 1805 and is the opioid against which all others are compared. Morphine binds to mu-opioid receptors in the brain and spinal cord, inhibiting the transmission of pain signals and producing analgesia. It is a first-line choice of opioid for moderate to severe acute, postoperative, and cancer-related pain (8).
Morphine undergoes first-pass metabolism in the liver and gut. It is well absorbed and distributed throughout the body. Its main metabolites are morphine-3-glucuronide and morphine-6-glucuronide. Its mean plasma elimination half-life after intravenous administration is about 2 hours. Approximately 90% of morphine is excreted in the urine within 24 hours (8).
Available Forms
Morphine is available in various forms.
- immediate-release tablets
- extended-release tablets
- oral IR solutions
- injectable solutions
- transdermal patches
Dosing and Monitoring
Morphine is hydrophilic and, as such, has a slow onset time. The advantage of this is that it is unlikely to cause acute respiratory depression even when injected. However, because of the slow onset time, there is more likelihood of morphine overdose due to the ability to “stack” doses in patients experiencing severe pain (Bistas, Lopez-Ojeda, & Ramos-Matos, 2023).
The dosing of morphine depends on the patient’s pain severity, previous opioid exposure, and other factors. It is usually initiated at a low dose and titrated upwards as needed. Monitoring pain relief, adverse effects, and signs of opioid toxicity is crucial. Reevaluate benefits and harms with patients within 1 to 4 weeks of starting opioid therapy or of dose escalation. General recommendations for initiating morphine (Bistas, Lopez-Ojeda, & Ramos-Matos, 2023).
Prescribe IR opioids instead of ER opioids.
Prescribe the lowest effective dosage, below 50 Morphine Milligram Equivalents (MME) /day.
Side Effects and Contraindications
Because morphine binds to opioid receptors in the brain and spinal cord, is metabolized in the liver and gut, and has a slow onset, the following side effects are common:
- Constipation
- Nausea
- Vomiting
- Sedation
- Dizziness
- Respiratory depression
- Pruritis
- Sweating
- Dysphoria/Euphoria
- Dry mouth
- Anorexia
- Spasms of urinary and biliary tract
Contraindications of morphine are:
- Known hypersensitivity or allergy to morphine.
- Bronchial asthma or upper airway obstruction
- Respiratory depression in the absence of resuscitative equipment
- Paralytic ileus
- Risk of choking in patients with dysphagia, including infants, children, and the elderly (8)
Concurrent use with other sedating medications: Amitriptyline, diazepam, haloperidol, chlorpromazine
Morphine interacts with the following medications:
- Ciprofloxacin
- Metoclopramide
- Ritonavir
Considerations for Nurse Practitioners
Assess for medical conditions that may pose serious and life-threatening risks with opioid use, such as the following:
- Sleep-disordered breathing, such as sleep apnea.
- Pregnancy
- Renal or hepatic insufficiency
- Age >= 65
- Certain mental health conditions
- Substance use disorder
- Previous nonfatal overdose
Fentanyl
Mechanism of Action and Metabolism
Fentanyl is a synthetic opioid more potent than morphine and was approved in 1968. Fentanyl is an agonist that works by binding to the mu-opioid receptors in the central nervous system. This binding inhibits the transmission of pain signals, resulting in analgesia. Fentanyl is often used for severe pain management, particularly in the perioperative and palliative care settings, or for severe pain in patients with Hepatic failure (8).
It is a mu-selective opioid agonist. However, it can activate other opioid receptors in the body, such as the delta and kappa receptors, producing analgesia. It also activates the Dopamine center of the brain, stimulating relaxation and exhilaration, which is responsible for its high potential for addiction (8).
Indications for fentanyl are as follows:
- Preoperative analgesia
- Anesthesia adjunct
- Regional anesthesia adjunct
- General anesthesia
- Postoperative pain control
- Moderate to severe acute pain (off-label)
Available Forms
- Fentanyl is available in various forms, including:
- transdermal patches
- injectable solutions
- lozenges
- nasal sprays
- oral tablets (8)
Dosing and Monitoring
Fentanyl is metabolized via the CYP3A4 enzyme in the liver. It has a half-life of 3 to 7 hours, and 75% of Fentanyl is excreted in the urine and 9% in feces.
The dosing of fentanyl depends on the route of administration and the patient’s needs. For example, transdermal patches are typically applied every 72 hours, while injectable solutions are titrated to achieve the desired analgesic effect. Monitoring should include assessing pain levels, respiratory rate, blood pressure, and sedation scores (8).
Fentanyl is most dosed as follows:
- Post-operative pain control
- 50 to 100 mcg IV/IM every 1 to 2 hours as needed; alternately 0.5 to 1.5 mcg/kg/hour IV as needed. Lower dosing should be considered in patients 65 and older.
PCA (patient-controlled analgesia): 10 to 20 mcg IV every 6 to 20 minutes as needed; start at the lowest effective dose for the shortest effective duration – refer to institutional protocols (8).
Moderate to severe acute pain (off-label) 1 to 2 mcg/kg/dose intranasally each hour as needed; the maximum dose is 100 mcg. Use the lowest effective dose for the shortest effective duration (8).
Side Effects and Contraindications
Common side effects of fentanyl include:
- respiratory depression
- sedation
- constipation
- nausea
- vomiting
- euphoria
- confusion
- respiratory depression/arrest
- visual disturbances
- dyskinesia
- hallucinations
- delirium
- narcotic ileus
- muscle rigidity
- addiction
- loss of consciousness
- hypotension
- coma
- death (8).
The use of fentanyl is contraindicated in patients in the following situations:
- After operative interventions in the biliary tract, these may slow hepatic elimination of the drug.
- With respiratory depression or obstructive airway diseases (i.e., asthma, COPD, obstructive sleep apnea, obesity hyperventilation, also known as Pickwickian syndrome)
- With liver failure
- With known intolerance to fentanyl or other morphine-like drugs, including codeine or any components in the formulation.
- With known hypersensitivity (i.e., anaphylaxis) or any common drug delivery excipients (i.e., sodium chloride, sodium hydroxide) (8).
Considerations for Nurse Practitioners
Nurse practitioners prescribing fentanyl should thoroughly assess the patient’s pain, medical history, and potential risk factors for opioid misuse. They should also educate patients about the proper use, storage, and disposal of fentanyl. It should be used cautiously in patients with respiratory disorders, liver or kidney impairment, or a history of substance abuse. Fentanyl is contraindicated in patients with known hypersensitivity to opioids and those without exposure to opioids.
Alcohol and other drugs, legal or illegal, can exacerbate fentanyl’s side effects, creating multi-layered clinical scenarios that can be complex to manage. These substances, taken together, generate undesirable conditions that complicate the patient’s prognosis (8).
Hydromorphone
Mechanism of Action and Metabolism
Hydromorphone is a semi-synthetic opioid derived from morphine. It binds to the mu-opioid receptors in the central nervous system. It primarily exerts its analgesic effects by inhibiting the release of neurotransmitters involved in pain transmission, thereby reducing pain perception. Hydromorphone also exerts its effects centrally at the medulla level, leading to respiratory depression and cough suppression (1).
Hydromorphone is indicated for:
- moderate to severe acute pain
- severe chronic pain
- refractory cough suppression (off-label) (1)
Available Forms
Hydromorphone is available in various forms, depending on the patient’s needs and severity of pain.
- immediate-release tablet
- extended release tablets
- oral liquid
- injectable solution
- rectal suppositories
Dosing and Monitoring
The immediate-release oral formulations of hydromorphone have an onset of action within 15 to 30 minutes. Peak levels are typically between 30 and 60 minutes with a half-life of 2 to 3 hours. Hydromorphone is primarily excreted through the urine.
The dosing of hydromorphone should be individualized based on the patient’s pain intensity, initiated at the lowest effective dose, and adjusted gradually as needed. Close monitoring of pain relief, adverse effects, and signs of opioid toxicity is essential. Patients should be assessed regularly to ensure they receive adequate pain control without experiencing excessive sedation or respiratory depression.
The following are standard dosages that should only be administered when other opioid and non-opioid options fail.
- Immediate-release oral solutions dosage: 1 mg/1 mLoral tablets are available in 2 mg, 4 mg, and 8 mg.
- Extended-release oral tablets are available in dosages of 8 mg, 12 mg, 16 mg, and 32 mg.
- Injection solutions are available in concentrations of 1 mg/mL, 2 mg/mL, 4 mg/mL, and 10 mg/mL.
- Intravenous solutions are available in strengths of 2 mg/1 mL, 2500 mg/250 mL, 10 mg/1 mL, and 500 mg/50 mL.
- Suppositories are formulated at a strength of 3 mg (1).
Side Effects and Contraindications
Hydromorphone has potential adverse effects on several organ systems, including the integumentary, gastrointestinal, neurologic, cardiovascular, endocrine, and respiratory.
Common side effects of hydromorphone include:
- Constipation
- Nausea
- Vomiting
- Dizziness
- Sedation
- respiratory depression
- pruritus
- headache
- Somnolence
- Severe adverse effects of hydromorphone include:
- Hypotension
- Syncope
- adrenal insufficiency
- coma
- raised intracranial pressure.
- seizure
- suicidal thoughts
- apnea
- respiratory depression or arrest
- drug dependence or withdrawal
- neonatal drug withdrawal syndrome
- Hydromorphone is contraindicated in patients with:
- known allergies to the drug, sulfites, or other formulation components.
- known hypersensitivity to opioids.
- severe respiratory depression
- paralytic ileus
- acute or severe bronchial asthma (1).
Caution should be exercised in patients with:
- respiratory insufficiency
- head injuries
- increased intracranial pressure.
- liver or kidney impairment.
Considerations for Nurse Practitioners
As nurse practitioners, it is crucial to assess the patient’s pain intensity and overall health status before initiating Hydromorphone. Start with the lowest effective dose and titrate carefully for optimal pain control. Regular monitoring for adverse effects, signs of opioid toxicity, and therapeutic response is essential. Educate patients about the potential side effects, proper dosing, and the importance of not exceeding prescribed doses. Additionally, nurse practitioners should be familiar with local regulations and guidelines regarding opioid prescribing and follow appropriate documentation and monitoring practices.
Additional Considerations
In terminal cancer patients, clinicians should not restrain opioid therapy even if signs of respiratory depression become apparent.
Hydromorphone requires careful administration in cases of concurrent psychiatric illness.
Specific Patient Considerations:
- Hepatic impairment and Renal Impairment: Depending on the degree of impairment, initiate hydromorphone treatment at one-fourth to one-half of the standard starting dosage.
- Pregnancy considerations: Hydromorphone can traverse the placental barrier and induce NOWS.
- Breastfeeding considerations: Nonopioid analgesic agents are preferable for breastfeeding women.
- Older patients: hydromorphone is categorized as a potentially inappropriate medication for older adults (1).
Tramadol

Mechanism of Action and Metabolism
Tramadol is a Schedule IV opioid medication with a higher potential for dependency and misuse than non-opioid medications. It binds to opioid receptors in the central nervous system, inhibiting the reuptake of norepinephrine and serotonin. It also has weak mu-opioid receptor agonist activity.
Tramadol is metabolized by the liver, mediated by the cytochrome P450 pathways (particularly CYP2D6), and is mainly excreted through the kidneys.
Tramadol is used for moderate to severe pain.
Available Forms of Tramadol include:
- Immediate-release-typically used for acute pain management.
- Extended-release-used for chronic pain.
Dosing and Monitoring
Tramadol has an oral bioavailability of 68% after a single dose and 90–100% after multiple doses and reaches peak concentrations within 2 hours. Approximately 75% of an oral dose is absorbed, and the half-life of tramadol is 9 hours (18).
Tramadol dosing should be individualized based on the patient’s pain severity and response.
The initial dose for adults is usually 50-100 mg orally every 4-6 hours for pain relief. The maximum daily dose is 400 mg for immediate-release formulations and 300 mg for extended-release formulations (18).
It is essential to monitor the patient’s pain intensity, response to treatment, and any adverse effects. Regular reassessment and adjustment of the dosage may be necessary.
Side Effects and Contraindications
Tramadol is responsible for severe intoxications leading to consciousness disorder (30%), seizures (15%), agitation (10%), and respiratory depression (5%). The reactions to Tramadol suggest that the decision to prescribe should be carefully considered.
Common Side Effects of Tramadol Include:
- Nausea
- Vomiting
- Dizziness
- Constipation
- Sedation
- Headache
- CNS depression
- Seizure
- Agitation
- Tachycardia
- Hypertension
- reduced appetite
- pruritus and rash
- gastric irritation
Serious side effects include:
- respiratory depression
- serotonin syndrome
- seizures
Contraindications
Tramadol is contraindicated in patients with:
- history of hypersensitivity to opioids
- acute intoxication with alcohol
- opioids, or other psychoactive substances
- Patients who have recently received monoamine oxidase inhibitors (MAOIs)
Additionally, the following can be observed in tramadol intoxication:
- miosis
- respiratory depression
- decreased level of consciousness
- hypertension
- tremor
- irritability
- increased deep tendon reflexes
Poisoning leads to:
- multiple organ failure
- coma
- cardiopulmonary arrest
- death
Considerations for Nurse Practitioners
Tramadol has been increasingly misused with intentional overdoses or intoxications. Suicide attempts were the most common cause of intoxication (52–80%), followed by abuse (18–31%), and unintentional intoxication (1–11%). Chronic tramadol or opioid abuse was reported in 20% of tramadol poisoning cases. Fatal tramadol intoxications are uncommon except when ingested concurrent with depressants, most commonly benzodiazepines and alcohol (18).
Tramadol poisoning can affect multiple organ systems:
- gastrointestinal
- central nervous system: seizure, CNS depression, low-grade coma, anxiety, and over time anoxic brain damage
- Cardiovascular system: palpitation, mild hypertension to life-threatening complications such as cardiopulmonary arrest
- respiratory system
- renal system: renal failure with higher doses of tramadol intoxication
- musculoskeletal system: rhabdomyolysis
- endocrine system: hypoglycemia, serotonin syndrome (18)
Cannabis
Mechanism of Action and Metabolism
Cannabis is classified as Schedule I. It contains various cannabinoids, with delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) being the most studied. THC primarily acts on cannabinoid receptors in the brain, producing psychoactive effects, while CBD has more diverse effects on the nervous system. These cannabinoids interact with the endocannabinoid system, modulating neurotransmitter release and influencing various physiological processes (32).
Similar to opioids, cannabinoids are synthesized and released in the body by synapses that act on the cannabinoid receptors present in presynaptic endings (32). They perform the following actions related to analgesia:
- Decrease the release of neurotransmitters.
- Activate descending inhibitory pain pathways.
- Reduce postsynaptic sensitivity and alleviate neural inflammation.
- Modulate CB1 receptors within central nociception processing areas and the spinal cord, resulting in analgesic effects.
- Attenuate inflammation by activating CB2 receptors (32).
- Emerging research shows cannabis is indicated for:
- Migraines
- chronic pain
- back pain
- arthritic pain
- pain associated with cancer and surgery.
- neuropathic pain
- diabetic neuropathic pain when administered early in the disease progression.
- sickle cell disease
- cancer
- inflammatory bowel disease (32)
Available Forms
Cannabis refers to products sourced from the Cannabis sativa plant. There are differences between cannabis, cannabinoids, and cannabidiol (CBD). Cannabinoids are extracted from the cannabis plants. Cannabinoid-based treatments, such as dronabinol and CBD, are typically approved medical interventions for specific indications. THC (9-tetrahydrocannabinol) is the psychoactive component of the cannabis plant. CBD is a non-psychoactive component (32).
Cannabis can be consumed in different forms, each with a different onset and duration. Patients may have individual preferences, including:
- smoking/vaporizing dried flowers.
- consuming edibles
- tinctures or oils
- applying topicals (32)
Dosing and Monitoring
Inhaling marijuana via the lungs by smoking or vaping causes maximum plasma concentration within minutes. Psychiatric effects begin within seconds to a few minutes after inhalation and peak after 15 to 30 minutes. The effect diminishes throughout 2 to 3 hours (32).
Oral ingestion of marijuana causes psychiatric effects that typically occur between 30 and 90 minutes and reach maximum effect after 2 to 3 hours. Ingested marijuana effects last about 4 to 12 hours (32).
Dosing cannabis is challenging due to variations in potency and individual responses. Start with low doses and titrate slowly to achieve the desired effect while minimizing side effects. Regular monitoring is crucial, including assessing symptom relief, adverse effects, and potential drug interactions. Encourage patients to keep a diary to track their cannabis use and its effects (32).
Side Effects and Contraindications
Cannabis can exacerbate mental health conditions such as anxiety and psychosis. Common side effects of cannabis include (32):
- Dizziness
- dry mouth
- increased heart rate
- impaired memory
- psychoactive effects
Contraindications include:
- Pregnancy
- Breastfeeding
- heart disease
- respiratory conditions
- history of substance abuse
- mental health disorders
Ask yourself...
- How do you address patients’ misconceptions about pain medications?
- What are the mechanisms of action for commonly prescribed pain medications?
- How do these mechanisms of action contribute to pain relief?
- What are the potential side effects and risks associated with commonly prescribed pain medications?
- How do you educate patients about the risks and benefits of pain medications?
- How do you manage patients who require high-dose opioids for pain management?
- Is medical cannabis legal in your State? If yes, are you familiar with the prescribing guidelines?
- Do you have any personal biases against the use of medical cannabis? Why or why not?
- What education is critical to provide to patients being started on hydrocodone?
- What education is critical to provide to patients being started on oxycodone?
- What education is critical to provide to patients being started on morphine?
- What education is critical to provide to patients being started on fentanyl?
- What education is critical to provide to patients being started on hydromorphone?
- What education is critical to provide to patients being started on tramadol?
- What education is critical to provide to patients being started on cannabis?
Case Study
Mary agrees to try an increased dose of Gabapentin. Mary would also like to see a counselor to discuss her past and get help with her anxiety. You made an appointment for Mary to see a Licensed Clinical Social Worker in your clinic.
You read the side effects and warnings for Gabapentin, and it is unsafe to use Gabapentin and Tramadol together since they are both depressants. You order a non-steroidal drug for Mary’s somatic knee pain and make a consult for imaging studies on her left knee. You also make a referral to Orthopedics.
You educated Mary about the side effects of Gabapentin and scheduled a follow-up appointment. The day after Mary began her treatment with the increased Gabapentin, you called Mary to follow up on its impact. Mary still has pain, but she is not having any untoward side effects. Gabapentin may not work immediately, so you will schedule a follow-up call in 3 days.

Ask yourself...
- In this case study, Mary has insurance. How might your practice be different were Mary not insured?
- In your experience, what are the possible reasons Mary’s knee pain was not included in her previous treatment record?
- Consider how your assessment of Mary’s needs differs from the above-mentioned case study.
- Explain the rationale for decisions made by the nurse practitioner in the case study mentioned above and if your decisions would differ.
Opioid Use, the Opioid Epidemic, and Statistics
The use and misuse of opioids has become a pressing public health concern, leading to a global epidemic. The history of opioid use, the opioid epidemic, and associated statistics provide essential context for healthcare professionals in addressing this public health crisis. More importantly, it is estimated that 1 in 4 patients receiving prescription opioids in primary care settings will misuse them. In addition, 50% of opioid prescriptions are written by primary care providers, including nurse practitioners (22). Understanding the factors contributing to the epidemic and the magnitude of its impact is crucial for effective prevention, intervention, and treatment strategies.
History of Opioid Use
Opioids have a long history of medicinal use, dating back to ancient civilizations. They have been a drug of choice for pain relief for thousands of years. The introduction of synthetic opioids in the 19th century, such as morphine and later heroin, revolutionized pain management. However, their potential for addiction and misuse soon became apparent (16).
The Opioid Epidemic
The opioid epidemic refers to the surge in opioid misuse, addiction, and overdose deaths. The epidemic gained momentum in the late 1990s with increased prescribing of opioids for chronic pain (43).
No doubt, increased prescribing put opioids in the hands of consumers, but increased prescribing resulted from a multifactorial influence. One of the main influences was aggressive marketing by pharmaceutical companies, which has been well publicized. However, due to the long history of underprescribing pain medications for fear of misuse and addiction, the medical community was primed to expand its opioid prescribing practices (31).
A historical event that increased comfort with prescribing opioids, in the writer’s opinion, was the introduction of the Medicare Hospice Benefit in 1986. Medical directors must be contracted or employed by hospices, and these medical directors had or soon gained pain management expertise. To further promote hospice and effective pain management, the hospice medical directors, with newly acquired skills, provided education throughout medical communities about pain management and specifically to decrease the fear of using opioids. Pharmacies and attending physicians grew accustomed to giving opioids for home use. Hospice care is for terminally ill patients, defined as a life expectancy of 6 months or less. Still, the reality is that hospice discharges 12 to 40% of patients for ineligibility and other reasons.
A more prominent factor in increasing opioid prescribing was the 1996 American Pain Society’s introduction of pain as “the 5th Vital sign.” Soon after, The Joint Commission promoted pain as “the 5th Vital Sign” and began compliance surveys in healthcare organizations requiring pain assessment details to be as prominent as blood pressure and heart rate. The Joint Commission cited a quote from 1968 by a nurse from the University of California Los Angeles, Margo McCaffrey, who defined pain as “…Whatever the experiencing person says it is, existing whenever s/he says it does.” The Joint Commission accreditation programs pursued pain management as part of the accreditation process throughout its healthcare accreditation programs, including hospice accreditation by 1989 per TJC Timeline (48).
The National Institute of Health published an article about the Joint Commission’s role in the opioid epidemic, particularly regarding the definition of pain, “This definition emphasizes that pain is a subjective experience with no objective measures. It also stresses that the patient, not the clinician, is the authority on the pain and that their self-report is the most reliable indicator of pain. This set the tone for clinicians: Patients are always to be trusted to report pain accurately” (45).
Statistics on the Opioid Epidemic
In the United States alone, over 500,000 people died from opioid overdoses between 1999 and 2017. The number of opioid-related overdose deaths continues to increase, with synthetic opioids, mainly illicitly manufactured Fentanyl, playing a significant role in recent years (46). Fentanyl-laced drugs, such as marijuana, are increasingly sold knowing and unknowingly to introduce medications with a high addiction rate, thus creating new consumers. This practice can potentially increase deaths due to the imprecise nature of manufacturing (16).
Opioid-related hospitalizations have also risen substantially. In 2014, there were approximately 1.27 million hospitalizations related to opioids in the United States. These hospitalizations not only place a burden on healthcare systems but also reflect the severe consequences of opioid misuse (3).

Ask yourself...
- Have you experienced changes to your practice because of the opioid epidemic? If so, what are the changes?
- What is your opinion on the validity of Margo McCaffrey’s definition of pain?
- What factors influence your willingness or unwillingness to prescribe opioids?
- How has the opioid epidemic changed over the years?
- Do the statistics regarding the opioid epidemic surprise you?
- Do the statistics above align with what you are seeing in practice?
Federal Regulations on Opioid Prescribing
The history of substance use disorder prevention that promotes opioid recovery and treatment for patients and communities can be traced back to the early 20th century. However, the current approach to addressing opioid addiction and promoting healing has evolved significantly in recent times (36).
In the early 1900s, health professionals treated opioid addiction with punitive measures, including incarceration and moralistic approaches. The focus was on punishing individuals rather than providing effective treatment. This approach persisted for several decades until the mid-20th century when the medical community started recognizing addiction as a medical condition rather than a moral failing (36).
The Controlled Substances Act (CSA), introduced in 1970, was a response to increasing drug abuse and illicit drug trafficking in the United States. The CSA is a federal law regulating the manufacture, possession, distribution, and use of certain substances, including drugs and medications, that can potentially cause abuse and dependence. Its primary purpose is to combat drug abuse, reduce drug-related crimes, and protect public health and safety. The Drug Enforcement Agency (DEA) plays a crucial role in enforcing the CSA by monitoring and controlling controlled substance production, distribution, and use (31).
In the 1990s, the significant increase in opioid prescribing, leading to a surge in opioid addiction and overdose deaths, prompted a shift in focus toward prevention. Efforts were made to educate healthcare providers about the risks of overprescribing opioids and to implement prescription drug monitoring programs to track and prevent abuse (36).
The Comprehensive Addiction and Recovery Act (CARA) was signed into law in 2016 to expand access to treatment and recovery services for opioid addiction. This legislation allocated funding for prevention, treatment, recovery, and support services while promoting evidence-based practices and programs (36).
The Centers for Disease Control and Prevention (CDC) published guidelines in 2016 for prescribing opioids for chronic pain, which was updated in 2022. These guidelines emphasize the importance of non-opioid alternatives, using the lowest effective dose for the shortest duration, and assessing the benefits and risks of continued opioid therapy (13).
Furthermore, the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT) was signed into law in 2018, providing additional resources to address the opioid crisis. This legislation expanded access to medication-assisted treatment (MAT), increased the availability of naloxone, a medication used to reverse opioid overdose, and enhanced support for recovery housing (36).
In recent years, there has been a growing recognition of the importance of a comprehensive approach to opioid addiction, including harm reduction strategies, increased access to naloxone, and the integration of mental health services. Communities and organizations have been working together to address the underlying issues contributing to addiction, such as poverty, trauma, and social determinants of health (50).
Overall, the history of substance use disorder prevention that promotes opioid recovery and treatment has evolved from a punitive approach to a more compassionate and evidence-based model. Efforts are now focused on prevention, early intervention, and expanding access to comprehensive treatment and support services for individuals and communities affected by opioid addiction (36).
The most current federal regulations on opioid prescribing for healthcare providers are the amendments to the CSA in 2018, which added new rules to limit the quantity and duration of opioid prescriptions for acute pain to seven days. In 2022, the CDC updated recommendations to the Clinical Practice Guidelines for Prescribing Opioids for Pain.
The 2022 CDC guidelines are summarized below (13):
- Non-opioid therapies should be considered the first-line treatment for chronic pain.
- Establish clear treatment goals with patients, including realistic pain management and functional improvement expectations.
- Conduct a thorough risk assessment for potential harms before initiating opioid therapy.
- When opioids are used, start with the lowest effective dose and consider immediate-release opioids instead of extended-release or long-acting opioids.
- Prescribe the lowest effective dose for the shortest duration possible, typically three days or less and rarely exceeding seven days.
- Reassess benefits and risks within one day after prescribing opioids, including checking the prescription drug monitoring database.
- Avoid prescribing opioids and benzodiazepines concurrently whenever possible due to the increased risk of overdose and death.
- Offer naloxone to patients at increased risk of opioid overdose, including those with a history of overdose, substance use disorder, or concurrent benzodiazepine use.
- When opioids are no longer needed, taper the dose gradually to minimize withdrawal symptoms.
- Arrange an evidence-based treatment for patients with opioid use disorder, including medication-assisted treatment (Naltrexone, Buprenorphine, or Methadone).
Ask yourself...
- What are the general guidelines for prescribing opioids for acute pain?
- How do these guidelines differ for chronic pain management?
- Discuss how federal regulations impact the practice of nurse practitioners in terms of opioid prescribing.
- Describe the potential benefits and challenges nurse practitioners face when adhering to federal regulations on opioid prescribing.
- How can nurse practitioners navigate and stay updated with evolving federal regulations surrounding opioid prescribing to ensure safe and effective care?
- How do you ensure appropriate documentation when prescribing controlled substances?
- What is the Comprehensive Recovery Act?
- How does the CDC manage the prescribing of opioids?
- Have you had patients use MAT? Did they find it effective?
- What does the Controlled Substances Act regulate?
Safe Prescribing and Prescription Monitoring Program
Prescription Drug Monitoring Programs (PDMPs) are state-run electronic databases that track.
The prescribing and dispensing of controlled substances: PDMPs are designed to improve patient care.
Care and safety are achieved by giving clinicians access to patients’ prescription histories, allowing them to make informed decisions when prescribing controlled substances. PDMPs help identify patients at risk of substance misuse or prescription drug overdose. They also enable clinicians to identify potential drug interactions and prevent opioid diversion (14).
PDMPs collect and store data from pharmacies and prescribers in a centralized database. Clinicians can access this database to review a patient’s prescription history, including the types of medications prescribed, the prescribers involved, and the dispensing pharmacies (14).
In many states, PDMP use is mandated by law, and nurse practitioners may be required to register and use the system. Understanding state-specific statutes and regulations regarding PDMP use is essential.
PDMPs have some limitations, such as incomplete data or delays in reporting. The CDC emphasizes that clinicians should use PDMP data for their clinical assessment and other relevant information to make informed decisions about prescribing controlled substances. Still, PDMP cannot be used as the sole basis for denying or providing treatment (14).
Case Study
After five days on Gabapentin, Mary was doing well, and her neuropathic pain had decreased to 3/10. However, Mary suffered a fall after her knee “gave out” and injured her knee and back. She was in severe pain, and her family drove her to the ER. The ER doctors saw Mary, and orthopedics were consulted. Mary has surgery scheduled for a knee replacement a week from now.
Mary was prescribed Vicodin because she was in excruciating pain, but her prescription only allowed enough medication for two days. Mary has made an appointment with you to renew her prescription.
You evaluate Mary because you know that concomitant use of Gabapentin and opioids puts Mary at risk for respiratory depression and possible side effects, including accidental overdose.
Mary stated she has been more alert the past 24 hours and is afraid her functional status will continue to decline if she does not have more Vicodin because the pain in her back and knee makes it difficult to stand. You assess Mary. Mary stated she occasionally drinks alcohol, but has not had a drink since she moved. She has no familial history of substance abuse or mental health disorders.
Mary’s mother stayed at her house to help her for the first 24 hours after Mary’s return from the ER, but Mary is now providing her care.
You check the PDMP database and see that Mary was prescribed eight pills she has taken over the last 48 hours.
Since Vicodin has been effective without untoward side effects, and Mary’s function is improving, you decide to refill the prescription. You will taper the dose to three Vicodin daily for two days and two for one day. Mary will also be near her appointment for a knee replacement.
Ask yourself...
- What are the potential benefits and drawbacks of using PDMPs in your practice?
- How can PDMPs help you identify potential drug abuse or diversion cases among your patients? Can you provide examples from your own experience?
- In what ways do PDMPs impact your decision-making process when prescribing controlled substances?
- What are the key considerations when prescribing controlled substances?
- How do you ensure responsible prescribing practices for controlled substances?
Preventing Opioid Use Disorder
As previously discussed, opioid addiction is a growing concern worldwide, affecting individuals from all walks of life. According to the CDC, “Anyone who takes prescription opioids can become addicted to them” (14).
As frontline healthcare professionals, nurse practitioners must recognize the signs of opioid addiction to provide timely intervention and support. This section will outline the key indicators of opioid addiction.
Physical Symptoms
Physical symptoms are often the first noticeable signs of opioid addiction. These symptoms may include constricted pupils, drowsiness, slurred speech, impaired coordination, and increased sensitivity to pain. Additionally, individuals struggling with opioid addiction may exhibit frequent flu-like symptoms, such as a runny nose, sweating, itching, or gastrointestinal issues.
Behavioral Changes
Opioid addiction can significantly impact an individual’s behavior. These may include increased secrecy, frequent requests for early prescription refills, doctor shopping (seeking prescriptions from multiple healthcare providers), neglecting personal hygiene, and experiencing financial difficulties due to excessive spending on opioids (37).

Social Isolation
Opioid addiction often leads to social withdrawal and isolation. Individuals struggling with opioid addiction may distance themselves from family, friends, and social activities they once enjoyed. They may exhibit erratic mood swings, become defensive or hostile when confronted about their drug use, and display a general lack of interest in previously important activities (30).
Psychological Changes
The psychological impact of opioid addiction is significant. Individuals with opioid addiction may exhibit increased anxiety, depression, irritability, and restlessness. They may also experience cognitive impairments, memory lapses, and difficulties in decision-making. Healthcare professionals should be attentive to these changes, as they can indicate opioid addiction (51).
Tolerance and Withdrawal Symptoms
The development of tolerance and withdrawal symptoms is a critical sign of opioid addiction. Individuals may require increased dosages of opioids to achieve the desired effect, indicating a growing tolerance. Furthermore, withdrawal symptoms such as muscle aches, nausea, vomiting, insomnia, and intense cravings for opioids may occur when the drug is discontinued or reduced abruptly (51).
Ask yourself...
- Discuss how nurse practitioners can contribute to preventing opioid use disorder.
- Explain how nurse practitioners effectively communicate the risks and signs of opioid misuse without stigmatizing or alienating patients.
- What are the signs of opioid addiction or misuse in patients?
- How do you approach patients who may be at risk for opioid addiction?
- How do you ensure appropriate documentation when prescribing controlled substances?
- What community resources do you have available to patients to help with OUD?
- What is a critical sign of opioid addiction?
- What are the psychological changes that are associated with OUD?
- What support groups or Narcotics Anonymous groups do patients have access to in your area?
- What is social isolation?
Opioid Overdose
The management of opioid overdose, withdrawal, and addiction requires a comprehensive approach that combines pharmacological interventions with psychosocial support. Naloxone remains a vital tool for reversing opioid overdose, while medications such as Methadone, buprenorphine, and naltrexone play crucial roles in withdrawal and addiction treatment (National Institute of Health, 2023). Nurse practitioners must stay vigilant and informed about the evolving landscape of medications. This section aims to provide a comprehensive review of medications and treatment strategies for opioid overdose, withdrawal, and addiction, and is excerpted from the NIH (40).
Naloxone
Mechanism of Action and Metabolism
Naloxone is an opioid receptor antagonist. It works by binding to opioid receptors and displacing any opioids present, thereby reversing the effects of opioid overdose. It has a higher affinity for opioid receptors than most opioids, effectively blocking their action.
Naloxone is indicated for emergency intervention of opioid overdose. It effectively reverses respiratory depression and other life-threatening effects. Studies suggest the potential benefits of combining naloxone with other medications, such as buprenorphine (see below), to improve outcomes. Initiatives promoting community-based naloxone distribution programs have shown promising results in reducing opioid-related deaths.
Available Forms
Naloxone is available in various formulations:
- Intranasal
- Intramuscular
- Intravenous
- auto-injectors.
The most used form is the intranasal spray, which is easy to administer and requires no specialized training. Intranasal naloxone formulations have gained popularity due to their ease of use and increased availability. A recent study showed that the non-FDA-approved compound spray was far less effective than either FDA compound (15).
Dosing and Monitoring
The recommended initial dose of naloxone for opioid overdose is 2mg intranasally or 0.4mg to 2mg intramuscularly or intravenously. If the patient does not respond within 23- minutes, additional doses may be administered every 2-3 minutes. Continuous monitoring of the patient’s respiratory status is essential, as repeat doses may be required due to the short half-life of naloxone.
Side Effects and Contraindications
Naloxone has been shown not to affect individuals without opioids in their system.
Common side effects of naloxone include
- Withdrawal symptoms: increased heart rate, sweating, and agitation
- nausea
- vomiting
- headache
Contraindications include known hypersensitivity to naloxone and situations where the use of naloxone may be unsafe or not feasible.
Considerations for Nurse Practitioners
Fentanyl and other opioids have a rapid onset, and the need to act quickly is paramount. As mentioned previously, the ease of use and higher plasma concentrations using the FDA-approved 4-mg FDANxSpray device compared with the locally compounded nasal sprays should be considered when ordering Naloxone (15).
Fentanyl and other potent synthetic opioids may require multiple administrations of naloxone to achieve reversal of an overdose (Chiang, Gyaw, & Krieter, 2019). As a nurse practitioner prescribing naloxone, it is crucial to assess the patient’s risk factors for opioid overdose, such as a history of substance use disorder or chronic pain management. Education regarding the proper administration of naloxone should be provided to the patients and their caregivers. Additionally, it is essential to provide resources for follow-up care, including addiction treatment and ongoing support.
Methadone
Mechanism of Action and Metabolism
Methadone is a long-acting opioid agonist that effectively suppresses withdrawal symptoms and reduces cravings. It binds to the same opioid receptors in the brain as other opioids. It relieves withdrawal symptoms and reduces cravings by blocking the euphoric effects of opioids, thus helping individuals with opioid dependence to achieve stability (33).
Available Forms
Methadone is available in oral tablets and liquid formulations. The oral tablet is the most used form and is typically administered once daily (33).
Dosing and Monitoring
Methadone dosing is individualized based on the patient’s response and needs. Initially, the dose often started low and gradually increased until the patient reached a stable dose. Dosing may need to be adjusted based on the patient’s response, adherence, and any changes in their overall health. Regularly monitoring the patient’s vital signs, urine drug screens, and assessment of their withdrawal symptoms and cravings is essential.
Side Effects and Contraindications
Common side effects of methadone include:
- Constipation
- dry mouth
- drowsiness
- sweating
- weight gain
- respiratory depression
Contraindications include:
- known hypersensitivity to methadone
- severe asthma
- respiratory depression
- certain heart conditions (33).
Considerations for Nurse Practitioners
As a nurse practitioner prescribing methadone, conducting a comprehensive assessment of the patient’s medical history, current medications, and substance use history is crucial. Opioid treatment programs or specialized clinics are often involved in methadone treatment, so collaboration and coordination of care with these programs are essential. Regularly monitoring the patient’s progress, adherence, and potential side effects or drug interactions is essential. Additionally, providing education on the risks and benefits of methadone and the importance of adherence to the prescribed regimen is crucial for successful treatment outcomes.
Buprenorphine
Mechanism of Action and Metabolism
Buprenorphine is a partial opioid agonist with a ceiling effect that minimizes the risk of overdose while reducing withdrawal symptoms. Buprenorphine is a partial opioid agonist that binds to the same receptors as other opioids but produces a weaker response. It has a high affinity for the mu-opioid receptors, which helps reduce cravings and withdrawal symptoms in individuals with opioid dependence.
Available Forms
Buprenorphine is available in different formulations, including sublingual tablets, buccal films, and extended-release injections. The sublingual tablets have different strengths, such as 2mg, 4mg, 8mg, and 12mg. Buprenorphine is taken as a daily tablet or weekly or monthly injection.
Dosing and Monitoring
The dosing of buprenorphine varies depending on the individual’s opioid dependence severity and treatment phase. Initially, a low dose (e.g., 2-4mg) is given, and it may gradually increase to a maintenance dose of 8-24 mg daily. Regular monitoring is essential to assess the patient’s response, adherence, and potential side effects.
Side Effects and Contraindications
Common side effects of buprenorphine include:
- Constipation
- Nausea
- Headache
- Insomnia
- Sweating
Serious side effects are rare but can include:
- Respiratory depression
- Allergic reactions
Buprenorphine is contraindicated in individuals with:
- Severe respiratory insufficiency
- Acute intoxication with opioids
- Known hypersensitivity
Considerations for Nurse Practitioners
Nurse practitioners can prescribe buprenorphine for opioid dependence treatment under the Drug Addiction Treatment Act (DATA). To become eligible, they must complete specific training requirements and obtain a waiver from the Substance Abuse and Mental Health Services Administration (SAMHSA). Nurse practitioners should assess patients thoroughly, including their opioid use history, comorbidities, and medication compatibility, while ensuring appropriate counseling and referral for comprehensive treatment (40).
Clonidine + Lofexidine
Mechanism of Action and Metabolism:
Both Clonidine and Lofexidine are alpha-2 adrenergic agonists. They work by stimulating alpha-2 receptors in the brain, which reduces sympathetic outflow and norepinephrine release. This results in decreased sympathetic activity, leading to various effects such as reduced blood pressure, decreased heart rate, and alleviated withdrawal symptoms (28).
Available Forms
Clonidine is available in oral tablets and patches. Lofexidine is available in oral tablets and is taken as needed (40).
Dosing and Monitoring
For opioid withdrawal, the Clonidine dose ranges from 0.1-0.3 mg every 4-6 hours. Lofexidine is usually initiated at 0.53 mg three times daily, and the dose can be increased to 2.88 mg daily. Monitoring blood pressure and heart rate is essential during treatment (40).
Side Effects and Contraindications:
Common side effects of both medications include:
- dry mouth
- sedation
- dizziness
- constipation
- orthostatic hypotension (40).
Both medications are contraindicated in patients with:
- Hypotension
- Bradycardia
- heart block
- history of hypersensitivity to the drugs (40).
Considerations for Nurse Practitioners:
An early study of lofexidine vs. clonidine for withdrawal symptoms showed that treatment with lofexidine resulted in lower withdrawal symptoms, fewer mood problems, less sedation, and hypotension. There were no significant differences in craving levels, morphine metabolites in urine, or dropout rates when both were compared.
Lofexidine can be a safe option for outpatient treatment as it does not lead to hypotension. However, nurse practitioners must closely monitor patients’ blood pressure and heart rate during treatment and educate them about possible side effects. If patients experience any concerning symptoms, they should inform their nurse practitioner immediately.
Gradual dose reduction of Clonidine is crucial to prevent rebound hypertension. Before prescribing either medication, nurse practitioners should assess for any contraindications or potential drug interactions (19).
Emerging Therapies for Withdrawal
- Extended-release naltrexone: Naltrexone is an opioid receptor antagonist that blocks the effects of opioids, reducing the risk of relapse. It is taken as a monthly injection.
- Alpha-2 adrenergic agonists: Emerging evidence suggests the potential use of dexmedetomidine and guanfacine for managing opioid withdrawal symptoms.
Medication-Assisted Treatment (MAT)
Methadone was introduced in the 1960s and marked a significant turning point in opioid addiction treatment or MAT. Along with counseling and behavioral therapies, MAT became the cornerstone of opioid addiction recovery.
Examples of medications used:
- Methadone
- Buprenorphine
- Naltrexone
Adjunctive Pharmacotherapies:
Antidepressants: Selective serotonin reuptake inhibitors and tricyclic antidepressants may help manage co-occurring depression and anxiety.
Anticonvulsants
Medications like Gabapentin and pregabalin show promise in reducing opioid cravings and improving treatment outcomes.

Ask yourself...
- What are the mechanisms of action for commonly prescribed addiction medications?
- What are the potential risks and benefits of using benzodiazepines for pain management?
- How do you assess and manage patients with co-occurring pain and substance use disorders?
- What are the guidelines for prescribing addiction medications like buprenorphine or methadone?
- How do these medications work in the treatment of opioid use disorder?
- What are the potential side effects and risks associated with addiction medications?
- How do you support patients in their recovery from opioid use disorder?
- How do you address patients’ concerns and fears about addiction medications?
- What are the federal guidelines around prescribing addiction medications for nurse practitioners?
- How do these guidelines influence your prescribing practices?
- Have you ever administered naloxone to a patient? What was their response?
- What is the minimum amount of time a patient can be on methadone?
- What education should you provide to a patient who is prescribed buprenorphine?
- Have you ever administered clonidine + lofexidine? What was your patient’s response?
- What is naltrexone?
- Have you seen an increased use of gabapentin in practice? How do you feel that has impacted pain management?
Other Substance Use Disorders
Patients in pain may struggle with Substance Use Disorders other than Opioid Use Disorder. Substance use disorders may often occur with mental health conditions such as anxiety, depression, and bipolar disorder. In addition, many individuals engage in polydrug use. Understanding the most common Substance Use Disorders aids in a comprehensive assessment of the patient and the development of appropriate treatment plans (28).
Alcohol Use Disorder (AUD):
The prevalence of AUD worldwide was estimated to be 9.8% in men and 5.5% in women in 2016 (28).
Cannabis Use Disorder (CUD):
the prevalence of CUD in the United States increased from 2.18% in 2001-2002 to 2.89% in 2012-2013. (28).
Cocaine Use Disorder:
According to the National Survey on Drug Use and Health (NSDUH), in 2019, approximately 1.9 million Americans aged 12 or older had cocaine use disorder in the past year (44).
Methamphetamine Use Disorder:
A study published in Drug and Alcohol Dependence reported that the prevalence of methamphetamine use disorder in the United States was estimated to be 0.2% in 2015-2016 (6).
Ask yourself...
- What are the options available for managing opioid addiction and withdrawal?
- How can nurse practitioners support patients in their recovery from opioid addiction?
- What strategies can nurse practitioners employ to effectively engage and build trust with patients reluctant to disclose or seek help for substance abuse disorders?
- How can nurse practitioners collaborate with other healthcare professionals and community resources to provide comprehensive care and support for patients with substance abuse disorders?
- What techniques or tools can nurse practitioners employ to start these sensitive conversations with new patients?
- How do you assess and manage patients experiencing opioid withdrawal symptoms?
- What are the non-pharmacological interventions for managing opioid withdrawal?
- How do you educate patients about the risks and benefits of addiction medications?
- How do you monitor patients on addiction medications for adherence and progress?
- What are the drug potential interactions with commonly prescribed addiction medications?
Drug Diversion and Illegal Opioids
Misuse of opioids is facilitated by diversion and is defined as “the transfer of drugs from lawful to unlawful use” (24). Most commonly, this occurs when family and friends share prescribed opioids with other family and friends. Opioids and other controlled drugs are also diverted from healthcare facilities. Statistics show that healthcare facility diversion has increased since 2015 (24)
Diversion affects patients, healthcare workers, healthcare facilities, and public health. Patients experience substandard care due to ineffective pain management and impaired healthcare workers. In addition, affected patients are at risk of infections from compromised syringes (24).
Healthcare employees who divert are at risk of overdose and death. If caught, they face criminal prosecution and malpractice suits. Healthcare facilities also bear the cost of diverted drugs via internal investigations, follow-up care for affected patients, regulatory fines for inadequate safeguards, and declining public trust (24).
Despite the enormous consequences of drug diversion, healthcare facilities have implemented few processes to detect and deter the diversion of controlled substances (24).
Ask yourself...
- What is the definition of diversion?
- What protocols can nurse practitioners implement to prevent drug diversion within their healthcare setting?
- Have you ever encountered drug diversion? What was your experience like?
- What documentation do you have to complete regarding the prescribing and disposal of controlled substances?
- What are some of the consequences of diversion that healthcare workers are at risk of?
Patient Teachings and Considerations
Opioids have significant side effects and carry a risk of addiction and overdose. Nurse practitioners can decrease the risks of misuse and addiction by educating patients on appropriate disposal, safe storage, and potential signs of addiction. Taking additional time to provide teaching nurse practitioners can promote patient safety, informed decision-making, and responsible opioid use.
Safe Storage and Disposal:
- Teach patients to store opioids securely, out of reach of children, pets, visitors, and non-caregiver family members, to prevent accidental ingestion or misuse (13). Only the caregiver, if applicable, or the patient should have access to pain medications.
- Instruct patients on proper disposal methods, such as using drug take-back programs or mixing opioids with undesirable substances (e.g., coffee grounds) before throwing them away (11) (13).
Medication Adherence:
- Emphasize the importance of taking opioids as prescribed, at the correct dose and frequency, to achieve optimal pain relief.
- Encourage patients to notify their healthcare provider if they experience inadequate pain control or side effects (35).
Potential Side Effects:
- Educate patients about common side effects of opioids, including constipation, nausea, sedation, and respiratory depression.
- Discuss strategies to manage side effects, such as maintaining adequate hydration, consuming a fiber-rich diet, and using over-the-counter laxatives as needed (11).
Risk of Dependence and Addiction:
- Explain the potential for opioid dependence and addiction, especially with long-term use or a history of substance abuse.
- Encourage patients to promptly report signs of opioid misuse, such as craving, loss of control, or continued use despite negative consequences (51).

Avoiding Alcohol and Other Central Nervous System Depressants:
- Instruct patients to avoid consuming alcohol or other medications that can enhance the sedative effects of opioids, increasing the risk of respiratory depression.
- Advise patients to contact the Nurse Practitioner before starting new medications, including over-the-counter drugs or herbal supplements (2).
Driving and Operating Machinery:
- Inform patients about the potential impairment caused by opioids, including reduced alertness, reaction time, and coordination.
- Advise patients to avoid driving or operating heavy machinery while taking opioids until they know how the medication affects them (14).
Ask yourself...
- What strategies can nurse practitioners employ to effectively communicate the risks and benefits of opioid use while ensuring they clearly understand the potential side effects and the importance of adhering to the prescribed regimen?
- How can nurse practitioners promote patient engagement and shared decision-making regarding opioid pain management, considering the potential for dependence and addiction?
- How can nurse practitioners assess a patient’s knowledge and understand the safe storage and disposal of opioids?
- What education do you provide patients regarding the safe disposal of controlled substances?
- What resources does your community have available for patients to dispose of unused controlled substances safely?
Case Study
You take some extra time with Mary to educate her on the taper dose of Vicodin, the potential for harm, and the risk of opioids, especially when used concomitantly with Gabapentin. You let Mary know it is unsafe to use alcohol, not only with Vicodin but also with Gabapentin. You let Mary know that Vicodin has a risk of dependency and misuse and, therefore, she will be monitored carefully. You also educate that Mary should store the Vicodin away from visibility by anyone but herself since she can self-administer her medication. You let Mary know that Vicodin can cause constipation and that she should increase her water intake and take a stool softener.
You ask Mary to call you if her pain is not adequately relieved or if her medications run out before the three days.
You let Mary know that if she does stop taking the Vicodin before she has completed all the medication, she should dispose of it by mixing the pills with liquid and coffee grounds to make them unpalatable to animals and others.
Mary complied with your education, completed her course of Vicodin, and was scheduled for surgery. Mary’s social worker helped her communicate with her new employer and delayed her start date until after her recovery.
During her recovery, Mary received physical therapy and a short course of pain medication managed by her orthopedist.
Mary returned to the clinic for a follow-up visit after completing her therapy and before starting work. Mary’s pain level in her knee is 3/10, and she already feels like she can walk further than pre-surgery. Gabapentin has continued to help Mary’s neuropathic pain in her back, and she reports 2/10. Mary looks forward to beginning her new job and is optimistic about the future.
Conclusion
Pain management is the leading cause of primary care appointments and chronic pain is the leading cause of disability. Yet, prescribing opioids for primary care patients is also a factor in drug misuse and the opioid epidemic. Nurse practitioners are challenged to appropriately treat pain and effectively control diversion, addiction, and death from overdose.
It is imperative that nurse practitioners use evidence-based practices to assess, appropriately intervene, and educate about the benefits and potential harm caused by treatment with opioids. Nurse practitioners must stay up to date with the current federal regulations regarding PDMPs, clinical prescribing guidelines, and emerging treatments for pain and opioid abuse disorders.
Tirzepatide for Type 2 Diabetes and Weight Management
Introduction
The emergence of the drug tirzepatide is becoming more popular and widespread and is being utilized among those with diabetes and also those who desire to lose weight. It is one of the newest diabetic drugs given by injection that also triggers dramatic weight loss in those who use the injections.
The U.S. Food and Drug Administration (FDA) approved tirzepatide in 2022 for individuals with diabetes, particularly Type 2 Diabetes. The FDA officials have not approved tirzepatide yet for weight loss, but they are currently tracking the medication and may have a recommendation for its approval by the end of this year. Clinical trials have shown that individuals with an elevated body mass index (BMI) and who did not have diabetes lost a considerable amount of weight when they received tirzepatide (1).
Advanced Practice Registered Nurses (APRNs) need to understand how to safely prescribe tirzepatide and the reasoning as to why it causes weight loss for specific individuals.
Drug Classification
Tirzepatide is part of a class of medications called glucose-dependent insulin tropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonists. It comprises a 39 amino acid linear synthetic peptide conjugate to selective receptor agonists in preclinical and clinical trials.
Tirzepatide is used for treating Type II diabetes in adults as an adjunct to diet and exercise. It is also used for weight loss in some individuals and has gained increased attention as a new therapeutic agent for glycemic and weight control.
Social media has had a significant influence and increased the desire to use tirzepatide, and while individual results vary, the weight loss in adults ranged from 12 – 25 pounds.
Online pharmacies, diet clinics, and medical spas are implementing thousands of ads on social media to capitalize on a surge of interest in the drug.
Ask yourself...
- Why has there seemed to be an increase in patients requesting this medication? What other medicines intended for type 2 diabetes are also being used for weight loss management?
- What are the ethical considerations regarding marketing this drug for weight loss when its primary use is for type 2 diabetes? Could this impact supply and costs?
Indications of Usage
The use of tirzepatide is being used for both Type II diabetes and weight control in certain patients. It has been a game changer for people living with Type II diabetes. The drug’s primary use is as an adjunct to diet and exercise to improve glycemic control in adults with diabetes.
The drug has also proven beneficial for weight loss in patients experiencing obesity, and those who are taking the highest dosage have shared a body weight reduction of 15.7% (2). Tirzepatide is an injectable prescription medication used together with diet and exercise, and it is not yet known if it can be used safely with patients who have had pancreatitis.
It is important to remember that it is not to be used for patients with Type I diabetes, but it is safe for Type II diabetic patients. Also, the safety of tirzepatide has yet to be discovered for children and those under 18; therefore, the medication should not be used for this age group.
In studies conducted with or without diabetic medicines, 75% – 90% of patients taking tirzepatide reached an overall A1C of less than 7% with an average starting A1C of 7.9 – 8.6% across the following dosages – 5mg, 10mg, and 15mg. The study results were measured at weeks 40 and 52 (3).
Ask yourself...
- What dietary and activity recommendations can you provide to patients using tirzepatide for weight loss?
- Is this drug intended for those who want to lose 5-10 pounds?
Use of Tirzepatide with Diabetic Patients
Tirzepatide can be used for patients with Type II diabetes in combination with a diabetic-friendly diet and exercise. The drug works by lowering the patient’s overall blood sugar and also improves the A1C results of patients over some time. The injection has been approved by the FDA to treat Type II diabetes and is administered once weekly (4).
It is considered the first in a new class of medications – a dual glucose-dependent insulin tropic polypeptide (GIP) and glucagon-like-peptide-1 (GLP-1) receptor antagonist. The mechanism of how it works mimics two gut hormones (GIP and GLP-1). These hormones are essential in how patients digest food and regulate blood glucose after meals. The hormones also play a role in making individuals feel fuller and curb specific food cravings.
The provider can prescribe tirzepatide before attempting other diabetic medications if a patient has a BMI of 30 or greater or 27 or greater with weight-related conditions and if the drug is combined with a personalized weight loss plan that addresses physical activity, nutrition, and lifestyle changes.
However, due to the cost and some insurance companies not covering the injection unless the patient has both diabetes and obesity, the provider must carefully consider prescribing this medication.

Case Study
The patient states this ‘miracle drug’ is worth paying for out of pocket!
Jeff Capron, a 53-year-old Boonville, New York, web developer, started taking tirzepatide in December 2022. His friend had reported good results with the medication, so Jeff looked into the research studies behind it and then spoke with his primary physician.
The physician said, “Yeah, let’s give it a shot,” even though he did not have much experience with it. The physician did not have an opinion one way or the other than looking at the data set and seeing no reason why they could not try it.
Jeff’s hemoglobin A1C went from 10.1% to 6% in 3 months, which was very promising. “I never had that kind of experience with any medication for diabetes.” There is a range in how much A1C reduction people experience with tirzepatide, but many people taking it can get their A1C under 7% — an ideal goal for people with Type 2 diabetes.
Jeff experienced constipation and a little trouble sleeping early, but both issues disappeared quickly. He says, “I wake up in the morning, and my fasting blood sugars are normal.”
The medication took effect, he says, within 12 hours. He compared the feeling to having a gastric bypass.
“You cannot overeat food. As soon as you overeat, you almost feel ill.” While it generally takes a few months to notice effects like A1C reduction and significant weight loss, side effects such as lower appetite may be felt immediately.
Weight loss was not his primary goal, but he lost about 35 pounds on the medication in the first five months. He also lost his sweet tooth. “I can maybe count three sweet things I have eaten since December.”
Jeff found that his appetite slowly recovered days after taking tirzepatide. “You take the shot every Sunday, and by Saturday, you start to get a lot of appetite,” he says. “It does not seem to affect your weight. If I eat a little bit more on Saturday night, on Sunday, the scale will not move one way or the other.”
Jeff is allergic to hornets, so he already carries an auto-injector. He was not worried about using another drug delivered through a needle. “It’s just a push button,” he says. It also helped that his wife is a nurse. “So, I had her with me the first time to ensure I was doing it right. I didn’t even feel it.”
When Jeff was first prescribed tirzepatide, his insurance covered it. The company has since removed that benefit. He has filed an appeal but pays about $1,000 monthly out of pocket for his weekly injections. He plans to keep paying as long as necessary.
He considers the financial burden well worth it. “I have never had a medication that worked as well before for chronic conditions,” Jeff says. “I’ve been blown away by it. For me, it’s a miracle drug. It got rid of my diabetes” (4).
Ask yourself...
- Can a provider willfully choose to prescribe tirzepatide before other diabetic medications are attempted?
- Would that impact his insurance coverage if Jeff did not meet the clinical criteria for using tirzepatide?
Use of Tirzepatide for Weight Loss Management
As mentioned, this medication is indicated for patients with a BMI of >30 or a BMI of >27 with qualifying comorbidities. Obesity can become a chronic lifetime disease, and for conditions such as these, the patient needs to implement therapy for the lifetime of the disease.
In a study conducted for tirzepatide, there was a dramatic increase in effectiveness compared to traditional nonsurgical interventions such as diet, exercise, and lifestyle changes. However, it has been noted that taking tirzepatide on an ongoing basis is recommended and necessary to maintain any weight loss achieved from the medication.
If a patient stops taking the drug, likely, it will no longer work (5).
Public health officials have expressed concerns about using the drug long-term. Still, data is currently lacking regarding long-term effectiveness, treatment duration, and maintaining weight reduction once the therapy is discontinued.
A recent trial consisted of 783 participants with a BMI greater than 30, and these participants agreed to take either a 10mg or 15mg dose of tirzepatide over 36 weeks. The injection is given once weekly, so this would equal a total of 36 injections.
By the end of 36 weeks, participants lost more than 21% of their body weight. After 36 weeks, participants continued on tirzepatide or received placebo treatment for the following year. The patients needed to be made aware of which treatment they were receiving.
Those still taking tirzepatide injections weekly after 88 weeks lost an additional 7% of their body weight, and those taking the placebo regained 15% at the end of 88 weeks (5).
Ask yourself...
- What is the minimum BMI needed to qualify to receive this drug for weight loss management?
- Is this medication indicated for long-term use for patients with a high BMI?
Common Side Effects and Contraindications
Side Effects
Patients vary immensely with different experiences and side effects related to tirzepatide; however, the following are the most common side effects experienced by those taking the medication:
- Nausea
- Decreased appetite
- Vomiting
- Diarrhea
- Indigestion
- Constipation
- Stomach Pain
Tirzepatide usually does not cause fatigue, leaving one feeling weak, tired, and low energy. However, fatigue can be a common side effect of Type II diabetes.
It is important to note that most individuals who experience nausea, vomiting, and diarrhea episodes do so while the dosage increases, and typically, the symptoms decrease over time. G.I. effects were more prominent in those taking tirzepatide than those taking the placebo. The individuals not in the placebo group were more likely to stop treatment due to the unpleasant side effects (3).

Ask yourself...
- Does tirzepatide cause fatigue in patients who use it?
Contraindications
Tirzepatide may cause thyroid tumors, including thyroid cancer, and it is essential to watch for possible symptoms, such as swelling or a lump in the neck, hoarseness, shortness of breath, or trouble swallowing.
Tirzepatide should also not be prescribed to any patient with Type 1 Diabetes.
One of the main ways that tirzepatide works is by stimulating the release of insulin from the pancreas, and due to this fact, there have not been many studies and clinical trials that include those with Type I diabetes.
However, this is not to say that prescribers have never ordered tirzepatide for those with Type I diabetes. Still, it is essential to note that if prescribed, it would be in addition to traditional insulin therapy.
- Personal or family history of a type of thyroid cancer known as medullary thyroid carcinoma (MTC).
- Any history of Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Patients who are allergic to the actual medication or any of its ingredients.
- Younger than 18 years of age

Ask yourself...
- What is the reason that tripeptide is contraindicated in those with Type I diabetes?
- Why is there a risk with patients who have a thyroid disorder?
Safe Prescribing Practices, Guidelines, and Considerations for Providers
Safe Prescribing Practices
As with all prescribed medications, safe standards of care must be implemented and followed to ensure patient safety is maintained. The same applies to providers considering prescribing tirzepatide, and specific criteria must be met beforehand. The following information discusses guidelines involving exclusion and inclusion criteria for providers to prescribe tirzepatide (6) accurately.
Guidelines
Exclusion Criteria – If present, the following indicates that the patient should not receive tirzepatide:
- Diagnosis of Type I diabetes
- Personal or family history of medullary thyroid carcinoma or with Multiple Endocrine Neoplasia syndrome type 2
- Severe gastrointestinal dysmotility
- History of pancreatitis
- Pregnancy
- Proliferative Diabetic Retinopathy (PDR), severe Nonproliferative Diabetic Retinopathy (NDR), clinically significant myalgic encephalomyelitis (M.E.), or diabetic macular edema (DME) unless the risks/benefits have been discussed with the patient and are documented in the patient’s health record along with monitoring plans and follow-up with an eye specialist who is informed at the time of initiation.
Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Diagnosis of Type II diabetes
- A BMI of 25 or greater
- Inadequate glycemic control on at least 1mg of semaglutide injection plus two or more glucose-lowering drugs
- Change needed to achieve goal A1C is less than 1%.
- Goal A1C should be based on those recommended in the Diabetic Guidelines.
- Adherence to current diabetic medications as evidenced by a review of the prescription refill history during the six months.
Additional Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Patients with atherosclerotic cardiovascular disease or chronic kidney disease
- Patients of childbearing potential who are using oral contraceptives
Inclusion Criteria for Weight Loss
- BMI of >30 or >27 with patient weight conditions.
Ask yourself...
- Would a patient with a BMI of 23 with no comorbidities qualify to use tirzepatide to lose 5-10% of their body weight? Why not?
- What impact can tirzepatide have on a person with a healthy weight and BMI of <25?
Considerations for Providers
There are specific considerations that prescribers must be aware of when contemplating if a patient should receive the medication tirzepatide. The following is imperative and must be considered each time the medication is prescribed to a patient:
- Clinical Indications – indicated for treating adults with insufficiently controlled diabetes mellitus as an add-on therapy to diet and exercise; as monotherapy when metformin is considered inappropriate due to contraindications or intolerance; and other medicinal products for treating Type II diabetes.
- Monitoring of medication – routine monitoring of serum calcitonin or thyroid ultrasound is of uncertain value but is recommended for early detection of Medullary Thyroid Cancer (MTC).
- Cost – the average price for tirzepatide ranges from $1,071-$1,351 without any coupons or insurance. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections.
- Benefits and Risks – One must evaluate the effectiveness of diabetes and the weight loss experienced. Some of the risks must be evaluated, such as increased cost of medication, unpleasant gastrointestinal side effects, poor insurance coverage, and drug shortages. The FDA has warned that the medicine can cause thyroid C-cell tumors in rats, and it is not sure whether tirzepatide causes similar tumors.
How long does it take for tirzepatide to begin working?
Tirzepatide will start to lower one’s blood sugar levels immediately, but it can take 8 to 12 weeks to reach one’s target A1C goal.
Compared to other diabetic treatments, studies have shown that it can take eight weeks to reach an A1C target of less than or equal to 7% and 12 weeks to get an A1C of less than or equal to 6.5%. Significant weight loss can occur as early as 28 weeks.
Safe Administration
It is essential to follow the correct steps for safe administration of tirzepatide as listed below:
- The recommended starting dosage is 2.5mg, injected subcutaneously once weekly. This dosage is for treatment initiation only, not for glycemic control.
- After four weeks, increase the dosage to 5mg, injected subcutaneously once weekly.
- If additional glycemic control is needed, increase the dosage in 2.5mg increments after at least four weeks on the current dose.
- The maximum dosage is 15mg, injected subcutaneously once weekly.
- If a dose is missed, instruct patients to administer it as soon as possible, within four days (96 hours) after the missed dose. If more than four days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once-weekly dosing schedule.
- If necessary, the day of weekly Administration can be changed as long as the time between the two doses is at least three days (72 hours).
- Before initiation, train patients and caregivers on proper injection techniques.
- Instruct patients using the single-dose vial to use a syringe appropriate for dose administration (e.g., a 1ml syringe capable of measuring a 0.5 mL dose).
- Administer the medication once weekly, any time of day.
- Inject the medication subcutaneously in the abdomen, thigh, or upper arm.
- Rotate injection sites with each dose.
- Inspect the medication visually before use. It should appear clear and colorless to slightly yellow. Do not use the medicine if particulate matter or discoloration is seen.
- When using the medication with insulin, administer it as separate injections and never mix. It is acceptable to inject tirzepatide and insulin in the same body region, but the injections should not be adjacent.
Does the tirzepatide injection hurt when administered?
Pain from the injection site has not been reported as a common side effect, but it may occur.
Due to the injection being given subcutaneously, slight pain or discomfort can occur.
Ask yourself...
- The patient asks you,” How long will this take to work?” How will you respond?
- The patient reports they have never used an injection before; what methods can you use to teach your patient how to administer this medication safely?
Alternatives to Tirzepatide for Weight Loss Management
In some instances, patients need to be aware of alternatives to tirzepatide in case they cannot take the actual injection for whatever reason. In cases such as these, there are alternative supplements that can be purchased over the counter, and they include the following (7):
- PhenQ – top OTC choice – comprehensive weight loss solution that targets specific body regions, facilitates prompt fat loss, and expedites the weight loss journey.
- PhenGold – the most potent OTC weight loss alternative – one of the top weight loss supplements that boost metabolism, making one less hungry, less tired, and an overall improved feeling.
- Capsiplex BURN – the best choice for men – helps to burn fat faster and keep blood sugar levels in check. It helps to keep one’s muscles, curbs hunger, gives one more energy, and torches stubborn fats.
- Trimtone – the best choice for women – helps women to lose weight, eat less, increase metabolism, burn extra calories, and boost energy.
- Prime Shred – best fat burner for men – boosts metabolism, keeps muscles intact, increases energy, and helps maintain focus.
The advanced practicing nurse or prescriber needs to inform patients about alternative options such as these in an effort for individuals to understand that other choices are available and can be used. Many individuals need to be more knowledgeable about alternatives besides tirzepatide due to the extra hype from social media sources that promote advertisements related to tirzepatide only but do not mention the other options.
Why does social media influence and encourage patients to take tirzepatide?
Social media trends can be helpful but can also become harmful by setting unrealistic expectations and promoting a diet culture mentality. They can create an unhealthy obsession with “clean” eating, especially in the younger populations.
Due to this, many individuals take the medication despite any occurrence or history of Type II diabetes, and the drug can ultimately become misused.
It has been noted that there is an influx of patients requesting this medication for weight loss instead of the intended purpose, which is to help control Type II diabetes.
Tirzepatide represents one of the most recent non-medical treatments aimed at managing the symptoms of Type II diabetes. While it is not indicated for weight management, diabetic patients who receive it frequently report a significant reduction in body weight.
Empirical evidence suggests the efficacy of tirzepatide in weight management, and certain physicians currently endorse the Administration of the medication as a therapeutic and effective means to overcome obesity.
What are some severe side effects of tirzepatide that can impact patient safety?
The Administration of tirzepatide can benefit many individuals, but some severe side effects must be mentioned.
These include thyroid tumors, thyroid cancer, pancreatitis, hypoglycemia, serious allergic reactions, kidney issues, severe stomach problems, vision changes, and gallbladder issues. All these side effects must be taken seriously and reported, as they can lead to life-threatening
Ask yourself...
- With what you have learned in this course, what education will you provide to patients requesting this medication for weight loss?
- Have you seen increased demand for this medication in your current practice?
- If you Google tirzepatide, your results will likely include links to telehealth services promoting this weight-loss medication. To determine eligibility, what special considerations need to be taken to assess a telehealth patient?
Conclusion
Medications like tirzepatide are game changers for those patients with type 2 diabetes that have failed other medications. Unfortunately, several companies seek to profit from its weight-loss benefits through aggressive marketing campaigns that limit the available supply and increase the costs for those who need it. As healthcare providers, we need to use sound clinical judgment and follow the exclusion/inclusion criteria and other guidelines before prescribing this medication, so we do not unintentionally cause harm while looking to appease our patients who request this.
Semaglutide and Type 2 Diabetes
Introduction
In 2017, the FDA approved the semaglutide injectable (Ozempic) for treating type 2 diabetes. The drug has experienced widespread acceptance due to its positive effects on weight loss and lowering chronic health risks. The drug has risen in popularity over the past few years, as many well-known actors/actresses/songwriters and others came forward, publicly sharing their weight loss journeys.
This rise in popularity has also resulted in significant shortages of this medication, negatively impacting the lives of the diabetic community, local pharmacies, and healthcare providers. The goal of this continuing education course is to educate and empower the healthcare provider in all aspects of this drug regimen: clinical indications, patient education, cost options, and benefit/risk analysis.
Ask yourself...
- Have you seen a rise in the use of semaglutide in your area?
- Have you had a shortage of semaglutide in your area?
Diabetes Overview
Diabetes is a chronic medical condition. Despite advances in diet, medications, and monitoring devices, diabetes diagnoses continue to grow at staggering rates. The Institute for Health Metrics and Evaluation (IHME) reports that over 529 million people worldwide are currently living with diabetes. That number is expected to grow to 1.3 billion in only 30 years. While the risk factors for diabetes are vast in number (poor diet, inadequate activity, obesity, sedentary lifestyles, daily stressors, and more), the sad reality is that this chronic medical condition will most likely linger on for generations to come despite our efforts to contain this health epidemic (1).
According to the latest research on diabetes, there are over 37 million people in the United States with diabetes as of 2022. Statistically, approximately 28 million of them have a confirmed diagnosis, while another estimated 8 million are experiencing symptoms without an official diagnosis. Diabetes currently ranks as the 7th leading cause of death in the United States (2).
Ask yourself...
- As a healthcare provider, what has been your experience with treating chronic medical conditions?
- Why do you think there is a continued increase in diabetes despite advances in medication and monitoring devices to treat this condition?
- Are you currently offering comprehensive care to your patients, including medication, diet, and activity counseling for their chronic health conditions?
- How would you describe diabetes to a patient who is newly diagnosed?
Types of Diabetes
In basic terms, diabetes is an impairment in one’s ability to either adequately produce or utilize insulin, which results in elevated levels of circulating glucose. Chronically elevated glucose levels affect blood vessels at every level, causing chronic inflammation and raising the risk of heart disease, stroke, blindness, and atraumatic amputations.
There are three main types of diabetes:
- Type 1 diabetes is thought to be an autoimmune disease. Approximately 5-10 percent of people with diabetes are diagnosed with type 1 diabetes. The diagnosis usually occurs in early childhood and results in a lifetime use of insulin to regulate blood glucose levels.
- Type 2 diabetes is thought to be related to dietary and lifestyle choices. It accounts for nearly 90-95 percent of diabetes diagnoses. Usually occurring later in life (adult-elderly population), it is believed to be related to factors such as diet, activity, weight gain, and related factors. Type 2 diabetes is usually controlled by diet and exercise, in addition to oral medications, although injectable insulin may be included in the treatment plan.
- Gestational diabetes refers to elevated glucose levels occurring during pregnancy for patients who are not diabetic at the onset of pregnancy. This version of diabetes usually resolves itself post-partum, although a woman may develop type 2 diabetes later in life, unrelated to pregnancy.
Type 2 diabetes in children: no longer a “later in life diagnosis”
Children are now being diagnosed with type 2 diabetes at an alarming rate. Despite widespread education and an increased awareness of diabetes, our up-and-coming generation is unhealthier than ever. Many families lack access to healthy food due to both general socioeconomic challenges and an increased rate of food insecurity. (19)
The CDC recommends that care providers have resources for diabetic patients and their families, such as food and nutrition programs, budget-friendly diabetes meal plans, information on how to save money on diabetes care, and coping strategies. (19)

Ask yourself...
- Are you able to articulate the different types of diabetes to patients?
- What resources can you offer to the families of children with type 2 diabetes?
- What is the difference between type 1 and type 2 diabetes?
Diabetes Signs and Symptoms, Diagnostic Testing
There are various ways to test for diabetes. A simple test is the fasting blood sugar (FBS)/ fasting glucose level.
The normal fasting glucose level is below 100mg/dl. The fasting glucose result of 100-125mg/dl indicates prediabetes, and results above 126mg/dl indicate diabetes.
The hemoglobin A1C blood test is another test used to confirm the diagnosis of diabetes. The patient does not need to be fasting for this test, so it is easier to order it regardless of the time of day. This blood test reflects the average glucose level over the period of 2-3 months.
The normal A1C level is below 5.7%. Test results between 5.7% and 6.4% indicate prediabetes and test results above 6.5% indicate diabetes.
A random glucose reading above 200mg/dl, done at any time of day, indicates diabetes.
Diabetes is diagnosed by blood tests, and for improved accuracy, it should be based on two separate readings, done (at least) a day apart. In the case of fasting and random blood tests, dietary intake (large amounts of carbohydrates in a single meal) may adversely affect test results. This is not the case when A1C testing is used for a confirmation diagnosis, as the results are an average of a 2-to 3-month span.
Target blood levels for a person with diabetes (3).
Target blood glucose levels for people with diabetes are as follows:
- Fasting glucose 80-130mg/dl.
- Postprandial blood glucose level- less than 180mg/dl
- A1C level 7-8%.
These target ranges are general guidelines. Patient-specific ranges will be dependent on a variety of factors, including preexisting comorbidities, overall health status, age, and activity levels.

The hallmark signs/symptoms of diabetes
- Polyuria- increased urination
- Polydipsia- increased thirst
- Polyphagia-increased hunger/appetite
The truth is, as healthcare providers, you will have patients who have no hallmark signs and symptoms of diabetes; the diagnosis will be found during annual preventive examinations, often unrelated to any chronic disease. For this reason, many insurance companies now cover numerous preventive screenings, including diabetes screenings, as part of their wellness and prevention initiatives. These tests are often approved based on a patient’s age or preexisting conditions rather than outright signs and symptoms.
Ask yourself...
- What are the typical glucose levels for non-diabetic versus diabetic patients?
- What hallmark symptoms can you identify when treating a potentially diabetic patient?
Lifestyle Interventions and the Diabetes Prevention Program
The initial diagnosis of diabetes can be managed in various ways, depending on the severity of the illness at the time of diagnosis. Lifestyle interventions (behavior modification education) are of utmost importance in the care and management of people with diabetes. Research over the past few decades has consistently shown that such interventions have immense positive effects on the successful long-term management of diabetes.
The official Diabetes Prevention Program was created in 2010 (4) and confirmed the effects of lifestyle interventions in managing diabetes: Lifestyle interventions decreased the incidence of type 2 diabetes by 58% compared with 31% in the metformin-treated group. Thus, these findings now serve as the blueprint, if you will, for all-inclusive, patient-specific disease management guidelines. These lifestyle interventions will be discussed in detail later in the program.
Additional Resources on Diabetes Prevention
Ask yourself...
- How do lifestyle interventions compare to other kinds of treatment for patients with type 2 diabetes?
Semaglutide
Semaglutide is an injectable drug used in the treatment of type 2 diabetes. It was approved by the FDA in May of 2017.
It is a once-a-week injectable and belongs to the drug class known as glucagon-like peptide-1 receptor agonists (GLP-1RAs) (5). It has been referred to as a “miracle weight loss drug” among those who are living with obesity despite frequent side effects, unusually high out-of-pocket costs, drug shortages, and weight regain when attempting to stop using the medication.
GLP-1 receptor agonist: Hormone Review
GLP-1 RAs are a class of medications used to treat Type 2 diabetes and, in some cases, obesity treatment. They are also known as GLP-1 receptor agonists, incretin mimetics, and GLP-1 analogs.
Ghrelin and Leptin (6)
Ghrelin and Leptin are hormones that greatly influence appetite and the sensation of fullness. Ghrelin is often referred to as the “hunger hormone.” It is responsible for many functions, including playing a key role in metabolism through glucose and insulin regulation.
Ghrelin, produced in your stomach, signals your brain when hungry and increases food intake.
Leptin, conversely, is produced in your fat cells and signals to the brain when you have eaten enough (by decreasing your appetite).
Glucagon-like peptide-1 receptors
Known as GLP1 receptors, Glucagon-like peptide-1 receptor proteins are located in the beta cells of the pancreas as well as in the neurons in the brain. GLP-1 receptors are involved in the regulation of blood glucose levels and affect the secretion of insulin. These cells encourage insulin release from the pancreas, increase the volume of beta cells, and reduce the release of glucagon. In doing so, they increase the feeling of fullness during and between meals, suppressing the appetite and slowing gastric emptying.
Ask yourself...
- What are some problems patients might face if they choose to take semaglutide?
- How do Ghrelin and Leptin relate to a patient’s appetite?
What is meant by receptor agonist and antagonist?
The term agonist refers to any substance that mimics the actions of a hormone in producing a specific response: a receptor antagonist blocks a response from occurring.
Opioids are examples of receptor agonists in that they produce responses such as analgesia.
Naloxone/Narcan is an example of a receptor antagonist in that it binds to a receptor site and decreases/blocks a response from occurring.
Semaglutide mechanism of action (7)
GLP-1 agonists work in several ways to positively affect glucose levels. Their mechanism of action includes the following:
- Increasing (stimulating) insulin secretion by the pancreatic beta cells.
- Decreasing the production of glucagon, a hormone that raises blood glucose levels
- Decreasing (slowing) gastric emptying
- Decreasing appetite (and thereby reducing food intake) by creating a sensation of stomach fullness
Through these mechanisms of action, semaglutide results in a lowering of serum glucose/A1C levels, which lowers the risk of cardiovascular events. Studies have also shown that semaglutide resulted in weight loss (approximately 8-14 pounds on average {dose dependent results}.
Ask yourself...
- What is the difference between an agonist and antagonist substance?
- How much weight do patients lose, on average, when taking semaglutide?
Side Effects of Semaglutide
Common side effects of semaglutide (8)
Common side effects may include any of the following:
- Nausea and vomiting
- Headache
- Diarrhea and stomach pain
- Upset stomach, indigestion, constipation, flatulence
These side effects usually subside within a few weeks as the patient acclimates to the medication.
Serious side effects of semaglutide
- Hypoglycemia- enhanced/worsened when used in combination with other diabetes medication. Symptoms may include drowsiness, confusion, weakness, irritability, and headache.
- Symptoms may include abdominal pain and distension, nausea and vomiting, fever, and back pain.
- Diabetic retinopathy. Symptoms may include blurred vision, vision loss, and diminished night vision.
- Kidney damage/injury/failure. Symptoms may include fatigue, nausea, diminished urine output, confusion, and edema of extremities.
- Gallbladder disease. Symptoms may include gallstones, abdominal pain, nausea and vomiting, and poor appetite.
Black Box Warning (9)
Semaglutide has a Black Box Warning for thyroid cancer. This is the most serious warning from the Food and Drug Administration (FDA) and is intended to alert consumers to the potential risks of a medication. This black box warning was issued when research found that the drug increased the risk of thyroid tumors in animals.
It is not known if semaglutide actually causes tumors in humans.
Contraindications
- Semaglutide is contraindicated in people with a personal or family history of MTC (medullary thyroid cancer) or in patients with multiple endocrine neoplasia syndrome type 2.
- Known hypersensitivity to semaglutide or any of the product components
Cautions
As noted under “serious side effects”, there have been reports of new illnesses or worsening of existing health conditions occurring “post-marketing”. Thus, healthcare providers are strongly encouraged to continue the ongoing surveillance of patients using semaglutide therapy. In addition, there is insufficient data available regarding the use of semaglutide by pregnant women. Women are, therefore, highly encouraged to stop any treatment with semaglutide for at least 2 months before a planned pregnancy.
Ask yourself...
- Can you name the 4 common side effects of semaglutide?
- What is the most severe warning associated with semaglutide?
Dosing
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM). It is also considered favorable for reducing the risk of cardiovascular events in adults with T2DM and a preexisting history of cardiovascular disease. This drug is FDA-approved for use in people with diabetes with a BMI of 27% or higher (a BMI of 25-29.9% is considered overweight).
Semaglutide (Ozempic) is an injectable prescription medication. It is available in 0.5mg, 1mg, or 2 mg doses once weekly.
The injection should be administered subcutaneously to the abdomen, thigh, or upper arm. Injection sites should be rotated and given as a single injection.
Start at 0.25 mg once weekly. After 4 weeks, increase the dose to 0.5 mg once weekly.
- If additional glycemic control is needed, increase the dose to 1 mg once weekly after at least 4 weeks on the 0.5 mg dose.
- If additional glycemic control is needed, increase the dose to 2 mg once weekly after at least 4 weeks on the 1 mg dose
Administer once weekly at any time of day, with or without meals. The maximum dose recommendation is 2mg/weekly once weekly.
Note: The initial 0.25-mg dose is intended for treatment initiation and is not effective for glycemic control
Missing Dose Guidelines
- If the missed dose is ≤5 days: Administer dose as soon as possible
- If missed dose >5 days: Skip the missed dose and administer the next dose on the regularly scheduled day; patients can then resume their regular once-weekly dosing schedule
Administration Day Guidelines (10).
The administration day each week can be changed, if necessary, as long as the time between 2 doses is at least 2 days (>48 hours)
Dose Availability (packaging)
- 2mg/1.5mL (1.34mg/mL); delivers doses of 0.25mg or 0.5mg per injection or four to eight doses per injection pen
- 4mg/3mL (1.34mg/mL); delivers 1mg per injection or 4 doses per injection pen
- 8mg/3mL (2.68 mg/mL); delivers 2mg per injection or 4 doses per injection pen
Treatment Goals- Effects on A1C and Weight (11)
A majority of adults who were placed on injectable semaglutide for diabetes management achieved a target A1C under 7% and were able to maintain it.
- Dose specific effects on A1C were as follows:
- 0.5mg dose injection yielded a 1.4% decrease
- 1.0mg dose injection yielded a 1.6% decrease
- 2.0 mg dose injection, in combination with diabetes pills, yielded a 2.1% decrease in A1C.
Adults taking semifluid injectables for diabetes management also noted weight loss.
- 8-pound weight loss reported with 0.5mg dose injection
- 10 pounds weight loss reported with 1.0mg dose injection
- Up to 14 pounds of weight loss was reported with a 2.0mg dose injection

Ask yourself...
- What should you tell a patient if they miss their injection by more than 5 days? What if it has been less than five days?
Prescribing insights: Long-Term therapy for a chronic condition?
Semaglutide is viewed favorably as a treatment option for Type 2 diabetes. It appears to lower A1C levels and body weight in the majority of patients, lowering their risk of future cardiovascular events.
The question of long-term medication use for a chronic health condition is being heavily discussed in the media. While a percentage of people can decrease or eliminate the need for chronic medications through significant lifestyle changes, there have been reports of weight gain in those who stopped taking this injectable medication.
Without intense lifestyle behavior modification education, there is a heightened risk of weight regain in the absence of such medications. Leaders in the treatment of obesity and related illnesses have commented that this drug is intended for long-term use.
Examples of this include the following:
“GLP-1 medications [like Ozempic] are designed to be taken long-term… They are chronic medications for the treatment of chronic conditions (both diabetes and obesity) (12)”. – Christopher McGowan, M.D., a gastroenterologist specializing in obesity medicine and endobariatrics
“As with many chronic conditions, most people who use the drugs for diabetes or weight loss will need to keep taking them to keep benefiting from them. Depending on your individual situation, and without sustained lifestyle changes, it is likely you would need to be on these medications indefinitely to maintain weight loss (13).” – Dr. Cecilia Low Wang, a UCHealth expert in endocrinology, diabetes and metabolism.
Ask yourself...
- Is semaglutide considered to be a long-term treatment for type 2 diabetes?
Cost Concerns
At this time, injectable semaglutide, FDA-approved for the treatment of Type 2 diabetes, has a self-pay price tag of $935.77 per month (4 injections). With FDA approval, many people with diabetes, insured under commercial plans, receive the drug for the cost of their copay. Those patients without coverage may use pharmacy discount cards that reduce the price, on average, to $814.55/month.
The following links are available to familiarize yourself with patient assistance programs related to semaglutide injectables.
Semaglutide Cost Savings Programs
The following links are provided to explore various semaglutide cost savings programs.
Ask yourself...
- What resources can you offer patients who are struggling to pay for semaglutide?
Emerging Concerns: Semaglutide and gastroparesis
In August 2023, a first-of-its-kind lawsuit was filed in Louisiana against the makers of semaglutide. The lawsuit states the makers of this injectable drug did not adequately warn patients about the risk of severe gastrointestinal issues/possible gastroparesis.
The plaintiff, in this case, had used both Ozempic and Mounjaro and experienced repeated episodes of severe gastrointestinal events, warranting trips to the emergency room and additional medications to alleviate her symptoms (14). While this lawsuit is in the developing stages, it bears mentioning in terms of concerns over long-term usage of the drug and possible complications.
While the drug labeling for semaglutide (Ozempic) does not specifically mention gastroparesis, the semaglutide/Mounjaro label does state that the drug has not been studied in patients with severe gastrointestinal disease and is, therefore, not recommended for these patients.
Up to 50% of people with diabetes have some degree of delayed gastric emptying, but most have no digestive symptoms or have only mild symptoms. For some people with diabetes, problems managing blood glucose levels may indicate delayed gastric emptying (15).
Healthcare providers should evaluate all patients with diabetes for possible symptoms of underlying gastroparesis, such as the feeling of fullness shortly after beginning a meal or the inability to finish a regular meal. Other symptoms of gastroparesis may include abdominal pain, nausea, bloating, vomiting, and anorexia.
Diabetes and gastroparesis
Uncontrolled or poorly controlled diabetes can affect nerve endings systemwide. Diabetes is a very common cause of gastroparesis. Although the condition is rare, it occurs more often in people with chronic conditions such as diabetes, autoimmune diseases, and nervous system disorders. Nerve endings are injured or damaged, cease to function properly, and result in delayed gastric emptying. The delay in gastric emptying can cause various symptoms, such as nausea, vomiting, bloating and distension, abdominal pain, and poor appetite.
In addition to underlying medical conditions, some medications may cause symptoms of gastroparesis (delays in gastric emptying and overall gastric motility. These medications include narcotics, antidepressants, and anticholinergics.
Left untreated, diabetic gastroparesis may lead to malnutrition, electrolyte imbalances, and poor glucose management and control.
Ask yourself...
- Why should nurses prescribing semaglutide watch out for symptoms of gastroparesis?
- What do you think are some ethical issues with semaglutide use for weight loss?
Diabetes Lifestyle changes: Patient education (16)
- Weight Management
- Healthy Eating
- Physical Activity
- Smoking Cessation
- Stress Management
Patient education regarding lifestyle changes is a priority. As with any chronic medication condition, the patient and their family/support system must be given every opportunity to educate and empower themselves on self-management of their disease process. Patients must be given the benefit of the doubt that they can indeed embrace their health and well-being and work with their healthcare provider to maximize their health outcomes.
Numerous lifestyle behaviors should be addressed and actively worked on for diabetes mellitus so that the patient receives the maximum health benefits. The following lifestyle behaviors are in no particular order; they all warrant discussion at every office visit.
Diet
A person with diabetes should be educated on the effects of food and nutrition on their glucose level. Referrals to a dietitian/nutritionist or Certified Diabetes Care Education Specialist (CDCES) should be considered a top priority. Well-balanced nutritional intake, appropriate carbohydrate awareness, calorie monitoring (if weight loss is appropriate for your specific patient), and medication/food interactions are all essential aspects of dietary lifestyle education. Many commercial insurance plans, as well as hospital community outreach programs, offer diabetes self-management classes.

Activity (17)
The CDC recommends a target goal of 150 minutes weekly. Patients should be educated on the positive effects of daily activity on overall health and well-being, stress management, and metabolism. Patients should find activities they are genuinely interested in, involve family and friends, and slowly build greater endurance through increased intervals of longer duration.
Sleep hygiene (18)
Patients should be educated on the positive effects of a good night’s sleep. The aim should be approximately 7-8 hours of restful sleep. Electronics should be powered down and (optimally) removed from the bedroom. A dark, well-vented, cool room temperature is encouraged, and large meals and late-evening caffeine should be avoided.
Medication adherence/ literacy
Medication education is critical to the health and well-being of a patient. Routine education of the patient and family members or support systems, when available, should be supportive and patient-specific. Patients should be assessed on language barriers, literacy issues, and related comprehension concerns. Medication education should include effects, side effects, treatment goals, and sick day management. Emergency care issues should also be discussed. Any monitoring equipment (continuous glucose monitors, accuchecks, lancets) should be reviewed with patients and confirmed with return verbalization and demonstration.
As discussed in this course, patients with chronic diseases must learn self-management techniques to optimize their health and well-being. They must become confident in understanding their disease process and take ownership of their health. In doing so, they minimize the risk of long-term complications, improve their self-worth, and actively invest (both time and money) in their future.
Ask yourself...
- How do sleep, diet, and activity levels affect the treatment of type 2 diabetes?
Ozempic Case Study
- 52-year-old female
- Height 67 inches
- Weight 225 pounds
- B/P 138/84, Heart rate 76 NSR
- BMI 35.2%
- Nonsmoker, occasional social drinker
- Multiple attempts at dieting without success.
- Diagnosed T2DM approx. 6 months ago, current A1C 7.5%; initial medication Metformin 500mg BID tablets; tolerated well. No GI upsets.
Today’s appointment is for evaluation and additional medication consideration (the patient requested this appointment)
The patient was diagnosed with T2DM approximately 6 months ago. Initial A1C 8.0%. Current A1C 7.7%
Despite an improved diet and adherence to the medication regimen, the patient voiced frustration at the lack of weight loss. Requesting additional medication. I have a neighbor friend who began injectable Ozempic and is having “really great results with it. I want to start on it as well”.
Ask yourself...
- What are your thoughts on prescribing semaglutide injectable for this patient?
- What objective health data points should be considered when prescribing semaglutide for this patient?
The patient has expressed frustration that despite taking her medications and adjusting her diet, she has not lost any weight in the past 6 months. She has “heard from her neighbor friend that the weight just melts off immediately,” and she is ready to start this medication.
Ask yourself...
- What concerns do you know about this patient’s understanding of weight loss related to semaglutide?
- What prescribing information, specific to semaglutide and weight loss, could you share with your patient regarding realistic weight loss targets?
- In addition to teaching your patient proper injection technique for the use of semaglutide, what other lifestyle education behaviors should you discuss at this point?
- What information should you share with your patient regarding the long-term use of semaglutide and the potential risks of stopping this medication (as it relates to weight regain)?
Your patient decides to go ahead with the semaglutide regimen, and your patient wants to know how long she will be taking this medication.
Ask yourself...
- What are some patient education guidelines regarding common side effects of this medication?
- How often is the dose increased? What is the maximum dose this patient can receive weekly?
- What talking points will you cover regarding the long-term use of this medication?
- How do you best prepare this patient for long-term success with this medication?
- What lifestyle behavior modification education would you discuss with your patient to give her the best chance at successfully managing her diabetes?
Medication Assisted Treatment (MAT)
Introduction
Medication-assisted treatment (MAT) is a treatment modality for substance use disorders. It combines counseling and behavioral therapies for addiction with medications used carefully to reduce the physical symptoms of cravings and withdrawal and assist clients in the recovery process. With half of the people 12 and older reporting use of an illicit substance at least once and 21 million Americans experiencing addiction, this is an important and relevant topic (4).
Historically, an intense stigma is attached to both addiction and some of the medications used to treat addiction. A thorough understanding of substance use disorders, available MAT therapies, and care of affected clients is an essential topic for nurses to be familiar with, particularly those working in psychiatry, pain management, or addiction medicine.
Overview of Addiction and Substance Abuse:
Drug and alcohol abuse and addiction are chronic, complicated issues involving persistent changes to the brain. There is a stigma or misunderstanding that people with substance abuse disorders can stop any time they want to or lack the willpower or moral fortitude to stop using. This is entirely untrue, and even people who are “recovering” and have not had any drugs or alcohol in years can easily relapse into addiction once those brain changes have occurred (5).
When a person uses drugs or alcohol, the brain’s reward center is flooded with dopamine. This provides a “buzz” or pleasurable sensation that may create the desire to use more of the same substance. Over time, and with regular use of the substance, the brain becomes accustomed to the flooding of dopamine and reduces the reward response, a process known as tolerance.
It will now take the same person a more significant amount of the substance to achieve the same “buzz” or “high” they used to feel. This process can also dull the pleasure response to activities not involving substance use, such as food, socialization, or sexual activity. Over time, the chemical changes in the brain can progress to include decreased functioning of learning, decision-making, judgment, response to stress, memory, and behavior (5).
To understand substance abuse disorders, it is first essential to understand some basic definitions. These terms are sometimes used interchangeably, but they mean different things and represent different stages of disease.
Definitions
- Substance Use: Substance use is any consumption of drugs or alcohol, regardless of frequency or amount. An occasional glass of wine or taking an edible at a party is an example of substance use. Substance use does not cause problems or dependency in many people (5).
- Substance Abuse: Substance abuse is the continued use of drugs or alcohol, even when they do cause problems. Conflict or problems at home, school, work, or legal issues related to the use of drugs or alcohol are signs of abuse. For example, being sent home from school for smoking in the bathroom or failing a drug test at work (5).
- Substance Dependence or Addiction: Dependence and addiction can be used interchangeably or is sometimes called substance use disorder. Addiction occurs when a person cannot stop drinking or using drugs despite creating problems in their life. People who are addicted may experience cravings until they use a specific substance, or they may experience uncomfortable physical symptoms, known as withdrawal if they do stop (5).
The American Psychiatric Association (APA) utilizes the following criteria to diagnose clients who suffer from addiction. The more criteria a client answers yes to, the greater their problem with substance use.
Six or more positive criteria are indicative of addiction.
- Using substances in more significant amounts or for more extended periods than intended
- Trying to stop using, but being unable to
- Increased amounts of time getting, using, or recovering from the use of the substance
- Experiencing cravings or urges to use.
- Continuing to use the substance despite problems with relationships or social situations.
- Missing work, social, or recreational obligations or activities because of substance use
- Participating in risky behavior because of substance use
- Continuing to use the substance despite psychological or physical health problems.
- Needing to use more substance over time to achieve the desired effect.
- Experiencing withdrawal symptoms when stopping the substance (1).

Ask yourself...
- Do you know anyone who suffers from a substance use disorder?
- Think about your biases (thoughts, opinions, attitudes) about addiction. Does any of the information above conflict with those biases?
- How does substance use become substance abuse?
- Define substance addiction.
- What is the criteria that indicates addiction?
Substance Abuse Statistics
Many factors go into gathering data on substance abuse disorders, from underreporting, the nuance between use, abuse, and addiction, and the large variety of substances available, with the legality of some substances varying by state or age.
The statistics below from 2020 are not meant to be an exhaustive list of substance use disorders in this country but rather an overview of some of the more prevalent addiction-related issues.
- 50% of people 12 years and older have used an illicit substance at least once.
- 5% of Americans 12 years and older have used drugs within the last month.
- This is a 3.8% increase from the previous year.
- About 50% of Americans 12 and over drink alcohol
- 4% of those people have an alcohol use disorder.
- About 20% of Americans use tobacco products or vape
- 18% of Americans over 18 used marijuana in the last 12 months
- 30% of those have some level of misuse or addiction.
- Marijuana is commonly involved in polysubstance use, paired with alcohol or other drugs.
- 7% of Americans over 12 misused opioids in the last 12 months
- 96% of those used prescription pain relievers
- Opioid prescriptions peaked in 2012, with 81.3 prescriptions per 100 people.
- The rate has declined recently due to increased attention to this crisis.
- In 2018, the rate was down to 51 prescriptions for every 100 people
- Fentanyl is now rising as a new and deadly concern.
- 5 million prescriptions were written for fentanyl in 2015.
- Fentanyl is involved in 53% of overdose deaths.
- 7% of all Americans misuse a prescription drug.
- 1% of those misuse stimulants
- 2% of those misuse sedatives
- 5% misuse painkillers
- Over 70,000 drug overdose deaths occur annually in the United States (4)
Ask yourself...
- Do the above statistics reflect what you currently see in practice?
- Have you seen an increase in substance use disorders since you have been in practice? Why do you think that is the case?
- Which substance have you most commonly seen cause complications in your practice?
Risk Factors
A combination of factors is involved in the risk of addiction, and no one factor can determine if someone will develop addiction or after how many uses this will occur.
The addiction process does occur more easily or progresses more rapidly for people with certain risk factors, including:
Genetics
There is a strong genetic correlation with addiction, indicating that biology plays a significant role in the disorder. Family history of addiction, gender, ethnicity, and comorbid mental health conditions can all influence the risk of addiction. (5)
- Children of addicts are eight times more likely to develop an addiction at some point.
- In 2020, among those using illicit or misusing prescription drugs, 22% were male and 17% were female.
- Only 20% of users in drug treatment programs are women.
- 9% of people with substance abuse disorders also have at least one mental health disorder (4)
Environment/Non-Genetic Demographics
The attitudes about drugs and alcohol from those in a person’s network and life experiences play a role in the risk of addiction. Substance use among friends, family, or coworkers increases the risk that a person will also use substances. Exposure to substance use from a young age relaxed parental attitudes about substance use, and peer pressure from friends can increase the risk. Certain stressful life circumstances, such as veteran status, history of sexual or physical assault, or being part of the LGBTQ community, can also increase risk. (5)
- 20% of people in urban areas used illegal drugs in 2020, compared to 5% in rural locations.
- 51% of Americans with an illegal pain relief medication obtained it from a friend or relative.
- 7% of LGBTQ Americans abuse illicit drugs.
- 2% of LGBTQ Americans abuse alcohol.
- 7% of Veterans abuse illicit drugs.
- 80% of Veterans abuse alcohol (4)
Developmental Stage
Substance use at any age can lead to addiction, but children and teens are at particular risk due to their underdeveloped brains. The parts of the brain responsible for decision-making, risk assessment, and self-control do not fully develop until the early 20s, putting teenagers at increased risk of dangerous behaviors. In addition, the effects of drugs and alcohol on the developing brain may mean that those parts of the brain never fully develop at all for teens with substance abuse disorders. (5)
- 70% of users who try an illegal substance before age 13 will develop a substance use disorder within the next seven years.
- This is for only 27% of people who first try an illegal substance after age 17.
- 47% of youths report trying an illegal substance by the time they graduate high school (4)
Ask yourself...
- Why do you think medication alone is not an adequate treatment for substance abuse disorders?
- How do genetics impact a patient’s risk for substance abuse?
- Is MAT something you have heard of before? Why do you think it is relatively uncommon despite being around for decades?
- What role does community nursing play in the education and reduction of substance abuse disorder?
- Which population group do you see as having the highest incidence of substance abuse?
Overview of Medication Assisted Treatment (MAT)
Treatment of substance abuse disorders is a complex and often tumultuous process. The nature of the brain changes that occur during addiction means that a person is never entirely “cured” but will always be considered “recovering” as the risk for relapse is always present. Effective treatment must be multifaceted and often involves removing triggers (such as people, places, and stressors) that may prompt a person to use again behavioral therapy, and medications to curb withdrawal symptoms and reduce cravings.
Medication Assisted Treatment (MAT) is a treatment that involves FDA-approved medications, in combination with behavioral therapy, in the recovery process for substance abuse disorders. Several medications are available for MAT, and evidence continues to emerge that the treatment is highly effective if used correctly.
However, it is a vastly underused and understudied treatment modality. MAT has been available in some form for over 50 years but is just starting to gain traction among the medical community (and policymakers) in recent years, with the federal government calling for more research and increased accessibility for the treatment (8).
The height of the opioid crisis in the last several years has highlighted the magnitude of drug addiction and deaths in the United States, bringing renewed attention to MAT as a treatment option. So, how does MAT work? Prescription medication is given to both stimulate the receptors seeking the abused substance and block the drug’s euphoric effects.
Over time, this normalizes brain chemistry and helps the person break the habit of using without the discomfort of cravings and withdrawal symptoms. Gradually, the prescription medication dosage is reduced, all the while in conjunction with behavioral therapy and lifestyle changes, and eventually, the client should be able to stop the medication altogether, often within 1-3 months (8).
MAT does require close supervision by a trained medical professional and an appropriate facility for treatment. It can be done on an inpatient, partial inpatient, or outpatient basis. There may be side effects to the medication, and there is a risk of misusing or developing addiction to the new drug, though the successful outcomes often outweigh this risk. Clients must also participate in behavioral therapy for a comprehensive and effective treatment plan. As with any treatment regimen, careful consideration of the client’s history and circumstances is essential (8).

Ask yourself...
- Why do you think medication alone is not an adequate treatment for substance abuse disorders?
- Is Medication Assisted Treatment (MAT) something you have heard of before? Why do you think it is relatively uncommon despite being around for decades?
Pharmacokinetics
Currently, there are three medications with FDA approval for MAT: buprenorphine, methadone, and naltrexone. Each will be discussed in depth below.
Buprenorphine
Mechanism of Action and Metabolism
Buprenorphine is an opioid partial agonist, acting on the same receptors as other opioids but with weaker effects. It can be used for the treatment of misuse of opioids, including:
- Heroin
- Fentanyl
- Oxycodone
- Hydrocodone
- Morphine
- Methadone (3)
Opiate receptors are G-protein coupled receptors (GPCRs) with four major types: Mu, Delta, Kappa, and opioid receptor like-1 (ORL1). Stimulation of these receptors results in varying levels of the following effects:
- Euphoria
- Relaxation
- Pain relief
- Sleepiness
- Sweating
- Constipation
- Impaired concentration
- Reduced sex drive (3)
Buprenorphine has a high affinity to the Mu-opioid receptor and is a partial agonist at this site, causing reduced opioid effects with a plateau or ceiling at higher doses. This limits dangerous effects and makes overdose unlikely. It also has slow dissociation from the site, allowing milder and more easily tolerated withdrawal effects compared to full agonists like morphine and fentanyl. Buprenorphine is also a weak kappa receptor antagonist and delta receptor agonist, reducing the craving sensation and improving tolerance to stress (3).
Buprenorphine has poor bioavailability when given orally due to the first-pass effect, where most of the drug is broken down in the liver and intestines. Because of this, sublingual or buccal are the preferred routes of administration and the most common forms in which the drug is manufactured. Transdermal patches and IV and IM forms exist, though not for use in MAT (3).
CYP34A enzymes break down buprenorphine, so other drugs, such as ketoconazole, may inhibit metabolism and increase available levels of buprenorphine. CYP34A inducers such as carbamazepine, topiramate, phenytoin, and barbiturates may speed metabolism and lower available levels. Once broken down, the med takes the form of norbuprenorphine and is excreted in the feces (3).
Available Forms
Buprenorphine is available by itself and with naloxone (in a 4 to 1 ratio). However, in oral form, naloxone is not readily absorbed, and buprenorphine is the only genuinely active ingredient. This combination is beneficial should clients try to inject their buprenorphine to get high; naloxone is a fast-acting opioid antagonist that is active when used intravenously and would block the opioid effect of buprenorphine, rendering it useless for recreational use and ensuring it has no street value.
The currently available preparations of buprenorphine for MAT include:
- Generic Buprenorphine/naloxone sublingual tablets
- Subutex – Buprenorphine sublingual tablets
- Suboxone – Buprenorphine/naloxone sublingual films
- Zubsolv – Buprenorphine/naloxone sublingual tablets
- Bunavail – Buprenorphine/naloxone buccal film (3)
Sublingual products dissolve within 2-10 minutes. Bloodstream absorption begins quickly, bypassing the first pass effect. Buprenorphine has a slow onset of action, peaking about 3-4 hours later. Metabolism is also slow, with the half-life lasting anywhere from 25 to 70 hours (an average of about 38 hours). This long half-life means the drug can be spaced out to every other day administration once weaning begins (3).
Dosing and Monitoring
Clients prescribed buprenorphine must stop using opioids for at least 12 to 24 hours before the first dose; this varies depending on which opioid they are stopping. For short-acting opioids like heroin and oxycodone, buprenorphine may be started 6-12 hours after the last dose. With longer-acting opioids such as morphine or extended-release preparations of oxycodone, buprenorphine should be delayed for about 24 hours. For the longest action opioids, fentanyl patch, 48 -72 hours must be between the last dose and buprenorphine initiation (3).
This initiation schedule means clients will be in the early stages of discomfort and withdrawal. Administration of buprenorphine when clients still have opioids in their bloodstream will lead to competition for receptor sites, rapidly replacing the opioid with buprenorphine and causing acute and more severe withdrawal symptoms.
Depending on the severity of a client’s addiction, they may complete the first step of abstaining and withdrawal in an inpatient setting. Once the initial withdrawal symptoms have passed and the initial dose of buprenorphine has been given, the client may be discharged home to continue buprenorphine initiation on an outpatient basis (3).
Initial doses are typically 2-4mg, with up to 4mg given to clients used to higher potency or larger doses of opioids. The dose is gradually increased to meet the client’s individual needs, with a maximum dosage of 24mg per day. The average client requires 8-12 mg per day and can reach this dose within the first 2-4 days. It is recommended that doses be supervised by a pharmacist at the dispensing pharmacy for the first two months of treatment to ensure compliance and clients are less likely to relapse (3).
The length of treatment with buprenorphine depends on each client’s case and, for some, may be indefinite. Clients who do wish to wean off buprenorphine can begin the process once they are stable and experiencing few or no cravings, and a minimum of 8 weeks from treatment initiation. Doses are moved to alternating days and eventually discontinued altogether (3).
Side Effects and Contraindications:
As with any medication, there are potential side effects, including:
Common Side Effects
- Nausea
- Vomiting
- Drowsiness
- Dizziness
- Headache
- Memory loss
- Sweating
- Dry mouth
- Miosis
- Postural hypotension
- Sexual dysfunction
- Urinary retention
Serious side effects
- CNS depression
- QT prolongation
- Reduced seizure threshold
- Potential for abuse or overdose (3)
Buprenorphine is contraindicated for clients with a past hypersensitive reaction to it. It should be used cautiously for clients with respiratory suppression, older adults, or for those with liver pathologies. Regular monitoring of liver enzymes via lab work is essential (3).
It is a Category C medication for pregnancy, and the risks versus benefits should be carefully weighed. Buprenorphine does cross the placenta and increases the risk of withdrawal symptoms and neonatal abstinence syndrome (NAS) after delivery. However, for pregnant clients with the highest risk of relapse and abuse of opioids, evidence does support that continuation of buprenorphine during pregnancy may improve maternal and fetal outcomes (3).
Buprenorphine may be abused by crushing tablets, snorting the powder, or dissolving it into an injectable solution. Safety measures against this include supervised administration by a pharmacist and the addition of naloxone, which blocks the buprenorphine effects. While the effect ceiling of buprenorphine makes overdose difficult, combining the drug with benzodiazepines, alcohol, or other drugs can compound the CNS depressant effects and increase the risk of overdose (3).
Clinicians need to have a comprehensive health history of clients before initiating buprenorphine so that all risks and potential interactions can be addressed appropriately.
Role of the Pharmacist
Pharmacists play a significant role in the success of MAT involving buprenorphine. Outpatient doses are monitored by the dispensing pharmacist daily, with at-home quantities being allowed on a limited basis (such as weekends or travel) and only for the most motivated and compliant clients. Vital signs are collected before each dosage, with careful monitoring for hypotension or bradypnea. The dose may be skipped for clients who experience excessive side effects, and the client can return the next day for their dose.
Clients presenting with signs of overdose (usually to the ED) may receive naloxone, which will reverse overdose symptoms within 1 hour. Overdose symptoms include dizziness, pinpoint pupils, hypotension, bradypnea, hallucinations, seizure, or unconscious state.
If a client misses a dose, does not show up for it, or is experiencing significant side effects from buprenorphine, the prescribing clinician should be notified so that the treatment plan can be revisited and revised if needed (3).
Considerations for the Prescriber
When considering which medication to prescribe for MAT, prescribers should understand that buprenorphine offers advantages over methadone.
- Lower risk of abuse
- Safer, including at higher doses.
- Therapeutic dose achieved quickly.
- Easier to taper.
- Can be obtained from any provider rather than a methadone clinic.
- Less stigma
The cost of a 30-day supply is around $300. Buprenorphine/naloxone combinations are a little more expensive at $400/month. While prior authorization is usually required, most commercial insurance and state Medicaid programs will cover the medication.
Buprenorphine is a Schedule III Controlled Substance; however, recent federal regulations have been aimed at approving access to MAT, and any provider with an active DEA license may prescribe buprenorphine as allowed by state regulations. Specialized clinics are not required (as they are with methadone), and it is dispensed at regular pharmacies.
Prescribers are encouraged to participate in additional training about MAT with buprenorphine, but it is not required. Detailed documentation must be completed, including the reason for prescribing, start and end dates of treatment, the pharmacy used, the credentials of who will supervise administration, and frequency of follow-up and compliance monitoring. The sublingual and buccal routes are the only forms of medication used for MAT; patches, IM, and IV preparations are not routinely used for MAT.
The success of buprenorphine treatment depends on the client’s education. Addiction potential, risk of combination with other CNS depressants, and side effects vs. signs of overdose should all be discussed with clients and their support system (3).
Ask yourself...
- Given the nature of substance abuse disorders, why do you think including an opioid antagonist like naloxone in preparations of buprenorphine is necessary for safety and compliance?
- What challenges do you see with a medication needing to be administered daily with pharmacist supervision?
- What are the risks of buprenorphine being given without this supervision?
- Consider the possible pros and cons of taking a medication like buprenorphine during pregnancy. Also, consider the risks of NOT taking the drug during pregnancy when a substance use disorder is present.
- What education should be provided to a patient who is prescribed buprenorphine?
Methadone
Mechanism of Action and Metabolism
Methadone is a synthetic opioid and a full agonist of the Mu-receptor site, stimulating the same effects as opioids.
- Euphoria
- Analgesia
- Sedation
It can be used as a potent analgesic for pain not responding to traditional medications, such as in clients with cancer or terminal illness, as well as for MAT and neonatal abstinence syndrome (NAS).
For this course, it will be discussed as a MAT agent, used in treatment for clients addicted to opioids such as:
- Heroin
- Fentanyl
- Oxycodone
- Hydrocodone
- Morphine
- Hydromorphone (2)
Methadone is a full agonist at the Mu-receptor, meaning it is a more potent and more easily addictive medication than partial agonists like buprenorphine. Methadone has a long half-life (8-60 hours), occupying the Mu-receptors and blocking short-acting opioids from making a client high. The longer half-life also leads to less severe cravings and withdrawal symptoms. Methadone is also an antagonist to the N-methyl-d-aspartate (NMDA) receptor, which adds to its pain relief action (2).
It has high oral bioavailability, is active in the bloodstream within 30 minutes of ingestion and remains elevated for around 24 hours. It is broken down via CYP3A4 and CYP2B6 enzymes and metabolized through the liver, making it a good option for clients with renal problems.
Medications such as ciprofloxacin, benzodiazepines, fluconazole, cimetidine, and fluoxetine may slow methadone metabolism, increasing the available drug and the side effects of overdose risk. Other medications may speed metabolism and decrease the effects of methadone, including phenobarbital, phenytoin, rifampin, ritonavir, and carbamazepine (2).
Available Forms
Methadone is available in many forms, including oral, IM, subcutaneous, IV, and intrathecal, though only the oral is typically used for MAT.
- Methadone – tablets
- DISKETS – dispersible/dissolvable tablet
- Methadone HCL Intensol – 10mg/ml suspension
- Methadone – dispersible tablet (2)
Dosing and Monitoring
Oral dosing is initiated at 30-40 mg/day with a slow titration of 10-20 mg/week until the optimal dosage is reached. The optimal dosage varies by client and depends on the drug they are replacing, tolerance to opioids, and side effects experienced. A dosage between 80- 150 mg/day is the typical goal. (2)
If parenteral methadone is given, it is usually 50%-80% of the oral dosage.
Blood sugar, EKG, and methadone blood levels should be checked regularly, every week for higher-risk patients, and every 3-6 months for those in good health and compliance. The target methadone blood level is around 400 ug/ml (2).
Side Effects and Contraindications
Potential side effects are directly related to stimulation of the opioid receptors and include:
- Diaphoresis
- Flushing
- Pruritus
- Nausea
- Dry mouth
- Constipation
- Sedation
- Lethargy
- Respiratory Depression
- QT prolongation
- Hypoglycemia (2)
Methadone should be considered with a comprehensive view of a client’s health history and other medications. Clients with CNS-related disease processes (trauma, increased ICP, dementia, or delirium) must be monitored closely or have other medication considered.
Methadone should not be used simultaneously as other opioids, benzodiazepines, alcohol, or antipsychotics due to increased CNS effects. Methadone is a Pregnancy Category C medication, and risks versus benefits should be weighed carefully. Infants exposed to methadone in utero are at increased risk of NAS after delivery (2).
Overdose can occur, and clients and support systems should be educated on signs of overdose.
- Lethargy
- Somnolence
- Stupor
- Coma
- Miosis
- Bradycardia
- Hypotension
- Respiratory sedation
- Cardiac arrest
Naloxone is used to reverse overdose (2).
Considerations for Prescribers and Clinics
Methadone is a Schedule II Controlled Substance, meaning it has a high abuse potential and must be carefully monitored. The Prescription Drug Monitoring Program (PDMP) is an electronic database used nationwide to register the distribution of controlled substances so that clients do not seek care at multiple clinics or pharmacies to obtain more of a controlled substance.
When prescribing methadone, providers should check the PDMP for both methadone and other prescription opioids so that they are fully aware of other medications clients may be receiving from other places. Regular urine drug screening should be performed to make sure clients are not using other substances not obtained by prescription and that they are testing positive for methadone, meaning they are genuinely taking it if administration is not observed (2).
At the beginning of treatment, methadone is given in the office under a nurse’s supervision, and then clients are monitored for adverse effects. Some take-home doses (up to 7 in the first two weeks) may be arranged for weekends or during travel, but this possibility is limited during the first few weeks of treatment. As treatment progresses and compliance is demonstrated, clients may self-administer more doses at home (up to 28 doses per month) and go longer between visits to the clinic. The total length of treatment varies but is often 1-2 years and can even be indefinite (7).
There are methadone clinics that work entirely in the scope of addiction management, but primary care providers may prescribe methadone as well. Prescribers must have an active DEA license and comply with state-based controlled substance regulations (2).

Ask yourself...
- Why do you think methadone is a Schedule II Controlled Substance while buprenorphine is only a Schedule III?
- What are the benefits of checking the serum level of methadone?
- What might the clinical presentation be for someone overdosing on methadone?
- Have you ever used the PDMP database before? What are the benefits of accessing this database?
- What education should you provide to a patient being prescribed methadone?
Naltrexone
Mechanism of Action and Metabolism
Naltrexone has been in use since the 1960s and is an opioid antagonist. It competes primarily with the mu-receptor but also serves as an antagonist at the kappa and delta receptors. As an antagonist, it competes with agonists such as opioids and alcohol and blocks the effects of agonists at those sites.
- Prevents euphoria.
- Prevents intoxication.
- Reduces tolerance (6)
Naltrexone also acts on the hypothalamic-pituitary-adrenal axis, modifying it to reduce cravings and suppress alcohol consumption.
It is FDA-approved for use in clinical practice for the treatment of:
- Alcohol use disorder
- Opioid use disorder (prescription and non)
Naltrexone is absorbed orally and undergoes extensive metabolism via the first-pass effect. However, this does not affect its potency as naltrexone’s active metabolite, 6β-naltrexone, acts as a potent opioid antagonist. The medication’s half-life is around 4 hours but can last up to 24 hours. If administered parenterally, it bypasses the first pass and is even longer acting, with a half-life of 5-10 days. Naltrexone is excreted by the kidneys (6).
Available Forms
Naltrexone is available in an oral tablet and IM injection. Available preparations include:
- Generic naltrexone tablets
- Revia (oral tablet)
- Depade (oral tablet)
- Vivitrol (solution for IM injection, extended-release) (6)
Dosing and Monitoring
Since naltrexone will compete for and block all opioid receptor sites, the risk for withdrawal symptoms is high, and clients must stop the use of alcohol or opioids for 7-10 days before beginning treatment to lessen the risk of withdrawal symptoms. A naltrexone challenge is recommended at the start of therapy.
This consists of administering small amounts of naltrexone subcutaneously or via IV and monitoring the client and their vital signs for signs of withdrawal, such as:
- Nausea
- Vomiting
- Diaphoresis
- BP changes
- Tachycardia
- Rhinorrhea
- Agitation
- Tremors
- Abdominal pain
- Pupillary dilation (6)
If a client fails the naltrexone challenge and has not been long enough since their last use of alcohol or opioids, the naltrexone initiation should be delayed, and the test should be repeated in 24 hours. If clients tolerate the naltrexone test and the negative result, they may begin naltrexone treatment (6).
For oral tablets, dosing usually starts at 25 mg for the first dose. Clients are observed for withdrawal symptoms and side effects; an additional 25 mg is given 1 hour later. After that, clients take 50 mg per day. Clients may continue with 50mg daily or take 100 mg every other day or 150 mg every 3rd day (6).
Alternatively, naltrexone may be given via IM injection for more extended action, improving compliance and reducing relapse. Particularly for alcohol or heroin dependence, data indicates that the IM route has much higher success rates than the oral route. If a client receives the IM injection, 380 mg is given to the gluteal muscle every four weeks (6).
Side Effects and Contraindications
Most common side effects of naltrexone include:
- GI irritation
- Diarrhea
- Abdominal cramps
- Nausea
- Vomiting
- Hypertension
- Headache
- Anxiety
- Low energy
- Joint or muscle pain
- Nervousness
- Sleep disruption
Less commonly, clients report:
- Loss of appetite
- Constipation
- Dizziness
- Irritability
- Depression
- Rash
- Chills (6)
Caution should be used for clients with liver function issues and renal impairment. It is Category C for use during pregnancy, and the risks versus benefits of use in pregnancy must be carefully considered. It also crosses into breast milk and must be considered carefully.
There is limited data about the overdose of naltrexone, and there may be very few symptoms if an overdose occurs. Clients should be monitored for signs of liver dysfunction, seizures, depression, and suicidal ideations. No antidote for naltrexone is currently available.
Naltrexone is contraindicated for clients who failed a naltrexone challenge, test positive for opioids or alcohol on drug screening, have a history of seizures, or have experienced a past hypersensitivity reaction to naltrexone.
Clients may switch from buprenorphine or methadone to naltrexone at some point in treatment. Both medications are agonists at the opioid receptor sites, so changing to naltrexone (an antagonist) may increase the risk of withdrawal symptoms for the first two weeks of treatment (6).
Considerations for Prescribers
Because naltrexone does not cause any euphoria or “high,” the abuse potential is non-existent. It is not a controlled substance and can be prescribed by any clinician with prescriptive authority. However, its use is typically only by those who work in mental health or addiction medicine. Clients can take the medication at home or go to the clinic for IM injections.
Many considerations for naltrexone use center around monitoring for side effects and treatment compliance. Baseline and periodic drug screening and liver function tests are prudent. Clients’ support persons should be educated on compliance and signs of relapse. The IM formulation should be considered for those with poor compliance or most at risk for relapse (6).
Ask yourself...
- Why might a client benefit from the IM formulation of naltrexone instead of the oral preparation?
- Why might compliance with an opioid antagonist be more complex than an opioid agonist like methadone or buprenorphine?
- How do side effects differ between naltrexone and the agonist medications like methadone?
- What does it mean if a client fails a “naltrexone challenge,” and how does this delay their care?
- What education should you provide to patients prescribed naltrexone?
Nursing Considerations
Nurses will encounter clients with addiction and even those receiving MAT in a variety of settings, including:
- Outpatient clinics for routine care of any health issues
- ED admission for acute problems not related to addiction.
- Inpatient hospitalization related to other health problems.
- Outpatient setting for participation in MAT or addiction management.
- ED admission for acute problems related to substance abuse or toxicity of MAT medication.
- Inpatient mental health admission for mental health and addiction issues
Regardless of the setting and if the client is being seen for an addiction issue or something else, it is crucial for nurses to be familiar with MAT medications and how they work to provide safe and competent care. Nurses may need to:
- Administer medication.
- Monitor lab results.
- Observe for side effects, toxicity, or withdrawal symptoms.
- Coordinate care within a multidisciplinary team
- Communicate with therapeutic and nonjudgmental techniques.

Ask yourself...
- Have you ever cared for a client in a non-addiction setting who had a MAT medication on their drug list?
- Did you have any biases or preconceived ideas about what this medication meant?
- Is there anything you have learned throughout this course that will change your care the next time you encounter a client receiving MAT?
Case Study
Justin is a 32-year-old male who presents to the ED with nausea, lethargy, and confusion worsening over the last 24 hours. Upon exam, the nurse notes diaphoresis, slurred speech, and pinpoint pupils. His vitals are RR 10, HR 54, BP 82/58, SPO2 97%, Temp 99.0.
He reports taking Wellbutrin 150mg daily for depression and smoking cessation, methadone 100mg daily for history of oxycodone abuse, and was started on ciprofloxacin 250mg BID for a UTI 2 days ago at urgent care.
His labs are significant for a WBC of 15,000 but otherwise regular. He tests positive for methadone, which is expected, but not for other substances. He reports being compliant with MAT and avoiding opioid use for nine months.
It is determined that Justin is experiencing methadone toxicity due to the slowed metabolism of the drug from the combination of methadone and ciprofloxacin. He is given naloxone in the ED, and within an hour, his symptoms have improved significantly, and his vital signs are typical. His antibiotic is switched to cefdinir, and he is discharged home in stable condition with instructions to follow up with his PCP within 1-2 days.
Ask yourself...
- Given Justin’s presentation, how could you differentiate between methadone toxicity and relapse?
- How might Justin’s condition have progressed if he had not sought emergency care?
- How would Justin’s case have been different if he had not tested positive for methadone?
- In what ways could Justin’s care before his ED visit have been improved to avoid this complication?
Conclusion
Substance use disorders are a long-standing and dangerous pathology experienced by millions of people each year. At the same time, the stigma of seeking help for such disorders has been eroding in recent years; there has also been a renewed push by the federal government to address the issue in evidence-based and meaningful ways, with access to effective treatment being at the top of the priority list.
Addiction treatment programs utilizing MAT will likely become much more popular in the coming years, and nurses will be on the front lines of this therapy. For nurses to provide competent and comprehensive care to this client population, up-to-date and accurate knowledge is necessary.
Hypertensive Agents
Introduction
Hypertension, or high blood pressure, is a common medical condition diagnosed and treated by healthcare professionals. According to the Centers for Disease Control and Prevention, around 34 million Americans are prescribed antihypertensive medications. Additionally, hypertension was a primary or contributing cause of more than 690,000 deaths in the United States in 2021 (6).
Healthcare providers must be knowledgeable of and follow current hypertension clinical practice guidelines. Understanding the different pharmacokinetics of antihypertensive medications is essential. This course outlines antihypertensive pharmacology and addresses pharmacokinetics, including mechanism of action, side effects, usage, and contraindications.
Definitions
- Hypertension
- High blood pressure is above normal. Normal is considered anything less than 120/80 mmHg (7).
- Antihypertensives
- Medications used to control hypertension and lower blood pressure (7).
- Hypertensive crisis
- Severely elevated blood pressure of either:
- Systolic pressure greater than 180 mmHg
- Diastolic greater than 120 mmHg (19).
- Severely elevated blood pressure of either:
- Hypertensive Emergency
- Acutely elevated blood pressure with signs of target organ damage (2).
Ask yourself...
- What is hypertension?
- What are antihypertensives?
- What is a hypertensive crisis?
- What is a hypertensive emergency?
Medication Overview
Antihypertensive medications are used for the treatment of hypertension and are used in both inpatient, outpatient, and emergency settings.
Some of the major antihypertensive medication classes include:
- Diuretics
- Beta-blockers
- Angiotensin-converting enzyme inhibitors
- Angiotensin II receptor blockers
- Calcium channel blockers
- Selective alpha-1 blockers
- Alpha-2 Receptor Agonists
- Vasodilators (3).
Different medical organizations have varying recommendations and hypertension treatment guidelines. Hypertension treatment clinical practice guidelines are available from organizations like the American Heart Association, the American College of Cardiology, and the European Society of Cardiology to name a few [21]. Healthcare providers should be aware of their healthcare institution’s recommendations for clinical practice guidelines and organizations.
All organizational guidelines share the same recommended treatment of starting antihypertensives immediately when:
- Blood pressure is greater than 140/90 mmHg for patients with a history of ischemic heart disease, heart failure, or cerebrovascular disease.
- Blood pressure is greater than 160/100 mmHg regardless of underlying medical conditions (21).
Again, healthcare providers should follow current and evidence-based clinical guidelines for initiating or titrating antihypertensive medications.
While most antihypertensives are prescribed in an outpatient setting, certain antihypertensives are indicated during hypertensive or medical emergencies. For example, intravenous (IV) vasodilators, like nitroprusside and nitroglycerin, and calcium channel blockers, like nicardipine, are used during hypertensive emergencies and crises.

Ask yourself...
- In what settings are antihypertensives used?
- What are the clinical guidelines for initiating hypertensive medications?
- Which medications are commonly used to treat hypertensive emergencies?
Diuretics
Diuretics are a class of drugs that help control blood pressure by removing excess sodium and water from the body through the kidneys. There are several types of diuretics, including thiazide, potassium-sparing, and loop, and all work to lower blood pressure differently (3).
Thiazide Diuretics
Thiazide diuretics remove excess sodium and water from the body by blocking the sodium-chloride (Na-Cl) channels in the kidneys’ distal convoluted tubule. As the Na-Cl channel becomes blocked, this inhibits sodium and water reabsorption into the kidneys. Concurrently, this causes a loss of potassium and calcium ions through the sodium-calcium channels and sodium-potassium pump (1).
Thiazide diuretics are approved by the Food and Drug Administration (FDA) for controlling primary hypertension and are available via the oral route. Some common thiazide diuretics are hydrochlorothiazide, chlorthalidone, and metolazone (3).
When initiating this medication, the healthcare provider should start with the lowest dose, usually 25mg daily, and then increase accordingly to aid with blood pressure control or if the patient has excess fluid retention, usually evidenced by leg swelling or edema (1).
Common side effects of thiazide diuretics include:
- Increased urination
- Diarrhea
- Headache
- Stomach and muscle aches (16).
As thiazide diuretics interfere with Na-Cl, Na-Ca, and Na-K channels, there is an increased potential for adverse effects, including:
- Hypotension
- Hypokalemia
- Hyponatremia
- Hypercalcemia
- Hyperglycemia
- Hyperlipidemia
- Hyperuricemia
- Acute pancreatitis
When prescribing thiazide diuretics, healthcare providers should avoid prescribing thiazide diuretics to patients with a sulfonamide allergy, since thiazides are sulfa-containing medications. Also, they should avoid prescribing these to patients with a history of gout [1].
Additionally, patients can experience a thiazide overdose if they take more than the amount prescribed. Patients with a suspected overdose may experience confusion, dizziness, hypotension, and other symptoms. These patients must seek emergency care, and poison control must be alerted [16].
Potassium-Sparing Diuretics
Potassium-sparing diuretics remove excess sodium and water from the body without causing potassium loss. Depending on the type, they interrupt sodium reabsorption by either binding to epithelial sodium channels or inhibiting aldosterone receptors. When catatonic sodium is reabsorbed, this creates a negative gradient, causing the reabsorption of potassium ions through the mineralocorticoid receptor (5).
Potassium-sparing diuretics are approved for controlling hypertension and are usually combined with other diuretics, like thiazide or loop diuretics, since they have a weak antihypertensive effect.
The common names of potassium-sparing diuretics are amiloride, triamterene, and spironolactone. These medications are available by intravenous or oral routes. Spironolactone is commonly used for treating primary aldosteronism and heart failure [5]. When first prescribing this class of medications, patients should be started on the lowest dose.
Common side effects can include:
- Increased urination
- Hyperkalemia
- Metabolic acidosis
- Nausea (4).
Healthcare providers should avoid prescribing this class of medications to patients with hyperkalemia or chronic kidney disease. They should also be avoided during pregnancy or in patients who are taking digoxin. Since potassium-sparing medications can cause hyperkalemia, periodic monitoring for electrolyte imbalances and potassium levels is necessary (4).
Loop Diuretics
Loop diuretics inhibit sodium and chloride reabsorption by competing with chloride binding in the Na-K-2Cl (NKCC2) cotransporter. The kidneys do not reabsorb potassium, which causes additional calcium and magnesium ion loss.
Loop diuretics are FDA-approved for the treatment of hypertension but are not considered first-line treatment. They can also be used for treating fluid overload in conditions like heart failure or nephrotic syndrome (12).
Loop diuretics are available via oral or IV routes, and furosemide, torsemide, and bumetanide are common forms (3).
Bioavailability and dosage differ for each type and route of loop diuretics. The bioavailability of furosemide is 50%, with a half-life of around 2 hours for patients with normal kidney function, and dosages start at 8mg for oral medication. Torsemide has a bioavailability of about 80%, a half-life of about 3 to 4 hours, and oral dosages start at 5mg (12).
Common side effects can include:
- Dizziness
- Increased urination
- Headache
- Stomach upset
- Hyponatremia
- Hypokalemia (13).
Loop diuretics can lead to several adverse effects, including toxicity, electrolyte imbalances, hyperglycemia, and ototoxicity. They have a black box warning stating that high dosages can cause severe diuresis. Therefore, a healthcare provider should closely monitor electrolytes, BUN, and creatinine values.
People with a sulfonamide allergy may also be allergic to loop diuretics, so this should be avoided if the patient is allergic. Loop diuretics also interfere with digoxin and therefore should be avoided. Other contraindications include anuria, hepatic impairment, and use during severe electrolyte disturbances (12).

Ask yourself...
- What is the pharmacokinetics of thiazide diuretics?
- What is the pharmacokinetics of loop diuretics?
- What is the pharmacokinetics of potassium-sparing diuretics?
- What are the common side effects and contraindications for each type of diuretic?
- What education would you provide to a patient being started on diuretics?
Beta-Blockers
Beta-blockers work by reducing the body’s heart rate and thus lowering cardiac output, resulting in lowered blood pressure (3). The mechanism of action for beta-blockers varies, depending on the receptor type it blocks, and are classified as either non-selective or beta-1 (B1) selective.
Non-selective beta-blockers bind to the B1 and B2 receptors, blocking epinephrine and norepinephrine, causing a slowed heart rate. Propranolol, labetalol, and carvedilol are common non-selective beta-blockers.
Alternatively, beta-1 selective blockers only bind to the B1 receptors of the heart, so they are considered cardio-selective. Some examples include atenolol, metoprolol, and bisoprolol. Sotalol is a type of beta-blocker that also blocks potassium channels and is, therefore, a class III antiarrhythmic (8).
Beta-blockers are not primarily used for the initial treatment of hypertension, but can be prescribed for conditions like tachycardia, myocardial infarction, congestive heart failure, and cardiac arrhythmias. It’s also approved for use in conditions such as essential tremors, hyperthyroidism, glaucoma, and prevention of migraines.
Beta-blockers are available in many forms, including oral, IV, intramuscular injection, and ophthalmic drops. Starting dosage and route are determined by the health condition being treated (8).
Common side effects of beta-blockers include:
- Bradycardia
- Hypotension
- Dizziness
- Feeling tired
- Nausea
- Dry mouth
- Sexual Dysfunction (17)
This class of medications can also lead to more severe adverse effects, such as orthostatic hypotension, bronchospasm, shortness of breath, hyperglycemia, and increased risk of QT prolongation, torsades de pointes, and heart block (8). Healthcare providers should avoid prescribing non-selective beta-blockers to patients with asthma. Instead, they can prescribe cardio-selective beta-blockers.
Additionally, the use of beta-blockers is contraindicated in patients with a history of bradycardia, hypotension, Raynaud disease, QT prolongation, or torsades de pointes. Healthcare providers must encourage patients to monitor their heart rate and blood pressure and follow administration parameters before taking beta-blockers daily, since it decreases their heart rate.
Overdose of beta-blockers is life-threatening, and healthcare providers must discuss the symptoms of an overdose and the need for emergency care (8).
Ask yourself...
- What is the pharmacokinetics of beta-blockers?
- What are the common side effects and contraindications of beta-blockers?
- What education would you provide to a patient being started on beta-blockers?
Angiotensin-Converting Enzyme Inhibitors
Angiotensin-converting enzyme (ACE) inhibitors prevent the body from producing angiotensin, a hormone that causes vasoconstriction. As angiotensin production is reduced, the blood vessels dilate, lowering blood pressure (3).
Moreover, ACE inhibitors act specifically on the renin-angiotensin-aldosterone system (RAAS) by preventing the conversion of angiotensin I to angiotensin II. They also decrease aldosterone, which in turn decreases sodium and water reabsorption (9).
ACE inhibitors usually end in the suffix—pril. Some common examples include lisinopril, benazepril, enalapril, and captopril, which also typically end in the suffix (3).
While ACE inhibitors are approved for treating hypertension, they are also FDA-approved for other uses or combination therapies for medical conditions such as:
- Systolic heart failure
- Chronic kidney disease
- ST-elevated myocardial infarction
One non-approved FDA use is treating diabetic nephropathy [9]. This class of medication is available in oral and IV forms, and dosages depend on clinical guidelines, underlying medical conditions, and route.
ACE inhibitors have common side effects, with some including:
- Dry cough
- Dizziness
- Hypotension (9)
This medication can also lead to adverse effects, such as syncope, angioedema, and hyperkalemia (9). As angioedema is an adverse effect, healthcare providers should understand that this class of medications is contraindicated in patients with a history of hypersensitivity to ACE inhibitors.
Additionally, ACE inhibitors are contraindicated in patients with aortic valve stenosis, hypovolemia, and during pregnancy. Individuals with abnormal kidney function should have renal function and electrolyte values monitored. If a patient develops a chronic dry cough, the healthcare provider should consider another antihypertensive medication class by following current guidelines (9).
Ask yourself...
- What is the pharmacokinetics of angiotensin-converting enzyme inhibitors?
- What are the common side effects and contraindications of angiotensin-converting enzyme inhibitors?
- What education would you provide to a patient being started on ACE Inhibitors?
Angiotensin II Receptor Blockers
Like ACE inhibitors, Angiotensin II Receptor Blockers (ARBs) act on the RAAS by binding to angiotensin II receptors, thus blocking and reducing the action of angiotensin II. Again, this lowers blood pressure by causing blood vessel dilation and decreasing sodium and water reabsorption [11]. ARBs typically end in the suffix -artan; common names are losartan, valsartan, and Olmesartan (3). Oral and IV routes of the medication are available, and again, dosages depend on the medication specifically and form (11).
All ARBs are FDA-approved for the treatment of hypertension, but a select few are approved for treating other medical conditions, such as:
- Candesartan for heart failure
- Irbesartan for diabetic nephropathy
- Losartan for proteinuria and diabetic nephropathy
- Telmisartan for stroke and myocardial infarction prevention
- Valsartan for heart failure and reduction of mortality in patients with left ventricular dysfunction (11).
Although not as common as ACE inhibitors, two side effects of ARBs are dry cough and angioedema.
Other common side effects include:
- Dizziness
- Hypotension
- Hyperkalemia (11).
Contraindications for use are pregnancy and renal impairment or failure. If a patient is on an ARB, the healthcare provider should closely monitor lab values for electrolyte imbalances and kidney function.
Additionally, if a patient is taking lithium, ARBs can increase lithium concentration, and therefore, lithium blood concentration should be frequently checked [11].
Ask yourself...
- What is the pharmacokinetics of angiotensin II receptor blockers?
- What are the common side effects and contraindications of angiotensin II receptor blockers?
- What education would you provide to a patient starting angiotensin II receptor blockers?
Calcium Channel Blockers
Calcium channel blockers (CCBs), also known as calcium channel antagonists, act by preventing calcium from entering the smooth vascular and heart muscles. In turn, this reduces heart rate and causes vasodilation (3).
They are further divided into two major categories, non-dihydropyridines and dihydropyridines, where there are differences in the mechanism of action. Non-dihydropyridines inhibit calcium from entering the heart’s sinoatrial and atrioventricular nodes and thus cause a cardiac conduction delay and reduce cardiac contractility.
Alternatively, dihydropyridines do not directly affect the heart but act as a peripheral vasodilator, lowering blood pressure. Both categories are metabolized by the CYP3A4 pathway (15).
Names of non-dihydropyridine CCBs are verapamil and diltiazem. Dihydropyridine CCBs typically end in the suffix -pine; common names are amlodipine and nicardipine. Both categories are available via oral and IV routes for administration. Oral dosages of non-dihydropyridine CCBs start at 30mg daily, and dihydropyridine CCBs begin at 30mg daily for immediate release (15).
Calcium channel blockers can be used to treat other medical conditions in addition to hypertension, including:
- Coronary spasm
- Angina pectoris
- Supraventricular dysrhythmias
- Pulmonary hypertension
- Hypertrophic cardiomyopathy
Non-dihydropyridine CCBs can cause side effects like bradycardia and constipation, while dihydropyridine CCBs can cause:
- Headaches
- Feeling lightheaded
- Leg swelling (15).
Both categories pose the risk of potential hypotension and bradycardia, so healthcare providers should closely monitor the patient’s blood pressure and heart rate when initiating or titrating the dosage.
Also, an overdose of this medication can lead to cardiac conduction delays, complete heart block, and cardiovascular collapse. Patients with possible symptoms of overdose should be sent to the emergency room immediately.
Additionally, healthcare providers should avoid prescribing CCBs to people with heart failure and sick sinus syndrome (15).
Ask yourself...
- What is the pharmacokinetics of calcium channel blockers?
- What are the common side effects and contraindications of calcium channel blockers?
- What education would you provide to a patient being started on calcium channel blockers?
Selective Alpha-1 Blockers
Selective alpha-1 blockers lower blood pressure by acting on the body’s sympathetic nervous system. They prevent norepinephrine from binding to the sympathetic nervous system’s alpha-1 receptors, causing smooth muscle relaxation and vasodilation, which leads to lowered blood pressure [18].
Selective alpha-1 blockers, which end in the suffix—sin, are available via the oral route. Examples are doxazosin, terazosin, and prazosin [3]. They are FDA-approved for the treatment of hypertension but are not considered first-line therapy. Additionally, this class of medications may be used to treat benign prostatic hyperplasia. Dosages can start as low as 1mg daily, depending on the drug selected.
Common side effects include:
- Hypotension
- Tachycardia
- Dizziness
- Headache
- Weakness (18).
As selective alpha-1 blockers can lead to orthostatic hypotension, the healthcare provider should instruct the patient to take this medication at night. They should also avoid prescribing to the elderly population when able because of hypotension and increased fall risk (18).
- What is the pharmacokinetics of alpha-1 blockers?
- What are the common side effects and contraindications of alpha-1 blockers?
- What education would you provide to a patient being started on alpha-1 blockers?
Alpha-2 Receptor Agonists
Alpha-2 receptor agonists work by decreasing the activity of the sympathetic nervous system to lower blood pressure. They inhibit adenylyl cyclase and decrease the formation of cyclic adenosine monophosphate (cAMP). Alpha-2 agonists also cause vasodilation by reducing the amount of available cytoplasmic calcium [20].
This class of medications is typically administered via the oral route but is also available in intravenous and transdermal forms. Two FDA-approved alpha-2 agonists for hypertension treatment are methyldopa and clonidine, and dosages are dependent on the name and route.
Methyldopa is commonly prescribed to patients with hypertension who are pregnant since it’s safe (20).
Common side effects of alpha-2 receptor agonists are:
- Dry mouth
- Drowsiness
- Fatigue
- Headache
- Sexual dysfunction (3).
Contraindications for use are orthostatic hypotension and autonomic disorders. Healthcare providers must avoid prescribing alpha-2 receptor agonists to individuals taking phosphodiesterase inhibitors (20).
Ask yourself...
- What is the pharmacokinetics of alpha-2 receptor agonists?
- What are the common side effects and contraindications of alpha-2 receptor agonists?
- What education would you provide to a patient started on alpha-2 receptor agonists?
Vasodilators
Vasodilators lower blood pressure by dilating the body’s blood vessels. They bind to the receptors of the blood vessel’s endothelial cells, releasing calcium. Calcium stimulates nitric oxide synthase (NO synthase), eventually converting to L-arginine to nitric oxide. As nitric oxide is available, this allows for GTP to convert to cGMP and causes dephosphorylation of the myosin and actin filaments. As this occurs, the blood vessels’ smooth muscles relax, leading to vasodilation and lowered blood pressure.
Nitrates and minoxidil are common vasodilators that act via this pathway. Hydralazine is another vasodilator, but its mechanism of action is unknown (10).
Sublingual, oral, and intravenous vasodilators are available. Like other classes of antihypertensives, vasodilator dosages depend on the form and treatment setting (10).
Nitrovasodilators like nitroprusside and nitroglycerin are used during hypertensive emergencies. Hydralazine is used for severe hypertension to prevent eclampsia or intracranial hemorrhage, and minoxidil is used for resistant hypertension (10)(3).
Side effects for each will vary, but nitrates commonly cause:
- Reflex tachycardia
- Headache
- Orthostatic hypotension (10)
Common side effects of hydralazine are headaches, heart palpitations, and myalgias. Minoxidil causes excessive hair growth, weight gain, and fluid retention (3). Additionally, nitroprusside can potentially cause cyanide toxicity.
Vasodilators have varying degrees of contraindications. Nitrates are avoided in patients with inferior myocardial infarction. Hydralazine should not be given to patients with coronary artery disease, angina, or rheumatic heart disease. Healthcare providers should be aware of contraindications and monitor patients’ blood pressure and potential side effects (10).
Combination Antihypertensives
Many antihypertensive medications, such as ACE inhibitors and thiazide diuretics, beta-blockers and diuretics, or calcium channel blockers and ACE inhibitors, come in combined forms. The mechanism of action for combination antihypertensives depends on the blend of medications (3).
Ask yourself...
- What is the pharmacokinetics of vasodilators?
- What education would you provide to a patient being started on vasodilators?
- What are the common side effects and contraindications of vasodilators?
Considerations for Prescribers
When prescribing antihypertensive medications, healthcare providers must consider several factors. The route is typically determined by the healthcare setting, and the dosage is determined by the underlying treatment goals. Again, healthcare providers should follow current guidelines when initiating or titrating antihypertensive medications.
Healthcare providers must complete a thorough health history and review lab values and contraindications. Monitoring kidney function and electrolyte values is imperative for patients taking antihypertensive medications.
While a single antihypertensive medication is recommended for initial treatment, there are some scenarios where combination therapy or combination antihypertensives are recommended (14).
Healthcare providers should also discuss the potential side effects of antihypertensives with patients and what to do if they are experiencing symptoms. For instance, if patients report syncope, they should be advised to go to the emergency room or be seen immediately for further evaluation. Also, healthcare providers must encourage patients to monitor their heart rate and blood pressure at home and abide by the administration parameters.
For example, patients taking beta-blockers should be instructed to measure their blood pressure and heart rate before taking their medication. They should not take the medication if their heart rate is below 60 beats per minute (14).
If a patient is experiencing side effects from an antihypertensive medication, then another alternative should be selected.
Ask yourself...
- What factors should healthcare providers consider when prescribing antihypertensives?
- What are the barriers to providing education to patients that you have encountered? How did you overcome those barriers?
- What precautions do you place patients on who are on a newly prescribed antihypertensive?
- What interventions can you encourage for patients who are at an increased risk of falls from taking antihypertensives?
- What resources are available to your patients to help them pay for their medications?
Upcoming Research
Research on antihypertensive medications has slowed throughout the years. Some clinical trials were performed on the potential of endothelin receptor antagonists to reduce hypertension. However, some studies found several unwanted side effects, and thus, clinical use was stopped for safety reasons.
An endothelin-A and endothelin-B receptor blocker, aprocinentan, has shown promise for treating resistant hypertension by lowering blood pressure and decreasing vascular resistance.
Research on sodium-glucose transport protein (SGLT2) inhibitors, which are typically used to treat type II diabetes mellitus, is also ongoing. SGLT2 inhibitors may promote blood pressure reduction through diuresis and reduce sympathetic tone (21).
Ask yourself...
- What new research is there about antihypertensives?
- How have you seen the use of antihypertensives change over time?
Conclusion
If hypertension is left untreated, it can lead to serious health complications, including death. When selecting antihypertensive treatment, healthcare providers should understand each drug class’s pharmacokinetics, potential side effects, and contraindications. They should also follow current clinical guidelines for an evidence-based approach.
Ask yourself...
- Which antihypertensive medication is often prescribed during pregnancy?
- Which lab values are important when monitoring patients on each antihypertensive medication?
- Which antihypertensive medications cause hypokalemia?
- Which antihypertensive medications cause hyperkalemia?
Migraine Management
Introduction
This course will guide you on a journey to unravel the complexities of migraines and empower you with the knowledge and skills needed for effective pharmaceutical interventions.
According to recent studies by (34), migraines involve changes in brain activity, neurotransmitter levels, and vascular function, resulting in throbbing headaches accompanied by sensitivity to light and sound. Diverse classes of migraine medications are available, such as Triptans and calcitonin gene-related peptide (CGRP) inhibitors. Understanding their mechanisms of action enables healthcare professionals to make informed decisions in prescribing and administering treatments (18).
Furthermore, recent research by (26) emphasizes the importance of recognizing warnings related to migraine medications. In this course, we will learn the details of medications for effective migraine management and delve into ways of ensuring patient safety when dealing with them.
Ask yourself...
- How might a comprehensive knowledge of migraine medications, including their mechanisms of action, enhance the ability of healthcare professionals to address the specific needs of patients experiencing migraines?
- In what ways can recognizing warnings related to migraine medications contribute to ensuring patient safety?
- What is your facilities current protocol for managing patients with a migraine?
Definition
Migraines are recurrent, pulsating headaches often accompanied by other symptoms such as nausea, sensitivity to light, and sensitivity to sound. According to recent studies by (17), migraines are recognized as a complex neurological disorder involving abnormal brain activity and a cascade of events leading to pain and associated symptoms.
The impact of migraines extends beyond physical pain, influencing various aspects of life, and recent literature by (14) highlights the profound effect on the quality of life, with disruptions in daily activities, work, and social interactions. For example, a professional experiencing frequent migraines might struggle to meet work deadlines and engage in social events. Recent advancements in diagnostic criteria by (15) emphasize the importance of a precise definition to ensure appropriate treatment strategies. Therefore, understanding the definition is crucial for accurate diagnosis and effective communication between healthcare providers and patients.

Ask yourself...
- What distinguishes migraines from common headaches, and how does understanding this difference impact the approach to their management?
- In what ways do migraines extend beyond physical pain, and how might this impact influence an individual’s overall quality of life?
- How can a precise definition of migraines contribute to accurate diagnosis, and why is accurate diagnosis essential for effective treatment planning?
- How does understanding the definition of migraines facilitate effective communication between healthcare professionals and patients?
- What is the most common symptom you have seen in patients with a migraine?
Migraine Medications
Understanding the various classes of migraine medications is like having a diverse toolkit to address the complexities of this neurological disorder. By exploring the different classes of drugs, healthcare professionals can tailor their approach and make informed decisions based on individual patient profiles and specific migraine characteristics (20).
It is crucial to recognize that migraine medications are not one-size-fits-all. Each patient is unique, and their response to medications may vary. Recent literature by (12) emphasizes the importance of an individualized approach when considering the best medication for each patient. Here’s a list of migraine medications in addition to important details to consider:
Triptans
Triptans are a class of medications specifically designed for the acute treatment of migraines. According to (39), they are not meant for preventive use but are highly effective in providing relief during an ongoing migraine attack. They work by narrowing blood vessels and inhibiting the release of certain chemicals in the brain associated with migraine symptoms (39). Let’s see more details below as described by (7), (39), and (29).
Drug Class
Triptans belong to the serotonin (5-HT) receptor agonist class and modulate the effects of serotonin receptors in the brain. The various types of Triptans include Sumatriptan, Rizatriptan, Eletriptan, and others. Each Triptan has unique characteristics, such as the onset of action and duration, allowing healthcare professionals to tailor prescriptions based on individual patient needs.
Benefits
Triptans offer several benefits in the management of migraines. One primary advantage is their ability to provide rapid and effective relief from migraine symptoms, including headache pain, nausea, and sensitivity to light and sound. The prompt onset of action is particularly valuable for individuals aiming to resume their daily activities quickly. Triptans are available in various formulations, including oral tablets, nasal sprays, and injectables, allowing for flexibility in administration.
Side Effects
While generally well-tolerated, Triptans may cause side effects. Common side effects include mild sensations of warmth or tingling, dizziness, and tightness or pressure in the chest. Healthcare professionals must consider the patient’s medical history and potential contraindications, such as cardiovascular issues, before prescribing Triptans. In rare cases, more severe side effects like chest pain and changes in heart rate may occur, necessitating immediate medical attention.
Clinical Effects
The clinical effects of Triptans are profound, offering relief to individuals experiencing acute migraine attacks. The primary outcomes include:
- Pain Relief: Triptans are highly effective in reducing the intensity of migraine-associated pain. By targeting migraines’ vascular and neuronal components, these drugs provide rapid relief, allowing patients to resume their normal activities.
- Relief of Associated Symptoms: Beyond pain relief, Triptans address accompanying symptoms such as nausea, photophobia, and phonophobia. This comprehensive effect enhances the overall patient experience during a migraine episode.
- Prevention of Migraine Progression: Triptans, when administered early in the migraine attack, can prevent the progression of the headache phase to more severe stages. This early intervention is crucial for optimizing outcomes and minimizing the impact of migraines on daily life.
- Improvement in Functional Impairment: Migraines often result in functional impairment, limiting individuals’ ability to perform daily tasks. Triptans restore functional capacity, allowing patients to regain control over their activities.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs, commonly known as NSAIDs, constitute a class of medications used in the treatment of migraines. According to (6), these drugs are characterized by their anti-inflammatory, analgesic, and antipyretic properties. NSAIDs are versatile, as they are not exclusively used for migraines but are also used for various other pain and inflammatory conditions (6). Let’s see more details below as described by (25), (40), (6), and (28).
Drug Class
NSAIDs encompass a broad class of medications, including well-known examples such as ibuprofen, naproxen, and aspirin. They function by inhibiting enzymes called cyclooxygenases (COX), thereby reducing the production of inflammatory prostaglandins. This mechanism provides relief from pain and mitigates inflammation associated with migraines.
Benefits
The primary benefit of NSAIDs in migraine management lies in their ability to alleviate pain and reduce inflammation. They are particularly effective for individuals experiencing mild to moderate migraines. NSAIDs offer a rapid onset of action, making them suitable for individuals seeking prompt relief. Additionally, these medications are available over the counter in many formulations, providing patient accessibility.
Side Effects
While NSAIDs are generally well-tolerated, they may cause side effects, especially with prolonged or excessive use. Common side effects include gastrointestinal issues such as stomach upset or ulcers. Healthcare professionals need to consider a patient’s medical history, including conditions like gastric ulcers, before prescribing NSAIDs. In rare cases, more severe side effects like cardiovascular events may occur, emphasizing the importance of cautious use.
Clinical Effects
The clinical effects of NSAIDs in migraine management encompass various aspects. Here’s a list of some of them.
- Pain Relief: NSAIDs are effective in providing pain relief during acute migraine attacks. Reducing prostaglandin levels alleviates headache symptoms and contributes to the overall comfort of individuals experiencing migraines.
- Inhibition of Inflammatory Responses: The anti-inflammatory properties of NSAIDs are particularly beneficial when migraines are associated with inflammatory processes. NSAIDs help mitigate inflammation, reducing the severity and duration of migraine attacks.
- Improvement in Associated Symptoms: Beyond pain relief, NSAIDs address associated symptoms such as nausea and photophobia, enhancing the overall patient experience during a migraine episode.
- Prevention of Migraine Progression: When administered early in the migraine attack, NSAIDs can prevent the progression of headaches to more severe stages. This early intervention is critical for optimizing outcomes and minimizing the impact of migraines on daily life.
Calcitonin Gene-Related Peptide (CGRP) Inhibitors
Calcitonin gene-related peptide (CGRP) inhibitors represent a modern class of medications revolutionizing the landscape of migraine management. According to (11), these drugs specifically target CGRP, a neuropeptide involved in dilating blood vessels and transmitting pain signals. By inhibiting CGRP, these inhibitors aim to modulate migraine pathways and reduce the frequency and severity of attacks (11). Let’s see more details below as described by (23) and (11).
Drug Class
CGRP inhibitors are a unique drug class designed explicitly for migraine prevention. Examples include Erenumab, Fremantumab, and Galcanezumab. These medications are administered via subcutaneous injections, typically monthly or quarterly. The focus on preventive therapy distinguishes CGRP inhibitors from acute treatment options like Triptans.
Benefits
The primary benefit of CGRP inhibitors lies in their efficacy in preventing migraines. Clinical trials have demonstrated a significant reduction in the frequency of monthly migraine attacks among individuals using CGRP inhibitors. This preventive approach is especially valuable for those with frequent and debilitating migraines, offering a chance to enhance their quality of life.
Moreover, CGRP inhibitors are well-tolerated with fewer side effects than preventive medications. They provide a targeted and specific intervention, addressing the underlying mechanisms of migraines without causing widespread effects on other bodily functions.
Side Effects
While generally well-tolerated, CGRP inhibitors may have some side effects. Local injection site reactions, such as redness or swelling, are common but typically mild. Healthcare professionals must monitor and address any adverse effects promptly. Additionally, ongoing research is essential to understand these medications’ long-term safety profile further.
Clinical Effects
The clinical effects of CGRP inhibitors are transformative in migraine management, offering a novel approach to prevention. Primary clinical effects include the following:
- Reduction in Migraine Frequency: One of the hallmark effects of CGRP inhibitors is a significant reduction in the frequency of migraine attacks. By consistently blocking CGRP receptors, these medications disrupt the migraine cascade, leading to a sustained preventive effect.
- Improvement in Migraine Severity: CGRP inhibitors not only reduce the frequency but also contribute to a decrease in the severity of migraine attacks. This comprehensive effect enhances the overall quality of life for individuals suffering from chronic migraines.
- Enhanced Functional Capacity: Migraines often result in functional impairment, limiting individuals’ ability to perform daily tasks. CGRP inhibitors restore functional capacity, allowing patients to regain control over their activities and participate more fully in their daily lives.
- Well-Tolerated Profile: CGRP inhibitors are generally well-tolerated, with a favorable side effect profile. This characteristic enhances patient adherence to preventive treatment, a critical factor in long-term migraine management.
Beta-Blockers
Beta-blockers are a class of medications that have found a significant place in migraine management. Initially developed for cardiovascular conditions, beta-blockers have demonstrated efficacy in preventing migraines by reducing the frequency and severity of attacks (32). According to (32), these medications work by blocking the effects of adrenaline, leading to reduced heart rate and blood pressure. Let’s see more details below as described by (32) and (27).
Drug Class
Beta-blockers encompass various medications, with examples such as propranolol, metoprolol, and timolol commonly prescribed for migraine prevention. These drugs fall into the broader category of antihypertensive medications but are repurposed for their preventive benefits in migraine care. Unlike acute treatments, which provide relief during an ongoing attack, beta-blockers are taken regularly to reduce the overall occurrence of migraines.
Benefits
Beta-blockers’ primary benefit in migraine management is their preventive action. Clinical studies have shown that they can significantly reduce the frequency of migraines, making them particularly suitable for individuals with chronic or frequent attacks. This preventive approach aims to enhance the overall quality of life for those who experience migraines regularly.
Beta-blockers are especially beneficial for individuals with comorbid conditions such as hypertension or heart disease. By addressing both cardiovascular concerns and migraines, these medications offer a comprehensive therapeutic approach.
Side Effects
While generally well-tolerated, beta-blockers may cause side effects that individuals must be aware of. Common side effects include fatigue, dizziness, and changes in sleep patterns. Healthcare professionals must monitor patients regularly, adjust the dosage, or consider alternative medications if side effects become problematic. Beta-blockers are typically avoided in individuals with certain heart conditions, emphasizing the importance of an individualized approach.
Clinical Effects
The clinical effects of beta-blockers in migraine management encompass various dimensions. See some examples below:
- Reduction in Migraine Frequency: Beta-blockers are known for significantly reducing the frequency of migraine attacks. This preventive effect is especially valuable for individuals experiencing chronic migraines, enhancing their overall quality of life.
- Alleviation of Migraine Severity: Beyond frequency reduction, beta-blockers contribute to a decrease in the severity of migraine attacks. This comprehensive effect enhances the overall comfort of individuals during migraine episodes.
- Improvement in Associated Symptoms: Beta-blockers have been shown to address associated symptoms such as nausea and sensitivity to light. By modulating the autonomic nervous system, these medications offer a holistic approach to migraine management.
- Cardiovascular Benefits: Beta-blockers provide additional benefits for individuals with comorbidities due to their primary use in cardiovascular conditions. This dual action allows for comprehensive management of both migraine and cardiovascular health.
Anticonvulsants
Anticonvulsants, originally developed to control seizures in epilepsy, have emerged as a valuable class of medications in the preventive management of migraines (9). According to (9), these drugs, also known as antiepileptic drugs (AEDs), work by stabilizing electrical activity in the brain and reducing the frequency and severity of migraine attacks. Let’s see more details below as described by (32) and (9).
Drug Class
Anticonvulsants comprise a diverse class of medications, including Topiramate, Valproic acid, and Gabapentin. While their primary use may be in epilepsy, the preventive benefits of certain anticonvulsants extend to migraines. These medications are taken regularly to provide ongoing protection against migraines.
Benefits
The primary benefit of anticonvulsants in migraine management is their preventive action. Clinical trials have demonstrated the efficacy of certain anticonvulsants, such as topiramate, in significantly reducing the frequency of migraines. This preventative approach is particularly suitable for individuals with chronic or frequent attacks, aiming to improve overall quality of life.
Anticonvulsants are especially valuable for individuals who may not find relief or experience intolerable side effects with other preventive medications. This drug class’s versatility allows healthcare professionals to tailor treatment plans based on individual patient characteristics and responses.
Side Effects
While generally well-tolerated, anticonvulsants may cause side effects that individuals must be aware of. Common side effects include drowsiness, dizziness, and gastrointestinal disturbances. Healthcare professionals must monitor patients regularly, adjust the dosage, or consider alternative medications if side effects become problematic. Additionally, certain anticonvulsants may have specific considerations, such as monitoring liver function in individuals taking Valproic acid regularly.
Clinical Effects
The clinical effects of anticonvulsants in migraine management encompass the following dimensions:
- Reduction in Migraine Frequency: Anticonvulsants are known for their ability to reduce the frequency of migraine attacks significantly. This preventive effect is particularly valuable for individuals experiencing chronic migraines, substantially improving their overall quality of life.
- Alleviation of Migraine Severity: Beyond frequency reduction, anticonvulsants contribute to a decrease in the severity of migraine attacks. This comprehensive effect enhances the overall comfort of individuals during migraine episodes.
- Improvement in Associated Symptoms: Anticonvulsants address associated symptoms such as nausea and sensitivity to light by modulating neurotransmission and neuronal excitability. This holistic approach contributes to a more comprehensive management of migraines.
- Beneficial in Comorbid Conditions: Due to their broader neurological effects, anticonvulsants can be helpful for individuals with comorbid conditions such as epilepsy or mood disorders. This dual benefit allows for comprehensive management and improves overall well-being.
Ask yourself...
- How do Triptans contribute to migraine management, and in what scenarios might healthcare professionals prioritize their use during acute migraine attacks?
- Can you differentiate the mechanisms of action between NSAIDs and Triptans in migraine management, and how might this understanding influence the choice of medication for a specific patient?
- What roles do CGRP inhibitors play in migraine pharmacotherapy?
- Consider a scenario where a patient experiences migraines with comorbid cardiovascular issues. How might the choice of medication be influenced by the need to prioritize both migraine relief and cardiovascular safety?
- What education would you provide to a patient prescribed a triptan medication?
Clinical Criteria for Prescribing
In migraine management, prescribing medications involves a comprehensive understanding of clinical criteria to tailor interventions effectively. This section explores the clinical factors guiding the prescription of migraine medications and the decision-making process for healthcare providers. Here are some of the factors.
Frequency and Severity of Migraine Attacks
An essential consideration in prescribing migraine medications is the frequency and severity of migraine attacks experienced by the patient. For instance, a patient suffering from frequent and severe attacks may benefit from preventive medications to reduce the overall frequency and intensity of migraines (31).
Individual Response to Pain and Associated Symptoms
The subjective experience of pain and associated symptoms during migraines varies among individuals. A patient who experiences intense nausea and vomiting may require medications with rapid onset and alternative formulations, such as nasal sprays or injectables, to address these specific symptoms effectively (8).
Impact on Daily Functioning and Quality of Life
Prescribing migraine medications involves considering the impact of migraines on a patient’s daily functioning and overall quality of life (24). For example, a working professional with migraines that significantly impede productivity may require acute medications with fast-acting formulations for quick relief during work hours.
Comorbid Conditions and Patient Preferences
Comorbid conditions and patient preferences are pivotal factors in prescribing migraine medications. A patient with comorbid cardiovascular issues may require careful consideration of medication options to mitigate potential risks (2).
Pharmacokinetics
Understanding the pharmacokinetics of migraine management medications enables healthcare professionals to tailor treatment plans based on individual patient characteristics, ensuring maximum therapeutic benefit. Let’s get into more details for each of the medications listed above:
Triptans
Absorption
Triptans exhibit distinct pharmacokinetic properties that influence their efficacy and onset of action. Following oral administration, Triptans are absorbed through the gastrointestinal tract, and the rate of absorption varies among different Triptans, contributing to differences in their clinical profiles. (39)
Distribution
Upon absorption, Triptans undergo distribution to reach target sites in the body, primarily the central nervous system. Their lipophilic nature allows them to penetrate the blood-brain barrier, enabling interaction with serotonin receptors implicated in migraine pathophysiology. The distribution of Triptans influences their ability to exert effects centrally and peripherally. (39)
Metabolism
Metabolism is a crucial aspect of triptan pharmacokinetics, occurring predominantly in the liver. The enzyme responsible for triptan metabolism is monoamine oxidase-A (MAO-A). (39)
Excretion
The final phase in the pharmacokinetic journey of Triptans is excretion, primarily through renal and biliary routes. Renal excretion eliminates the unchanged drug and its metabolites, while biliary excretion expels metabolites via the bile into the gastrointestinal tract. The interplay between metabolism and excretion contributes to the overall pharmacokinetic profile of Triptans. Variations in renal function may influence the elimination of the half-life of certain Triptans, impacting the duration of their therapeutic effect. (39)
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Absorption
Following oral administration, NSAIDs are absorbed in the gastrointestinal tract, with the rate and extent varying among different agents. For instance, ibuprofen exhibits rapid absorption, making it suitable for prompt relief during acute migraine attacks. On the other hand, naproxen has a longer duration of action due to slower absorption, making it well-suited for sustained pain relief. (28)
Distribution
Upon absorption, NSAIDs embark on a journey of distribution throughout the body. Their lipophilic nature allows for penetration into various tissues, including inflamed areas. The distribution influences the drug’s ability to reach target sites, such as the central nervous system, where NSAIDs exert their analgesic and anti-inflammatory effects. This property is particularly relevant in the context of migraines, where the inflammatory component contributes to pain. (28)
Metabolism
Metabolism plays a role in shaping the pharmacokinetic profile of NSAIDs, occurring primarily in the liver. Enzymes such as cytochrome P450 contribute to the biotransformation of NSAIDs into metabolites. The metabolism of NSAIDs can vary among individuals, impacting factors such as drug efficacy and potential side effects. For example, the metabolism of certain NSAIDs, like diclofenac, can be influenced by genetic polymorphisms, contributing to interindividual variability in drug response. (21)
Excretion
The final phase of the NSAID journey involves excretion, predominantly through the kidneys. Unchanged NSAIDs and their metabolites are eliminated via urine. Considerations of renal function are crucial in the context of NSAID use, as impaired kidney function can lead to prolonged drug half-life and increased risk of adverse effects. Regular monitoring of renal function is essential, especially in individuals with conditions that may affect kidney health. (21)
Calcitonin Gene-Related Peptide (CGRP)
Absorption
Administered via subcutaneous injections, CGRP inhibitors such as Erenumab and Fremanezumab enter the bloodstream directly, allowing for precise control over drug levels. This mode of administration ensures a reliable and consistent absorption rate, contributing to the predictability of therapeutic outcomes. (11)
Distribution
Following absorption, CGRP inhibitors are distributed throughout the body, focusing on target sites implicated in migraine pathophysiology, like the central nervous system. (11)
Metabolism
Unlike many traditional medications, CGRP inhibitors follow a different path in terms of metabolism. Due to their biotechnological origin as monoclonal antibodies, these drugs do not undergo significant hepatic metabolism. Instead, proteolytic enzymes break them down into smaller peptides and amino acids, which occur systemically. This unique metabolic pathway aligns with the specificity of CGRP inhibitors, minimizing interactions with hepatic enzymes and potential drug-drug interactions. (37)
Excretion
The final phase of the CGRP inhibitor journey involves excretion, primarily through the kidneys via renal clearance. This aspect is particularly relevant when considering individual patient factors such as renal function, as impaired kidney function can affect the clearance of CGRP inhibitors and influence their duration of action. (37)
Beta-Blockers
Absorption
Administered orally, beta-blockers like propranolol and metoprolol are absorbed through the gastrointestinal tract. Depending on the condition of the gastrointestinal tract, the rate and extent of absorption can vary, impacting the time it takes for these medications to reach therapeutic levels in the bloodstream. (27)
Distribution
Once absorbed, beta-blockers embark on distribution throughout the body. Their lipophilic nature enables penetration through cell membranes, allowing them to reach target tissues, including the heart and blood vessels. In the context of migraine management, the distribution properties of beta-blockers are crucial for their ability to modulate the autonomic nervous system centrally and peripherally, leading to the desired preventive effects against migraines. (27)
Metabolism
The metabolism of beta-blockers occurs primarily in the liver, where enzymes play a role in their biotransformation. Genetic polymorphisms in these enzymes can contribute to interindividual variability in drug metabolism. (27)
Excretion
The final phase of the beta-blocker journey involves excretion, predominantly through the kidneys, where unchanged beta-blockers and their metabolites are eliminated via urine. The renal excretion of these drugs is relevant when considering individual patient factors, such as renal function, as impaired kidney function can affect the clearance of beta-blockers and influence their duration of action. (27)
Anticonvulsants
Absorption
Typically administered orally, anticonvulsants like topiramate and valproic acid are absorbed through the gastrointestinal tract. The rate and extent of absorption play a crucial role in determining the onset of action and overall effectiveness. (9)
Distribution
Following absorption, anticonvulsants undergo distribution throughout the body. Their lipophilic nature allows them to penetrate the blood-brain barrier, reaching target sites in the central nervous system relevant to migraine pathophysiology. The distribution properties of anticonvulsants contribute to their ability to modulate neuronal excitability centrally and exert preventive effects against migraines. (9)
Metabolism
Metabolism is a crucial aspect of anticonvulsant pharmacokinetics. This occurs predominantly in the liver and enzymes play an important role in the biotransformation of anticonvulsants into metabolites. The metabolism of anticonvulsants can vary among individuals, impacting factors such as drug efficacy and potential side effects. For example, valproic acid undergoes extensive hepatic metabolism, and variations in enzyme activity can lead to interindividual variability in drug response. (30)
Excretion
The final phase in the anticonvulsant journey involves excretion, primarily through the kidneys. Unchanged anticonvulsants and their metabolites are eliminated via urine. This renal excretion is relevant when considering individual patient factors such as renal function, as impaired kidney function can affect the clearance of anticonvulsants and influence their duration of action. (30)
Ask yourself...
- How do the pharmacokinetics of Triptans, specifically their absorption and distribution, contribute to their efficacy in managing acute migraine attacks?
- Can you explain the key pharmacokinetic parameters of nonsteroidal anti-inflammatory drugs (NSAIDs) used in migraine management and how they influence drug effectiveness?
- What role do pharmacokinetic factors play in the onset and duration of action of calcitonin gene-related peptide (CGRP) inhibitors?
- In the context of migraine medications, how does the beta-blockers’ pharmacokinetics influence their absorption, distribution, metabolism, and excretion in the body?
- What education would you provide to a patient who is taking NSAIDS for migraine management?
- What education would you provide to a patient who is taking CGRP medications?
- What education would provide to a patient who is taking beta-blockers?
Warnings Related to Migraine Medications
Understanding potential warnings related to migraine medications is essential for healthcare professionals to ensure safe and effective treatment. Here are some factors to consider.
Medication Safety
Migraine medications, whether preventive or acute, come with specific warnings that need careful attention. For instance, some medicines may have contraindications for individuals with certain medical conditions or those taking specific medications concurrently. According to (34), healthcare providers must be vigilant in assessing patient medical histories to identify potential contraindications.
Addressing Cardiovascular Risks
Certain migraine medications, such as Triptans, may pose cardiovascular risks, especially in individuals with pre-existing cardiovascular conditions (35). Therefore, healthcare providers must assess patients’ cardiovascular health and consider alternative medications or dose adjustments for those at higher risk. For example, a patient with a history of myocardial infarction may be advised to avoid Triptans, and a different class of medication, like NSAIDs, may be recommended.
Pregnancy and Lactation Considerations
Warnings related to pregnancy and lactation are paramount. Some migraine medications may have potential risks during pregnancy, and healthcare providers must carefully weigh the benefits and risks when prescribing for pregnant or lactating individuals. For instance, valproic acid is associated with an increased risk of congenital disabilities, and alternative medications with a safer profile may be preferred for pregnant individuals seeking migraine relief (1).
Managing Medication Overuse Headaches (MOH)
A significant warning associated with migraine medications is the risk of medication-overuse headaches (34). To prevent this problem, healthcare providers must educate patients about the importance of adhering to prescribed dosages and avoiding excessive use of acute medications. Alternative strategies, such as lifestyle modifications and preventive drugs, can be crucial in managing and preventing MOH.

Ask yourself...
- What are some standard warnings associated with using Triptans in migraine management, and how should healthcare providers address these warnings?
- Can you identify specific cardiovascular risks associated with certain migraine medications, and what should be considered when prescribing these medications to patients with pre-existing cardiovascular conditions?
- What warnings are typically associated with the use of valproic acid in migraine management, particularly concerning specific patient populations such as pregnant individuals?
- How do healthcare providers manage and educate patients about the risk of medication-overuse headaches associated with certain migraine medications, and what preventive measures can be implemented to minimize this risk?
Alternatives to Migraine Medications
According to (5), integrating alternative methods provides additional tools for healthcare professionals and empowers individuals seeking a more comprehensive and personalized approach to migraine care. Here are some alternative approaches:
Holistic Lifestyle Modifications
Holistic management of migraines involves lifestyle modifications that can significantly impact the frequency and severity of attacks. For instance, incorporating regular physical activity, maintaining a consistent sleep schedule, and managing stress through practices like mindfulness and yoga have shown promise in reducing migraine occurrence (5). Educating patients about these lifestyle changes empowers them to actively participate in their migraine management.
Biofeedback and Relaxation Techniques
Biofeedback and relaxation techniques offer non-pharmacological interventions that enhance self-awareness and control over physiological responses (22). These approaches teach individuals to recognize and manage stress triggers, ultimately reducing the frequency of migraines. For example, biofeedback training that monitors muscle tension and provides real-time feedback can effectively prevent migraines (22).
Acupuncture and Acupressure
According to (22), traditional Chinese medicine practices like acupuncture and acupressure have gained recognition for their potential in migraine management. Acupuncture involves the insertion of thin needles into specific points on the body, while acupressure applies pressure to these points. Research suggests that these methods reduce the frequency and intensity of migraines, providing an alternative avenue for individuals seeking non-pharmacological options (22).
Nutritional Approaches
Dietary modifications and nutritional approaches also play a role in holistic migraine management. For example, it can be beneficial to identify and avoid potential trigger foods, such as those containing tyramine or artificial additives (10). Additionally, ensuring adequate hydration and incorporating anti-inflammatory foods into the diet may contribute to overall well-being and migraine prevention (10).
Ask yourself...
- What benefits do biofeedback and relaxation techniques offer in reducing the frequency and intensity of migraines?
- How can biofeedback and relaxation techniques be integrated into a comprehensive migraine management plan?
- What role do dietary modifications and nutritional approaches play in the holistic management of migraines?
- How can healthcare professionals guide patients in identifying trigger foods and making informed nutritional choices?
Nursing Considerations
Nurses play a pivotal role in treating individuals with migraines, contributing to the preventive and acute management aspects through various considerations. Here are some critical considerations.
Assessment and Patient Education
A thorough assessment includes evaluating migraines’ frequency, duration, and severity and identifying triggers and associated symptoms (3). Additionally, nurses play a key role in patient education, ensuring individuals clearly understand their migraine condition, the prescribed medications, and potential side effects. For instance, educating a patient about the importance of early intervention with acute medications during a migraine attack empowers them to take timely action.
Monitoring and Adverse Event Management
Nurses actively monitor individuals undergoing migraine treatment, the response to medications and any potential adverse events. Regular monitoring includes assessing the effectiveness of preventive measures, tracking the frequency of migraine attacks, and identifying patterns that may require adjustments in the treatment plan (34). If adverse events or side effects occur, nurses are instrumental in managing them promptly, collaborating with healthcare providers to ensure the safety and well-being of individuals.
Supportive Care and Holistic Approach
Nursing considerations extend beyond medication management to supportive care and a holistic approach. Nurses provide emotional support, helping individuals cope with the impact of migraines on their daily lives. Moreover, they collaborate with other healthcare professionals to integrate holistic approaches such as lifestyle modifications, stress management, and alternative therapies into the overall care plan. According to (34), this collaborative and patient-centered approach enhances the effectiveness of migraine management.
Documentation and Communication
Accurate and thorough documentation of relevant patient information, medication administration details, and treatment responses is a fundamental nursing responsibility in migraine management (34). Clear and concise communication between nursing staff, healthcare providers, and other healthcare team members also ensures continuity of care (34).
Ask yourself...
- How can a thorough patient assessment contribute to the safe and effective administration of migraine medications?
- How do nursing responsibilities extend beyond medication administration to encompass patient education?
- When monitoring individuals undergoing migraine treatment, what are the essential aspects that nurses should observe?
- How does the collaborative communication between nursing staff, healthcare providers, and other team members contribute to the continuity of care in migraine management?
- What education would you provide to a patient who is taking anticonvulsants for migraine management?
Upcoming Research
Staying ahead of the curve is essential for healthcare professionals to provide cutting-edge care and optimize outcomes for individuals with migraines. Upcoming research includes the following:
Advancements in Targeted Therapies
Recent research has unveiled promising advancements in targeted therapies for migraine management. For example, research about monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway is effective in preventing migraines (12).
Digital Health and Telemedicine in Migraine Care
Integrating digital health technologies and telemedicine is a burgeoning trend in migraine management research. Smartphone applications for tracking migraine patterns, wearable devices for monitoring physiological parameters, and virtual consultations enable a more comprehensive and patient-centric approach (12). This shift toward digital solutions enhances data collection and facilitates remote monitoring and timely interventions, particularly in scenarios where in-person visits may be challenging (12).
Genetic and Personalized Medicine Approaches
Advancements in genetic research are paving the way for personalized medicine in migraine care. Understanding the genetic underpinnings of migraines can guide the development of targeted interventions tailored to an individual’s unique genetic profile. This customized approach could revolutionize treatment strategies, allowing for more precise and effective interventions based on the genetic factors contributing to a person’s migraines (13).
Exploration of Lifestyle and Environmental Influences
Upcoming research increasingly focuses on the intricate interplay between lifestyle, environmental factors, and migraines, and studies examining the impact of factors such as diet, sleep patterns, and ecological triggers contribute valuable insights (13). For instance, research may reveal specific dietary components that act as triggers or protective factors for migraines, allowing healthcare professionals to offer targeted lifestyle recommendations.
Ask yourself...
- What recent research findings have emerged regarding migraine prevention?
- How are digital health technologies and telemedicine being incorporated into upcoming research on migraine management?
- In personalized medicine, how is genetic research influencing upcoming approaches to migraine care?
- How can consideration of lifestyle factors and environmental influences contribute to a more holistic approach to migraine management?
Conclusion
In conclusion, this course focused on empowering learners not only with knowledge about migraine management but with the practical insights and skills crucial for excellence in migraine care. The journey doesn’t end here; it extends into the realm of compassionate practice, where the combination of scientific understanding and safety measures transforms care providers into formidable advocates for those navigating the complexities of migraines.
Asthma Treatment and Monitoring
Introduction
When hearing the phrase asthma, what comes to mind? If you’re an advanced practice registered nurse (APRN) with prescriptive authority, you’ve definitely heard of asthma before. Even as a nurse or maybe before nursing school, conversations about prescription drug use and respiratory health existed every so often.
Presently, patients seek guidance and information on various health topics from APRNs, including medication management and respiratory health. The information in this course will serve as a valuable resource for APRNs with prescriptive authority across all specialties, education levels, and backgrounds to learn more about medications that can treat and manage asthma.
Defining Asthma
What Is Asthma?
Asthma is a non-communicable, chronic health condition that affects the lungs’ airways and millions of people nationwide. Asthma is often diagnosed in childhood and can resolve in adulthood or continue for the rest of a patient’s life. Several studies postulate the cause of asthma, but there is no definitive cause.
Genetics, age, environmental exposures, smoking, and a history of allergies are thought to play a role in asthma severity and development. Clinical presentation of asthma often includes trouble breathing, chronic airway inflammation, and airway hyperresponsiveness. Assessment for asthma often includes patient history, clinical presentation, spirometry testing, and pulmonary function tests (PFTs).
What Are the Stages of Asthma?
Since asthma is a chronic condition, several established guidelines can be used to determine its severity and explore possible medication options. Treatment and management vary depending on the stage of asthma and the patient’s response to existing therapy.
The four stages of asthma are intermittent, mild, moderate, and severe. Based on the 2020 National Asthma Education and Prevention Program (NAEPP) guidelines, here are the standard criteria for each stage of asthma (2).
Intermittent asthma is characterized by the following clinical presentation and assessment (2):
- Patient history of respiratory symptoms, such as cough, trouble breathing, wheezing, or chest tightness <2 times a week
- Asthmatic flare-ups are short-lived with varying intensity
- Symptoms at night are <2 a month
- No asthmatic symptoms between flare-ups
- Lung function test FEV 1 at >80% above normal values
- Peak flow has <20% variability am-to-am or am-to-pm, day-to-day
Mild persistent asthma is characterized by the following clinical presentation and assessment (2):
- Patient history of respiratory symptoms, such as cough, trouble breathing, wheezing, or chest tightness 3-6 times a week
- Asthmatic flare-ups may affect activity level and can vary in intensity
- Symptoms at night are 3-4 times a month
- Lung function test FEV1 is >80% above normal values
- Peak flow has less than 20-30% variability
Moderate persistent asthma is characterized with the following clinical presentation and assessment (2):
- Patient history of respiratory symptoms, such as cough, trouble breathing, wheezing, or chest tightness, daily
- Asthmatic flare-ups may affect activity level and can vary in intensity
- Symptoms at night are >5 times a month
- Lung function test FEV1 is 60%-80% of normal values
- Peak flow has more than 30% variability
Severe persistent asthma is characterized with the following clinical presentation and assessment (2):
- Patient history of respiratory symptoms, such as cough, trouble breathing, wheezing, or chest tightness, continuously
- Asthmatic flare-ups affect activity level and often vary in intensity
- Asthmatic symptoms at night are constant
- Lung function test FEV1 is <60% of normal values
- Peak flow has more than 30% variability
Based on patient history, clinical presentation, and these criteria, treatment can be administered to decrease the patient’s symptoms. If a patient presents with symptoms outside your scope of work or understanding, you can always refer them to a pulmonologist or asthma specialist.
Often, more severe cases of asthma and asthma emergencies require increased frequency and dosing of asthma-related medications. Health care provider discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history, pulmonary function, and health history before prescribing asthma medications (1).
What Are Asthmatic Emergencies?
Asthmatic emergencies occur when a patient has asthma symptoms that are beyond what they typically experience and is unable to function without immediate medical intervention. They can occur as a result of a patient being unable to access their asthmatic medications, being exposed to a possible allergen, or being under increased stress on the body.
Asthmatic emergencies often require collaborative medical intervention, increased dosages of medications discussed below, and patient education to prevent future asthmatic emergencies (1).
What If Asthma Is Left Untreated?
Depending on the clinical presentation and severity of asthma, it can cause several long-term complications if left untreated. If asthma is not correctly managed, several complications, such as chronic obstructive pulmonary disease (COPD), decreased lung function, permanent changes to the lungs’ airways, and death, can occur (1, 2).
Ask yourself...
- What is moderate persistent asthma?
- What is mild persistent asthma?
- What is intermittent asthma?
- What is severe, persistent asthma?
- What is considered an asthmatic emergency?
Asthma Medications
What Are Commonly Used Medications to Manage Asthma?
Commonly used medications to manage asthma include inhaled corticosteroids, oral corticosteroids, short-acting beta agonists (SABAs), long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LABAs), adenosine receptor antagonists, leukotriene modifiers, mast cell stabilizers, and monoclonal antibodies. The dosage, frequency, amount of asthma management medications, and medication administration route can vary depending on clinical presentation, patient health history, and more.

How and Where are Asthma Medications Used?
Depending on the patient, asthma medications can be used routinely or as needed to manage asthma symptoms. They can be used at home, in public places, and in health care facilities. Depending on the specific asthma medication and dosage, these medications can be taken by mouth or via an external device, such as an inhaler, subcutaneous injection, or intravenous solution (1).
What Are the Clinical Criteria for Prescribing Asthma Medication?
Clinical criteria for prescribing asthma medication can depend on a patient’s clinical presentation. Assessment of lung health and patient history is essential to determining the dosage and medications needed for adequate asthmatic symptom control.
Clinical guidelines from reputable organizations, such as the National Asthma Education and Prevention Program (NAEPP), the National Institutes of Health (NIH), the Global Initiative for Asthma (GINA), and the American Academy of Family Physicians (AAFP) can provide insight into the latest recommendations for asthma management (1, 2). In addition, local laws or health departments might have suggestions for asthma medication guidelines.
What Is the Average Cost for Asthma Medications?
The cost of asthma medications can significantly vary depending on the type of medication, insurance, dosage, frequency, medication administration route, and other factors. Cost is a leading reason many patients cannot maintain their medication regimen (3). If cost is a concern for your patient, consider reaching out to your local pharmacies or patient care teams to find cost-effective solutions.
Ask yourself...
- What are some common signs of asthma?
- What are some common medications that can be prescribed to manage asthma?
- What are some factors that can influence asthma development and severity?
Inhaled Corticosteroids Pharmacokinetics
Therapy should be guided by the healthcare provider’s professional discretion and the patient’s condition. Consider reviewing a patient’s medication history and health history before prescribing asthma medications.
Drug Class – Inhaled Corticosteroids
Commercially available inhaled corticosteroids include: ciclesonide (Alvesco HFA), fluticasone propionate (Flovent Diskus, Flovent HFA, Armon Digihaler), budesonide (Pulmicort Flexhaler), beclomethasone dipropionate (QVAR RediHaler), fluticasone furoate (Arnuity Ellipta), and mometasone furoate (Asmanex HFA, Asmanex Twisthaler).
Clinical criteria for prescribing an inhaled corticosteroid include adherence to the latest clinical guidelines, patient medical history, clinical presentation, and drug availability (4).
Inhaled Corticosteroids: Method of Action
Inhaled corticosteroids have an intricate action mechanism involving several immune system responses. They decrease the existing initial inflammatory response by decreasing the creation and slowing the release of inflammatory mediators. Common inflammatory mediators include histamine, cytokines, eicosanoids, and leukotrienes. Inhaled corticosteroids can also induce vasoconstrictive mechanisms, which can lead to less blood flow, resulting in less discomfort and edema (4).
In addition to anti-inflammatory properties, inhaled corticosteroids can create a localized immunosuppressive state that limits the airways’ hypersensitivity reaction, which is thought to reduce bronchospasms and other asthma-associated symptoms. It is important to note that inhaled corticosteroids often do not produce therapeutic effects immediately, as many patients may not see a change in their asthma symptoms for at least a week after beginning inhaled corticosteroid therapy (4).
Inhaled Corticosteroids Side Effects
Every medication has the possibility of side effects, and inhaled corticosteroids are no exception. Common side effects of inhaled corticosteroids include oral candidiasis (thrush), throat irritation, headache, and cough.
Patient education about rinsing their mouth and oral hygiene after use is essential to avoid the possibility of thrush and other oral infections and irritations. More severe side effects can include prolonged immunosuppression, reduction in bone density, and adrenal dysfunction (4).
Inhaled Corticosteroids Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with inhaled corticosteroids, so additional medication, increased dosage, a change in frequency, or a new medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of inhaled corticosteroids?
- What are some patient considerations to keep in mind when prescribing inhaled corticosteroids?
Oral Corticosteroids Pharmacokinetics
The health care provider’s professional discretion and the patient’s condition should guide therapy. Consider reviewing a patient’s medication history and health history before prescribing asthma medications.
Drug Class – Oral Corticosteroids
Commercially available oral corticosteroids include methylprednisolone, prednisolone, and prednisone. Clinical criteria for prescribing an oral corticosteroid include adherence to the latest clinical guidelines, patient medical history, clinical presentation, and drug availability (4).
Oral Corticosteroids Method of Action
Methylprednisolone and prednisolone have a method of action as intermediate, long-lasting, synthetic glucocorticoids, have COX-2 inhibitory properties, and inhibit the creation of inflammatory cytokines (5).
Prednisone is a prodrug to prednisolone and has anti-inflammatory and immunomodulating glucocorticoid properties. Prednisone has a method of decreasing inflammation by reversing increased capillary permeability and suppressing the movement of certain leukocytes (6).
Oral Corticosteroids Side Effects
Every medication has the possibility of side effects, and oral corticosteroids are no exception. Methylprednisolone and prednisolone have possible side effects of skin changes, weight gain, increased intraocular pressure, neuropsychiatric events, neutrophilia, immunocompromised state, fluid retention, and GI upset.
Consider monitoring symptoms and overall health of patients on systemic corticosteroids to assess for long-term side effects (5). Prednisone has possible side effects of changes in blood glucose, changes in sleep habits, changes in appetite, increased bone loss, an immunocompromised state, changes in adrenal function, and changes in blood pressure (6).
Oral Corticosteroids Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with oral corticosteroids, so additional medication, increased dosage, a change in frequency, or a new medication class might need to be considered (6).
Ask yourself...
- What are some possible side effects of oral corticosteroids?
- What are some patient considerations to keep in mind when prescribing oral corticosteroids versus inhaled corticosteroids?
Short-Acting Beta Agonists (SABAs)
Health care provider professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing asthma medications.
Drug Class – SABAs
Common commercially available SABAs include albuterol sulfate (ProAir HFA, Proventil HFA, Ventolin HFA), albuterol sulfate inhalation powder (ProAir RespiClick, ProAir Digihaler), levalbuterol tartrate (Xopenex HFA), and levalbuterol hydrochloride (Xopenex) (4).
SABAs Method of Action
Short-acting beta-agonists (SABAs) have a rapid onset as broncho-dilating medications. SABAs, especially albuterol in emergent situations, are often used to quickly relax bronchial smooth muscle from the trachea to the bronchioles through action on the β2-receptors.
While SABAs are effective bronchodilators in the short term for asthma symptoms, they do not affect the underlying mechanism of inflammation. As a result, SABAs are often used for short-acting intervals, such as a few hours, and have limited capabilities to prevent asthma exacerbations alone (4). SABAs can be administered via metered-dose inhalers, intravenous dry powder inhalers, orally, subcutaneously, or nebulizers.
SABAs Side Effects
Every medication has the possibility of side effects, and SABAs are no exception. Because of the beta receptor agonism, possible SABA side effects include increased heart rate, chest pain, chest palpitations, body tremors, and nervousness (4). Chronic side effects are not typically observed because of the short half-life of SABAs.
SABAs Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with SABAs, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of short-acting beta agonists?
- What are some patient considerations to keep in mind when prescribing SABAs?
Long-Acting Beta Agonists (LABAs)
Therapy should be guided by the healthcare provider’s professional discretion and the patient’s condition. Consider reviewing a patient’s medication history and health history before prescribing asthma medications.
Drug Class – LABAs
Common commercially available LABAs are salmeterol and formoterol (7).
LABAs Method of Action
Long-acting beta-agonists (LABAs) have a rapid onset like SABAs, but also have a longer half-life. LABAs are often used as asthma maintenance medications to relax bronchial smooth muscle from the trachea to the bronchioles through action on the β2-receptors. While SABAs are effective bronchodilators in the short term for asthma symptoms, LABAs are effective in the long term.
Like SABAs, LABAs do not affect the underlying mechanism of inflammation. LABAs can be administered via metered-dose inhalers, intravenously, dry powder inhalers, orally, subcutaneously, or via nebulizer. LABAs are often effective for 12-hour durations (7).
LABAs Side Effects
Every medication has the possibility of side effects, and LABAs are no exception. Like SABAs, because of the beta receptor agonisms, possible LABA side effects include increased heart rate, chest pain, chest palpitations, body tremors, and nervousness (7). Other, more prolonged side effects can include changes in blood glucose and potassium levels with prolonged LABA use (7).
LABAs Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with LABAs, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
In addition, combination inhaled corticosteroid/LABA medications can be considered, such as fluticasone propionate and salmeterol (Advair Diskus, Advair HFA, AirDuo Digihaler, AirDuo RespiClick, Wixela Inhub), fluticasone furoate and vilanterol (Breo Ellipta), mometasone furoate and formoterol fumarate dihydrate (Dulera), and budesonide and formoterol fumarate dihydrate (Symbicort) (4).
Ask yourself...
- What are some possible side effects of LABAs?
- What are some patient considerations to keep in mind when prescribing SABAs compared to LABAs?
Long-Acting Muscarinic Antagonists (LAMAs)
Health care provider’s professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing asthma medications.
Drug Class – LAMAs
Common commercially available LAMAs include two inhalation powders via inhalers: tiotropium bromide (Spiriva Respimat) and fluticasone furoate, umeclidinium, and vilanterol (Trelegy Ellipta) (4).
LAMAs Method of Action
Both drugs mentioned above are long-acting muscarinic antagonists (LAMAs). LAMAs work to alleviate asthmatic symptoms by antagonizing the type 3 muscarinic receptors in bronchial smooth muscles, resulting in relaxation of muscles in the airway (4). Because LAMAs are long-acting, they are not recommended for cases of acute asthma exacerbations or asthmatic emergencies (4).
LAMAs Side Effects
Possible LAMA side effects include urinary retention, dry mouth, constipation, and glaucoma (4).
LAMAs Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with LAMAs, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of LAMAs?
- What are some patient considerations to keep in mind when prescribing LAMAs?
Adenosine Receptor Antagonists Pharmacokinetics
Therapy should be guided by the health care provider’s professional discretion and the patient’s condition. Consider reviewing a patient’s medication history and health history before prescribing asthma medications.
Drug Class – Adenosine Receptor Antagonists
The commercially available adenosine receptor antagonist for asthma management is theophylline as a pill or intravenous (8).
Adenosine Receptor Antagonists: Method of Action
Theophylline’s method of action is to act as a nonselective adenosine receptor antagonist and a competitive, nonselective phosphodiesterase inhibitor and to reduce airway responsiveness to histamine, allergens, and methacholine (8).
Adenosine Receptor Antagonists Side Effects
Common side effects of theophylline include GI upset, headache, dizziness, irritability, and arrhythmias (8).
Adenosine Receptor Antagonists Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with theophylline, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of adenosine receptor antagonists?
- What are some patient considerations to keep in mind when prescribing adenosine receptor antagonists?
Leukotriene Modifiers Pharmacokinetics
Health care provider’s professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing asthma medications.
Drug Class – Leukotriene Modifiers
Commercially available leukotriene modifiers include montelukast (Singular) and zafirlukast (Accolate), which are oral pills taken once a day. Zileuton (Zyflo CR) is a 5-lipoxygenase inhibitor modifying leukotriene activity (4).
Leukotriene Modifiers: Method of Action
Montelukast and zafirlukast work to control asthma-related symptoms by targeting leukotrienes, which are eicosanoid inflammatory markers. Montelukast works by blocking leukotriene D4 receptors in the lungs, thus allowing decreased lung inflammation and increased relaxation of lung smooth muscle (9).
Zafirlukast works by being a competitive antagonist at the cysteinyl leukotriene-1 receptor (CYSLTR1) (10). Zileuton is a 5-lipoxygenase inhibitor, and 5-lipoxygenase is needed for leukotriene creation. Blocking 5-lipoxygenase decreases the formation of leukotrienes at several receptors. As a result of decreased leukotriene production, there is decreased inflammation, decreased mucus secretion, and decreased bronchoconstriction (11).
Leukotriene Modifiers Side Effects
Possible side effects of montelukast include headaches, GI upset, and upset. Neuropsychiatric events, such as nightmares, changes in sleep, depression, and suicidal ideation, are more severe side effects associated with montelukast.
Possible side effects of zafirlukast include headache, GI upset, and hepatic dysfunction (9).
Possible side effects of zileuton include hepatic dysfunction, changes in sleep, changes in mood, headaches, and GI upset. When the leukotriene modifiers, neuropsychiatric side effects are to be monitored for, in particular, for suicidal ideation (9,10,11).
Leukotriene Modifiers Alternatives
Some patients might not report their symptoms alleviating with leukotriene modifiers, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).

Ask yourself...
- What are some possible side effects of leukotriene modifiers?
- What are some patient considerations to keep in mind when prescribing leukotriene modifiers?
Mast Cell Stabilizer Pharmacokinetics
Health care provider professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing asthma medications.
Drug Class – Mast Cell Stabilizer
A commercially available mast cell stabilizer is cromolyn, available via metered-dose inhaler and nebulizer solution (12).
Mast Cell Stabilizer Method of Action
Cromolyn inhibits the release of inflammatory mediators from cells, such as histamine and leukotrienes (12).
Mast Cell Stabilizer Side Effects
Every medication has the possibility of side effects, and cromolyn is no exception. Common side effects of cromolyn include dry throat, throat irritation, drowsiness, dizziness, cough, headache, and GI upset (12).
Mast Cell Stabilizer Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with mast cell stabilizers, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of mast cell stabilizers?
- What are some patient considerations to keep in mind when prescribing mast cell stabilizers?
Monoclonal Antibody Pharmacokinetics
Health care provider professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing asthma medications.
Drug Class – Monoclonal Antibody
Commercially available monoclonal antibodies include Omalizumab (Xolair), mepolizumab (Nucala), reslizumab (Cinqair), benralizumab (Fasenra), dupilumab (Dupixent), and tezepelumab-ekko (Tezspire). Omalizumab, mepolizumab, benralizumab, dupilumab, and texepelumab-ekko are available via subcutaneous injection. Reslizumab is available via intravenous solution (4).
Monoclonal Antibody Method of Action
Omalizumab is an anti-IgE monoclonal antibody that works by inhibiting the binding of IgE to mast cells and basophils. As a result of decreased bound IgE, activation and release of mediators, such as histamine, in the allergic response are decreased (13).
Mepolizumab, reslizumab, and benralizumab are interleukin (IL)-5 antagonists. These IL-5 antagonists inhibit IL-5 signaling, allowing for a decrease in the creation and survival of eosinophils. However, the full method of action for IL-5 antagonists is still unknown, as more evidence-based research is needed (4, 15).
Dupilumab is an IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. This inhibition of the IL-4Rα subunit allows for the decrease of IL-4 and IL-13 cytokine-induced inflammatory responses (14).
Tezepelumab-ekko is an IgG antibody that binds to the thymic stromal lymphopoietin (TSLP) and prevents TSLP from interacting with the TSLP receptor. Blocking TSLP decreases biomarkers and cytokines associated with inflammation. Knowing this, the full method of action for Tezepelumab-ekko is still unknown, as more evidence-based research is needed4,15.
Monoclonal Antibody Side Effects
Possible side effects of omalizumab include injection site reactions, fracture, anaphylaxis, headache, and sore throat (13). Possible side effects of mepolizumab, reslizumab, and benralizumab include injection site reactions, headache, and hypersensitivity reactions (4). Possible side effects of dupilumab include joint aches, injection site reactions, and headache (14). Possible side effects of tezepelumab-ekko include injection site reactions and headache (14).
Monoclonal Antibody Alternatives
While there are clinical criteria for asthma medications, everyone can respond to medications differently. Some patients might not report their symptoms alleviating with monoclonal antibodies, so additional medication, increased dosage, a change in frequency, or an additional medication class might need to be considered (4).
Ask yourself...
- What are some possible side effects of monoclonal antibodies?
- What are some patient considerations to keep in mind when prescribing monoclonal antibodies?
Nursing Considerations
Nurses remain the most trusted profession for a reason, and APRNs are often pillars of patient care in several health care settings. Patients turn to nurses for guidance, education, and support. While there is no specific guideline for the nurses’ role in asthma education and management, here are some suggestions to provide quality care for patients currently taking medications to manage asthma or concerned about possibly having asthma.
Take a detailed health history. Respiratory symptoms, such as a cough or trouble breathing, are often dismissed in health care settings or seen as “common symptoms with everyone.” If a patient is complaining of symptoms that could be related to asthma, inquire more about that complaint.
Ask about how long the symptoms have lasted, what treatments have been tried, if these symptoms interfere with their quality of life, and if anything alleviates any of these symptoms. If you feel like a patient’s complaint is not being taken seriously by other health care professionals, advocate for that patient to the best of your abilities.
Review medication history at every encounter. Oftentimes, in busy clinical settings, reviewing health records can be overwhelming. Millions of people take asthma medications at varying dosages, frequencies, and times of day. Many people with asthma take more than one medication to manage their symptoms.
Ask patients how they feel on the medication, if their symptoms are improving, and if there are any changes to medication history.
Be willing to answer questions about asthma, respiratory health, and medication options. Society stigmatizes open discussions of prescription medication and can minimize symptoms of asthma, such as a chronic cough.
Many people do not know about medication options or the long-term effects of undiagnosed or poorly managed asthma. Be willing to be honest with yourself about your comfort level discussing topics and providing education on asthma medications and asthma clinical assessment options.
Inquire about a patient’s life outside of medications, such as their occupation, living situation, and smoking habits. Household exposures, such as carpets or pets, can trigger asthma. Occupations with high exposure to smoke can also trigger asthmatic symptoms. Smoking, living with someone who smokes, or residing in an area with high levels of pollution can also influence asthma symptoms.
Discuss possible solutions to help with symptoms, such as improving ventilation, increasing air quality, and wearing masks when possible.
Communicate the care plan to other staff involved for continuity of care. Care often consists of a team of nurses, specialists, pharmacists, and more for several patients, especially those with severe asthma. Ensure that patients’ records are up to date for ease in record sharing and continuity of care.
Stay current on continuing education related to asthma medications, as evidence-based information is constantly evolving and changing. You can then present your new learnings and findings to other healthcare professionals and educate your patients with the latest information. You can learn more about the latest research on asthma and asthma-related medications by following updates from evidence-based organizations.
How can nurses identify if someone has asthma?
Unfortunately, it is not always possible to look at someone with the naked eye and determine if they have asthma. While some people might have visible asthmatic symptoms, such as wheezing or trouble breathing, asthmatic clinical presentation can significantly vary from person to person.
APRNs can identify and diagnose if someone has asthma by taking a complete health history, listening to the patient’s concerns, and offering pulmonary function testing.
What should patients know about asthma medications?
Patients should know that anyone can experience side effects from medications for asthma management, just like any other medication. They should seek medical care if they notice any mood changes, experience sharp headaches, or feel like something is concerning.
Nurses should also teach patients to advocate for their health to avoid untreated or undetected asthma and possible chronic complications from asthma or asthma-related medications.
Here are essential tips for patient education in the inpatient or outpatient setting:
- Tell the health care provider of any existing medical conditions or concerns (need to identify risk factors).
- Tell the health care provider of any existing lifestyle concerns, such as tobacco use, other drug use, sleeping habits, occupation, diet, and menstrual cycle changes (need to identify lifestyle factors that can influence asthmatic medication use, asthma severity, and asthma management).
- Tell the health care provider if you have any changes in your breathing, such as pain with deep breathing or persistent coughing (potential asthma exacerbation symptoms or possibility of asthma medications not being as effective for treatment).
- Tell the nurse or health care provider if you experience any pain that becomes increasingly severe or interferes with your quality of life.
- You can track your health, medication use, and health concerns via an app, diary, or journal (self-monitoring for any changes).
- Tell the health care provider right away if you are having thoughts of hurting yourself or others (a possible increased risk of suicidality is a potential side effect of montelukast use).
- Take all prescribed medications as indicated and ask questions about medications and possible other treatment options, such as non-pharmacological options or surgeries.
- Tell your health care provider if you notice any changes while taking medications or receiving other treatments to manage asthma (potential worsening or improvement of your health situation).
Ask yourself...
- What are some problems that can occur if medications are not asthma properly?
- What are some possible ways you can obtain a detailed, patient centric health history?
- What are some possible ways APRNs can educate patients on asthma and air quality?
Research Findings
There is extensive publicly available literature on asthma and asthma-related medications via the National Institutes of Health and other evidence-based journals (1,2,4).
What are some ways for people who take asthma medications to become a part of research?
Patients interested in participating in clinical trial research can seek more information on clinical trials from local universities and health care organizations.
Ask yourself...
- What are some reasons someone would want to enroll in clinical trials?
Case Study
Susie is a mom to a 15-year-old named Jill. She arrives for a new patient visit at the pediatric asthma and allergy specialist practice. Susie reports that she has noticed Jill has been having trouble sleeping at night and coughing more during the day for the past month. Jill plays soccer with her school, but her mom is concerned that coughing and trouble sleeping are interfering with her sports.
Susie knows that her dad has asthma, and Jill spends a lot of time with her aunt, who smokes cigarettes. Jill has a history of generalized anxiety disorder and reports no smoking, no drinking, and no recreational drugs. Jill also wants to learn more about her lung health, as she wants to play soccer professionally one day.
Ask yourself...
- What are some specific questions you’d want to ask about Jill’s coughing and respiratory health?
- What are health history questions you would want to highlight?
- What are some tests or lab work would you suggest performing?
Case Study (Continued)
Susie also shares that she is dating a new partner who vapes at home, and they recently got a pet puppy. She states that Jill had trouble sleeping at night and coughing a lot when she was younger, but Susie thought Jill grew out of it. Susie wants to learn more about whether Jill has asthma like her grandfather, if there are any ways to manage this cough, and if any tests can determine Jill’s lung health in the office today.
Ask yourself...
- What sort of tests can be done in-office to assess pulmonary function?
- What sort of environmental exposures can trigger respiratory conditions?
Case Study (Continued)
Susie agrees to have Jill do allergy testing and an in-office pulmonary function test for teenagers. Jill also wants to learn more about spirometry, which you mentioned earlier, and how to monitor her health outside of the office since she’s busy with school and soccer and doesn’t want to come to the office whenever there’s a problem.
Susie is open to medication options for Jill, but doesn’t want anything that will interfere too much with her social time with her friends. Susie and Jill also want to know if this is a health condition that Jill will have forever or if she will grow out of it.
Ask yourself...
- Knowing Susie’s concerns and Jill’s age, what are some talking points about reducing possible asthma triggers?
- How would you explain asthma as a chronic health condition to an adolescent patient?
- Given Jill and Susie’s concerns about medications, what are some possible options to consider after reviewing Jill’s pulmonary function test results and patient history?
Conclusion
Asthma is a chronic condition affecting many people from childhood to adulthood. As more medications for asthma come onto the market and more evidence-based approaches to asthma and lung care emerge, APRNs will be at the forefront of primary care and asthma care across the lifespan.
SSRI Use in Major Depressive Disorder
Introduction
When hearing the phrase selective serotonin reuptake inhibitors, what comes to mind? If you’re an advanced practice registered nurse (APRN) with prescriptive authority, you’ve heard of SSRIs before. Even as a nurse or maybe before nursing school, conversations about prescription drug use and mental health existed every so often.
Presently, patients seek guidance and information on various health topics from APRNs, including medication management, women’s health, and mental health. The information in this course will serve as a valuable resource for APRNs with prescriptive authority across all specialties, education levels, and backgrounds to learn more about SSRIs and major depressive disorder (MDD).
Defining SSRIs
What Are SSRIs?
Selective serotonin reuptake inhibitors, known as SSRIs, are a type of pharmacological drug class. SSRIs have existed for the past several decades as a class of prescription medications that can manage major depressive disorder (MDD) and other mental health conditions (1).
While this course focuses explicitly on SSRI use in MDD management, SSRIs are also Food and Drug Administration (FDA) approved to manage obsessive-compulsive disorder (OCD), panic disorder (PD), post-traumatic stress disorder (PTSD), and social anxiety disorder (SAD). In addition, several off-label uses for SSRIs include management for binge eating disorder and menopausal vasomotor symptoms.
How and Where Are SSRIs Used?
SSRIs are commonly prescribed to manage MDD and other mood disorders in the U.S. and around the world in pediatric, adult, and geriatric populations (1, 2). SSRIs can be taken by mouth as a pill, capsule, or liquid oral solution. Presently, SSRIs cannot be offered via intravenous, rectal, buccal, or injection routes.
What Are the Clinical Criteria for Prescribing SSRIs?
Clinical criteria for prescribing SSRIs can vary depending on the intention for the SSRI. In the case of MDD, several factors can play a role in the clinical criteria for prescribing SSRIs. A patient’s adherence to swallowing a pill daily, dosage given the patient’s weight, medical history, and MDD concerns, and prior experience with other medications can influence prescribing SSRIs. When considering prescribing SSRIs for MDD management, consider assessing the patient for MDD first, taking a detailed health history, and discussing the risks versus benefits of starting SSRIs for this patient (1, 3).
What Is the Average Cost for SSRIs?
The cost of SSRIs can significantly vary depending on the type, insurance, dosage, frequency, and other factors. Cost is among the leading reasons many patients cannot maintain their medication regimen (4). If cost is a concern for your patient, consider reaching out to your local pharmacies or patient care teams to find cost-effective solutions.
What Is Major Depressive Disorder (MDD)?
Major depressive disorder (MDD) is a mental health condition in which a person has consistent appetite changes, sleep changes, psychomotor changes, decreased interest in activities, negative thoughts, suicidal thoughts, and depressed mood that interfere with a person’s quality of life (5). According to the Diagnostic and Statistical Manual of Mental Disorders, a patient must have at least five persistent mood-related symptoms, including depression or anhedonia (loss of interest in activities once enjoyed), that interfere with a person’s quality of life to be formally diagnosed with MDD. Note that MDD does not include a history of manic episodes, and pediatric populations can present with more variable MDD symptoms (5). As an APRN, you can assess for MDD by doing a detailed patient health history or having a patient complete the Patient Health Questionnaire-9 (PHQ-9) – a depression assessment tool (5).
Ask yourself...
- What are some medication administration options for SSRIs?
- What populations can be prescribed SSRIs?
SSRI Pharmacokinetics
Drug Class SSRIs
Selective serotonin reuptake inhibitors, known as SSRIs, are a type of pharmacological drug class part of the antidepressant drug class. They can be prescribed at various dosages depending on the patient’s history, severity of major depressive disorder (MDD), other medication use, and other factors based on patient-centered decision making. Currently, SSRIs that are FDA approved for MDD management include paroxetine, sertraline, citalopram, escitalopram, vilazodone, and fluoxetine. SSRIs can be prescribed for the oral route and are available via capsule, tablet, or liquid suspension/solution. SSRIs can be taken at any time of day. They can be taken with or without food, though vilazodone in particular is recommended with food. SSRIs are often prescribed to be taken once a day, sometimes twice a day, depending on the severity of MDD. Health care providers’ professional discretion and patient condition should guide therapy (1).
SSRIs are metabolized by and known to affect the cytochrome P450 system. CYP2D6 inhibitors include escitalopram, citalopram, sertraline, paroxetine, and fluoxetine. Fluoxetine and fluvoxamine are inhibitors of CYP2C19. Fluvoxamine is an inhibitor of CYP1A2. Review a patient’s medication and health history before prescribing SSRIs (1).
SSRIs Method of Action
SSRI action method has been subject to several studies, especially in the last few years. Serotonin is a neurotransmitter that plays a role in mood and other bodily functions. It can be measured in plasma, blood, urine, and CSF (6). It is important to note that serotonin is rapidly metabolized to 5-hydroxyindoleacetic acid (5-HIAA) (6). SSRIs work by inhibiting serotonin reuptake at specific chemical receptors, thereby increasing serotonin activity and concentration (1). SSRIs inhibit the serotonin transporter (SERT) at the presynaptic axon terminal.
By obstructing the SERT, a higher amount of serotonin (5-hydroxytryptamine or 5HT) remains in synaptic clefts. This higher amount of serotonin can then stimulate postsynaptic receptors for a more extended period (1). While SSRIs can increase serotonin activity, there is some evidence that suggests the possibility of long-term SSRI use reducing serotonin concentration (6). In addition, the clinical response to SSRIs in patients with MDD can take anywhere from a few to several weeks to emerge (7). While some research suggests initial improvements in mood, evidence remains inconclusive regarding the exact time SSRIs can take to provide a therapeutic response for patients (7). Also, while research suggests that SSRIs can increase serotonin levels, there is still mixed evidence on the exact method of action for SSRIs (7).
As a result, it is essential to counsel patients that SSRIs can take a few weeks to provide a therapeutic response and to monitor mood and symptoms while taking SSRIs.
SSRI Side Effects
Every medication has the possibility of side effects, and SSRIs are no exception. Fortunately, SSRIs are known to have fewer side effects than other drug classes of antidepressants, such as monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants (TCAs). The most commonly known side effects of SSRIs include weight gain, sleep changes, headache, gastrointestinal issues, drowsiness, orthostatic hypotension, and sexual function changes (1).
Sleep changes can include an increased desire to sleep, increased sleeping time, or insomnia. Gastrointestinal issues include an upset stomach, nausea, or dry mouth. Mood changes, such as anxiety, are possible side effects as well. Sexual function changes can consist of erectile dysfunction, libido changes, impaired orgasmic response, and vaginal dryness (1, 8).
There are more serious possible side effects of SSRIs as well. For instance, SSRIs have the potential side effect of QT prolongation, which, if left untreated or undiagnosed, can lead to fatal cardiac arrhythmias (1, 8). In particular, the SSRI citalopram has been shown to have a higher risk of QT prolongation compared to other SSRIs. Also, like any other medication that can increase levels of serotonin in the body, there is a possibility of serotonin syndrome as a complication of SSRI use. Possible clinical manifestations of serotonin syndrome include increased blood pressure, increased sweating, increased reflexes, and increased dry eyes (8). Due to the vast and varied range of side effects, patient counseling, monitoring, and education are essential when prescribing SSRIs.
SSRI Black Box Warning
In 2004, the FDA issued a black box warning for SSRIs and other antidepressant medications due to the possible increased risk of suicidality in pediatric and young adult populations (up to age 25). When considering SSRI use in patients under 25 and knowing MDD is a risk factor for suicidality, having a conversation with the patient about risks versus benefits must be considered. However, in the past several years since the FDA’s warning, there is no clear evidence showing a correlation between SSRIs and the increased risk of suicidality (1, 8). The health care provider’s professional discretion and the patient’s condition should guide therapy.
SSRI Alternatives
MDD can be a complex, chronic condition to manage with varying clinical presentation and influence on a patient’s quality of life. There are several alternatives to SSRI use, such as: (1, 9)
- Other prescription drugs
- Serotonin-norepinephrine reuptake inhibitors (SNRIs). SNRIs include milnacipran, venlafaxine, desvenlafaxine, duloxetine, and levomilnacipran.
- Atypical antidepressants. Commonly known atypical antidepressants include bupropion and mirtazapine.
- Tricyclic antidepressants (TCAs). TCAs commonly include amitriptyline, desipramine, imipramine, clomipramine, doxepin, and nortriptyline.
- Monoamine oxidase inhibitors (MAOIs). Commonly known MAOIs include phenelzine, tranylcypromine, isocarboxazid, and selegiline.
- Psychotherapy, such as cognitive behavioral therapy (CBT) or interpersonal therapy
- Electroconvulsive therapy (ECT)
- Vagus Nerve Stimulation (VNS)
- Transcranial Magnetic Stimulation (TMS)
Ask yourself...
- What are some possible side effects of SSRIs?
- What are some pharmacological alternatives to SSRIs?
Nursing Considerations
Nurse’s Role
What Is the Nurse’s Role in SSRI Patient Education and Management?
Nurses remain the most trusted profession for a reason, and APRNs are often pillars of patient care in several health care settings. Patients turn to nurses for guidance, education, and support. While there is no specific guideline for the nurses’ role in SSRI education and management, here are some suggestions to provide quality care for patients interested in or currently taking SSRIs to manage current or suspected major depressive disorder (MDD).
- Take a detailed health history. Oftentimes, mental health symptoms, such as depressive thoughts or anxiety, are dismissed in health care settings, even in mental health settings. If a patient is complaining of symptoms that could be related to major depressive disorder, inquire more about that complaint. Ask about how long the symptoms have lasted, what treatments have been tried, if these symptoms interfere with their quality of life, and if anything alleviates any of these symptoms. If you feel like a patient’s complaint is not being taken seriously by other health care professionals, advocate for that patient to the best of your abilities.
- Review medication history at every encounter. In busy clinical settings, reviewing health records can be overwhelming. While a vast number of people take SSRIs, many are no longer benefiting from them. Ask patients how they are feeling on the medication, if their symptoms are improving, and if there are any changes to their medication history.
- Ask about family history. If someone is complaining of symptoms that could be related to MDD, ask if anyone in their immediate family, such as their parent or sibling, has experienced similar conditions.
- Be willing to answer questions about mental health and SSRIs. Society can often stigmatize open discussions of prescription medication and mental health. SSRIs are no exception. Many people do not know about the benefits and risks of SSRIs, the long-term effects of unmanaged MDD, or possible treatment options. Be willing to be honest with yourself about your comfort level discussing topics and providing education on SSRIs and MDD.
- Communicate the care plan to other staff involved for continuity of care. MDD management often consists of a team of mental health professionals, nurses, primary care specialists, pharmacies, and more for several patients. Ensure that patients’ records are up to date for ease in record sharing and continuity of care.
- Stay up to date on continuing education related to SSRIs and mental health conditions, as evidence-based information is constantly evolving and changing. You can then present your findings to other health care professionals and educate your patients with the latest information. You can learn more about the newest research on SSRIs and mental health by following updates from evidence-based organizations.
Identifying Major Depressive Disorder
How can nurses identify if someone has major depressive disorder?
Unfortunately, it is not possible to look at someone with the naked eye and determine if they have MDD. APRNs can identify and diagnose if someone has MDD by taking a complete health history, listening to patients’ concerns, having patients complete the PHQ-9 questionnaire, and communicating any concerns to other health care professionals (9).
Patient Education
What should patients know about SSRIs?
Patients should know that anyone has the possibility of experiencing side effects of SSRIs, just like any other medication. Patients should be aware that they should seek medical care if they notice any mood changes, experience sharp headaches, or feel like something is concerning. Due to social stigma associated with mental health and SSRI use, people may be hesitant to seek medical care for fear of being dismissed by health care professionals (1, 6). In addition, side effects (that interfere with the quality of life) are often normalized (1, 6). However, as more research and social movements discuss mental health and SSRI use more openly, there is more space and awareness for SSRI use and mental health.
Nurses should also teach patients to advocate for their own health to avoid the progression of MDD and possible unwanted side effects of SSRIs. Here are important tips for patient education in the inpatient or outpatient setting.
- Tell the health care provider of any existing medical conditions or concerns (need to identify risk factors)
- Tell the health care provider of any existing lifestyle concerns, such as alcohol use, other drug use, sleeping habits, diet, and menstrual cycle changes (need to identify lifestyle factors that can influence SSRI use and MDD)
- Tell the health care provider if you notice any changes in your mood, behavior, sleep, sexual health (including vaginal dryness or erectile dysfunction), or weight (possible changes that could hint at more chronic side effects of SSRIs)
- Tell the health care provider if you have any changes in urinary or bowel habits, such as increased or decreased urination or defecation (potential risk for SSRI malabsorption or possible unwanted side effects)
- Tell the nurse or health care provider if you experience any pain that increasingly worsens or interferes with your quality of life.
- Keep track of your mental health, medication use, and health concerns via an app, diary, or journal (self-monitoring for any changes)
- Tell the health care provider right away if you are having thoughts of hurting yourself or others (a possible increased risk of suicidality is a potential side effect for SSRI use)
- Take all prescribed medications as indicated and ask questions about medications and possible other treatment options, such as non-pharmacological options or surgeries.
- Tell the health care provider if you notice any changes while taking medications or on other treatments to manage your MDD (potential worsening or improving mental health situation)

Ask yourself...
- What are some possible ways you can obtain a detailed, patient-centric health history?
- What are some possible ways APRNs can educate patients on SSRIs and major depressive disorder?
Research Findings
What Research on SSRIs exists presently?
There is extensive publicly available literature on SSRIs via the National Institutes of Health and other evidence-based journals.
What are some ways for people who take SSRIs to become a part of research?
Patients interested in participating in clinical trial research can seek more information on clinical trials from local universities and health care organizations.
Ask yourself...
- What are some problems that can occur if SSRIs are not managing major depressive disorder symptoms adequately?
- What are some reasons someone might want to enroll in SSRI clinical trials?
Case Studies
Case Study Part 1
Susan is a 22-year-old Black woman working as a teacher. She arrives for her annual exam at the local health department next to her place of work. She reports nothing new in her health, but she says she’s been feeling more tired over the past few months. Susan reports having trouble sleeping and eating, but doesn’t feel too stressed overall. She heard one of her friends talking about SSRIs and wants to try them, but she’s never taken prescription medications long-term before. She also thinks she might have some depression because she looked at some forums online and resonated with a lot of people’s comments.
Ask yourself...
- What specific questions would you want to ask about her mental health?
- What are some health history questions you’d want to highlight?
- What lab work would you suggest performing?
Case Study Continued
Susan agrees to complete bloodwork later this week and thinks she might have a family history of depression. She said that no one in her family talks about mental health, but she heard about depression from her friends recently, and her family a long time ago. She’s back in the office a few weeks later, and her labs are within normal limits. Susan states she’s still feeling fatigued and more hopeless these days. She denies thinking about hurting herself or others.
Ask yourself...
- How would you discuss Susan’s mental health concerns?
- How would you explain to Susan the influence of lifestyle, such as sleep, diet, and environment, on mood?
Case Study Cont.
Susan completed the PHQ-9 questionnaire and had a high score. After discussing her responses with her, you diagnose her with MDD. Susan admits that she is open to trying SSRIs. She is also open to seeing a therapist, as she states that she’s never been to therapy. She would like resources on any therapy services, medication options, and non-pharmacological options to help her manage her condition.
Ask yourself...
- Knowing Susan’s concerns, what are some possible non-pharmacological management options for her MDD?
- What are some major SSRI side effects to educate Susan on?
Conclusion
Major depressive disorder is a complex, chronic health condition that affects many people nationwide. SSRIs are often a first-line pharmacological option for MDD management. However, clinical presentation and symptom management with SSRIs can vary widely. While some patients would prefer a low-dose SSRI, others will need a higher dose and possibly extra medication management. Education and awareness of SSRIs can influence the lives of many people.
Anticoagulant Therapy
Introduction
Anticoagulants are a class of medications that prevent and treat blood clots, or venous thromboembolism (VTE).
The Centers for Disease Control and Prevention (CDC) estimates that around 900,000 people in the United States are affected by some form of venous thrombosis yearly. Furthermore, the CDC estimates around 60,000 to 100,000 Americans die from some form of venous thromboembolism each year.
They further state that sudden death is the first symptom that occurs in about 25% of people who have a pulmonary embolism (4). Thus, healthcare providers must know the signs and symptoms of venous thromboembolism and the available anticoagulants for prevention and treatment.
Understanding the different pharmacokinetics of anticoagulant medication is essential during drug selection. This course outlines anticoagulant pharmacology and addresses pharmacokinetics, including mechanism of action, side effects, usage, and contraindications.
Definitions
Anticoagulants are medications used to prevent and treat blood clot formation, commonly called “blood thinners” (5).
Venous Thromboembolism – a condition where a blood clot forms in a vein, including deep vein thrombosis and pulmonary embolism, and appears during periods of hemostasis (5).
Ask yourself...
- What are anticoagulants?
- Have you seen an increase in patients taking anticoagulants?
- What is a venous thromboembolism?
- What policies and procedures does your facility have regarding anticoagulation therapy?
- What documentation must you complete when patients are prescribed anticoagulants?
Medications Overview
Anticoagulant medications are used for the prevention and treatment of venous thromboembolism in both inpatient and outpatient settings.
The major anticoagulant medication classes include:
- Unfractionated Heparin
- Low Molecular Weight Heparin
- Vitamin K Dependent Antagonists
- Direct Oral Anticoagulants
- Direct Thrombin Inhibitors
- Direct Factor Xa Inhibitors (14).
Unlike anticoagulants, antiplatelets act on platelet formation. Although commonly mistaken for anticoagulants, they are not a part of this class (14).
Depending on the type of anticoagulant, they have other indications for use, such as:
- Atrial Fibrillation Stroke Prevention
- Left Ventricular Thrombus
- Left Ventricular Aneurysm
- Prosthetic Heart Valve
- Venous Thromboembolism Treatment
- VTE prevention in people with cancer
- Pulmonary Embolism
- Pregnancy
- Heparin-Induced Thrombocytopenia
Ask yourself...
- What settings are anticoagulants used in?
- What medical conditions can anticoagulants be used for treatment and prevention?
- What are the different classes of anticoagulant medications?
Pharmacokinetics
Unfractionated Heparin
Unfractionated heparin, also known as heparin, is a medication used to prevent excess blood coagulation. It’s used to prevent and treat thrombotic events like deep vein thrombosis (DVT) and pulmonary embolism (PE).
Heparin is also used in patients with atrial fibrillation to prevent blood clot formation and during medical procedures, like dialysis, continuous renal replacement therapy, extracorporeal circulation, and heart catheterizations and surgeries. It’s also used for various off-label indications and usually in inpatient settings, such as acute coronary syndrome, percutaneous coronary intervention (PCI), or as a bridge medication when converting to oral anticoagulants (15).
Heparin works by binding to an antithrombin that inactivates thrombin and thus blocks the clotting cascade. More specifically, by inactivating thrombin factor IIa and factor Xa, fibrinogen is not converted to fibrin, which in turn prevents clot formation (15).
Heparin is available via intravenous (IV) and subcutaneous (SQ) forms. Intravenous heparin is infused via continuous infusion until therapeutic levels are achieved, while the SQ form is administered intermittently for VTE prophylaxis. Starting dosages of IV heparin usually begin with a bolus of 80 units per kilogram (kg), then a continuous infusion rate of 18 units/kg/hour. However, this depends on the treatment’s underlying medical condition and is titrated to achieve therapeutic levels.
In hospital settings, heparin flushes are available to lock and maintain IV-line patency. Additionally, subcutaneous dosages of heparin are weight-based and dependent on the medical condition (15).
Common side effects of heparin include:
- Bleeding
- Thrombocytopenia
- Injection site reactions
- Hyperkalemia (15).
As bleeding is a common side effect, nurses and healthcare providers must monitor patients for evidence of bleeding. Some signs or symptoms of bleeding can include petechiae, bruising, nosebleeds, hematuria, and bright red or dark, tarry stools. Moreover, heparin-induced thrombocytopenia (HIT) usually occurs within five days of initiating heparin therapy, causing serious adverse effects, like myocardial infarction, stroke, DVT, PE, and even death (15).
Before ordering this medication, healthcare providers should be aware of this anticoagulant’s contraindications. Heparin is contraindicated in individuals with a platelet count of less than 100,000/mm or with an active, uncontrolled bleed, except if they have disseminated intravascular coagulation (DIC). It should also be avoided in patients with a history of HIT or who cannot have routine blood monitoring of heparin therapeutic levels.
Monitoring the patient’s hemoglobin, hematocrit, and vital signs is essential to detecting a possible hemorrhage (15).
As heparin affects clotting time, healthcare providers must order therapeutic monitoring for activated partial thromboplastin time (aPTT) and activated clotting time (ACT). Before patients are initiated on a heparin infusion, a baseline aPTT level is drawn and then monitored every 6 hours until a therapeutic aPTT level is achieved. After two or more aPTT therapeutic results, an aPTT is drawn every 24 hours.
The therapeutic range of heparin is typically 1.5 to 2 times the normal range, and titration algorithms depend on the healthcare facility’s protocols. ACT monitoring is less specific than aPTT and is typically reserved for PCI or cardiac bypass (15).

Ask yourself...
- What is the pharmacokinetics of unfractionated heparin?
- What are the common side effects and contraindications for unfractionated heparin?
- Which lab value(s) require routine monitoring when administering this medication?
- What education should you provide to a patient on unfractionated heparin?
- What precautions should a patient take with heparin?
Low Molecular Weight Heparin
Low molecular weight heparin (LMWH) is another medication that prevents excess blood coagulation. It is also used to prevent venous thromboembolic disease in hospitalized patients and to treat DVT and PE. Low molecular weight heparin is also approved to treat medical conditions such as ST-elevation myocardial infarction (STEMI), unstable angina, and extracorporeal clot prevention.
Common names of LMWH include enoxaparin and dalteparin (13).
Low molecular weight heparin’s mechanism of action works on the body’s clotting cascade by activating antithrombin III. Once antithrombin III is activated, it binds to factor Xa, which inhibits thrombin activation. Therefore, clot formation is prevented since fibrinogen is not converted to fibrin. This medication’s mechanism of action slightly differs from heparin, as LMWH inhibits factor Xa only, while heparin acts on factor IIa and factor Xa [13].
Low molecular weight heparin is available via subcutaneous injection. Dosages of this medication are typically calculated by body weight and depend on whether it’s being used for prevention or treatment. LMWH is usually administered once or twice daily and is safe during pregnancy.
Some adverse effects of LMWH include:
- Bleeding
- Heparin-induced Thrombocytopenia
- Injection site reactions
- Osteoporosis (13).
Some other side effects are spontaneous fractures, hyperaldosteronism, and hypersensitivity reactions. Before prescribing this medication, healthcare providers must be knowledgeable of LMWH contraindications. It’s contraindicated in patients with hemorrhagic disorders, peptic ulcer disease, cerebral hemorrhage, trauma, and recent eye or nervous system surgeries.
Additionally, healthcare providers should caution when prescribing LMWH to patients with chronic kidney disease, as it increases the risk of accumulation in their system. Lower doses may be required for these individuals. A benefit of low molecular weight heparin compared to unfractionated heparin is that the patient’s aPTT blood levels do not need to be monitored (13).

Ask yourself...
- What is the pharmacokinetics of low molecular weight heparin?
- What are the common side effects and contraindications of low molecular weight heparin?
- Where should low-molecular-weight heparin be injected?
- What education should you provide to patients being started on LMWH?
- What is the dosage of LMWH based on?
Vitamin K Dependent Antagonists
Vitamin K antagonists (VKAs) are a class of anticoagulant medications used in the prevention and treatment of blood clots. Warfarin is a commonly prescribed VKA and other less common VKAs are acenocoumarol, fluindione, and phenprocoumon (7).
Warfarin is approved for the treatment and prevention of pulmonary embolism, venous thrombosis, and thromboembolic complications of heart valve replacement and atrial fibrillation. It’s also used to reduce mortality risk after a myocardial infarction or stroke. Prevention of transient ischemic attack and stroke is an additional off-label indication (11).
Vitamin K antagonists inhibit the liver’s vitamin K epoxide reductase complex 1 (VKORC1). This reduces the amount of vitamin K reserves and therefore reduces the production of the body’s clotting factors that require vitamin K. Some reduced clotting factors include factors II, VII, IX, and X (7).
Vitamin K antagonists are available orally and are typically taken once daily in the afternoon or evening. Warfarin is rapidly absorbed, and the onset of action is about 24 to 72 hours. The medication’s therapeutic effects aren’t generally seen until five to seven days after taking the drug. The typical starting dosage is 5 mg, but it might be less (2.5 mg) in patients who are elderly or who have liver disease (11).
Some common side effects of this class of medications include:
- Bleeding or bruising
- Nausea
- Vomiting
- Abdominal pain
- Altered sense of taste (11).
As VKAs alter the body’s ability to clot, they can cause more serious adverse effects, such as:
- Significant bleeding or hemorrhage
- Purple toe syndrome
- Warfarin-induced skin necrosis
- Calciphylaxis (11).
Warfarin and other VKAs have several contraindications, such as gastrointestinal bleeding, cerebral aneurysm, dissecting aortic aneurysm, or other hemorrhagic conditions. Additionally, if the patient has had a recent nervous system, eye, or trauma surgery, then this medication should not be prescribed. Threatened abortion, malignant hypertension, and regional or lumbar anesthesia blocks are contraindications as well (11).
When prescribing VKAs, healthcare providers must order therapeutic monitoring and assess the patient’s prothrombin (PT) or international normalized ratio (INR). INR is usually the preferred method where a baseline level is initially drawn and subsequent levels are drawn within a week. The therapeutic range depends on the underlying medication condition, where the goal can range from an INR of 2 to 3 for most patients or from 2.5 to 3 for patients with a mechanical mitral valve.
Once the patient has reached their therapeutic goal, levels are typically drawn every four weeks unless there have been changes in their medications. Additionally, patients who take VKAs should have their hemoglobin and hematocrit levels and liver and kidney function checked at least every 6 months (11).
Ask yourself...
- What is the pharmacokinetics of vitamin K-dependent antagonists?
- What are the common side effects and contraindications of vitamin K-dependent antagonists?
- Which lab value(s) require routine monitoring when administering vitamin K antagonists?
- What education should you provide to a patient being started on a vitamin K antagonist?
- What is the reversal for warfarin?
Direct Oral Anticoagulants
Direct oral anticoagulants (DOACs) are a class of anticoagulant medications mainly used to prevent thrombosis formation. There are two main classes of DOACs, which include direct thrombin inhibitors and direct factor Xa inhibitors (6).
Direct Thrombin Inhibitors
Direct thrombin inhibitors (DTIs) are used to treat and prevent thrombosis in patients with heparin-induced thrombocytopenia, acute coronary syndrome, or during percutaneous coronary intervention. Common names of direct thrombin inhibitors include argatroban, bivalirudin, fondaparinux, and dabigatran (9). Additional off-label uses may consist of anticoagulation during dialysis or renal replacement therapy, and it can be used as an alternative anticoagulant for patients who are resistant to heparin (10).
Direct thrombin inhibitors block coagulation activities involved in fibrin clot formation. There are two types of direct thrombin inhibitors: univalent DTIs that act by binding to the active site of thrombin and bivalent DTIs that bind to two sites, the active thrombin site and exosite I.
As the thrombin pathways are blocked and cleavage of fibrinogen to fibrin is prevented, this ultimately inhibits clot formation and coagulation (9). Some DTIs, like argatroban, are metabolized primarily via the cytochrome P450 enzyme (10).
This class of anticoagulant medications is typically available in intravenous form. It is also available in oral form, but dabigatran is the only available oral form. Starting dosages for DTIs depend on the treated medical condition (9).
Some side effects of DTIs may include:
- Bleeding
- Gastrointestinal symptoms and bleeding
- Hypotension
- Dyspnea
- Fever
- Sepsis (10).
When DTIs are used during PCI, some potential side effects are back pain, nausea, chest pain, and headache. Contraindications include increased bleeding, a history of medication hypersensitivity, lumbar puncture, and spinal anesthesia (10).
As most patients treated with DTIs have HIT, aPTT monitoring is common. The goal is 1.5 to 3 times the patient’s baseline. ACT is used for patients who undergo PCI, and levels are obtained according to the hospital’s protocol or policy (10).
Direct Factor Xa Inhibitors
Direct factor Xa inhibitors, such as apixaban, rivaroxaban, and edoxaban, are used to prevent and treat several blood clotting disorders. They are only available in oral form (9).
These medications are often used to reduce the risk of stroke in patients with atrial fibrillation or other cardiac arrhythmias. Direct Xa inhibitors also prevent and treat conditions like DVT or PE [1]. Rivaroxaban is approved for secondary prevention and adjunct therapy after acute coronary syndrome and peripheral artery disease [12].
Direct factor Xa inhibitors directly bind to factor Xa, preventing it from cleaving prothrombin to thrombin. This blocks the propagation phase of the coagulation cascade [9]. It is metabolized via the liver and CYP3A4/5 and CYP2J2 mechanisms [12].
Starting dosages of direct factor Xa inhibitors depend on the underlying medical condition. Rivaroxaban is usually started at 20mg daily for atrial fibrillation stroke prevention or 10mg daily for VTE prevention [12].
Some side effects of direct factor Xa inhibitors may include:
- Dizziness
- Hemorrhage
- Abdominal Pain
- Pruritis
- Nausea (1)(12)
Direct factor Xa inhibitors have several drug interactions and thus, the healthcare provider must review any potential interactions before initiating this medication. Common drugs that have potential interactions are aspirin, ketoconazole, phenytoin, rifampin, and carbamazepine (1).
They are also contraindicated in patients with hepatic and renal impairment, antiphospholipid syndrome, and increased bleeding risk. Most have black box warnings of increased risk of thrombotic adverse events and spinal or epidural hematoma. They should also be avoided in pregnant or breastfeeding patients (12).
Although this class of medications does not require routine blood monitoring, the healthcare provider should consider obtaining baseline aPTT, PT, and kidney and liver function.
If the patient is scheduled for surgery with a moderately high risk of bleeding, direct factor Xa inhibitors should be held for at least 48 hours before the procedure (1).
Ask yourself...
- What is the pharmacokinetics of direct thrombin inhibitors?
- What are the common side effects and contraindications of direct thrombin inhibitors?
- What is the pharmacokinetics of direct factor Xa inhibitors?
- What are the common side effects and contraindications of direct factor Xa inhibitors?
- What education should you provide patients being started on direct oral anticoagulants?
Considerations for Prescribers
When prescribing anticoagulant medications, healthcare providers must consider and review several factors. First, the route and dosage are typically determined by the setting, inpatient versus outpatient, if it’s being used for prevention or treatment, and the underlying medical condition.
Additionally, healthcare providers should strive to follow current guidelines, approved uses, and hospital protocols when initiating or adjusting these medications. Healthcare providers must review the patient’s medical history, baseline lab values, contraindications, and recommended therapeutic medication range as discussed above.
Low molecular weight heparin is often considered safe during pregnancy (13). Certain anticoagulants, especially warfarin, should be avoided in patients who are elderly, prone to falls, or have reduced kidney function (11). Anticoagulants are often combined with antiplatelet medications to produce better therapeutic results in certain medical conditions (8).
Healthcare providers should also review the potential common and adverse side effects of anticoagulants with patients. Additionally, as bleeding is a serious adverse effect, they should review potential signs of bleeding, such as dizziness, uncontrolled bleeding to the skin, blood in the stool or urine, and other symptoms (15). Patients should be instructed to seek immediate medical treatment if they experience any of these symptoms, fall at home, or have another traumatic injury.
In addition, they should be instructed on how to control bleeding when cuts occur to the skin [11]. A common side effect of LMWH is pain and bruising around the injection site. Therefore, patients should understand the importance of rotating injection sites, and if side effects become a concern, then an oral alternative should be considered (13).
As periodic blood monitoring is required for many anticoagulants, such as INR, PT, or aPPT, the healthcare provider must review the importance of completing these labs and coming to their scheduled visits (15,11). Suppose the patient is non-compliant with laboratory monitoring. In that case, the healthcare provider should consider other alternatives, such as direct oral anticoagulants that do not require routine bloodwork or DTIs with a wider therapeutic range (6)(9).
In addition, the healthcare provider must be knowledgeable about the potential drug interactions for each medication and dietary concern. For example, patients on vitamin K antagonists should reduce their intake of foods high in vitamin K since it counteracts the therapeutic effects of the medication. They should avoid vitamin K-rich foods such as spinach, kale, and broccoli (7). Co-administration of other anticoagulants, aspirin, and non-steroidal anti-inflammatory medications also increases bleeding risk (11).
Most anticoagulant medications can potentially cause toxicity, and patients should seek emergency medical treatment. Therefore, healthcare providers must be aware of the signs and symptoms of toxicity and the reversal agent, or antidote, for each medication.
Protamine sulfate is the reversal agent for heparin and low molecular weight heparin. Protamine sulfate is administered via IV push and, if administered too rapidly, can lead to pulmonary edema, vasoconstriction, and pulmonary hypertension (15). Vitamin K is the antidote for vitamin K antagonists (11). Andexanet alfa is the reversal agent for direct factor Xa inhibitors, and idarucizumab is approved for the reversal of dabigatran specifically (12).

Ask yourself...
- What factors should healthcare providers consider when prescribing anticoagulants?
- What are the reversal agents for each anticoagulant class?
- Which anticoagulant medication is often prescribed during pregnancy?
- What health conditions and lab values are essential when selecting anticoagulants?.
- What barriers to providing adequate education to patients have you encountered? How do you overcome those?
- Some anticoagulant therapies are costly. What resources can you access to help patients pay for their medications?
- What groups are at higher risk for complications related to anticoagulants?
Upcoming Research
There have been many new anticoagulant medications introduced over the past several years and research continues to evolve for this type of medication. More recently, a newer class of anticoagulant medications called anti-factor XI and Xia inhibitors, affect the factor XI pathway for clot formation. However, it’s still being researched and there are ongoing clinical trials (3).
Moreover, in November 2023, the American Heart Association released promising information about abelacimab, a monoclonal antibody that acts as a factor XI inhibitor. This medication has been shown to reduce stroke risk in patients with atrial fibrillation. The same report stated that this medication reduced the bleeding risk by more than 60% (2).
Potential factor VII and VIII inhibitors are also being researched (16).
Ask yourself...
- What new research is there about anticoagulants?
- Which new class of medications has shown promise in reducing bleeding risk in patients with atrial fibrillation
Conclusion
Anticoagulants are typically indicated for preventing and treating blood clotting conditions, including deep vein thrombosis and pulmonary embolism. However, they have several additional approved and off-label uses. When selecting an anticoagulant, healthcare providers should understand the pharmacokinetics, potential side effects, and contraindications. They should also follow current clinical guidelines and their facility’s protocols for a more evidence-based approach.
SNRIs for Depression
Introduction
Depression can significantly interfere with daily activities and diminish the quality of life among patients who experience it. Major depressive disorder (MDD) is one of the leading causes of the burden of worldwide diseases (5). At the present stage, the first-line treatment of MDD is selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). Research has found that SNRIs showed faster antidepressant effects than SSRIs (5).
The serotonin norepinephrine reuptake inhibitors (SNRIs) are a family of antidepressants that inhibit the reuptake of both serotonin and norepinephrine; however, it is essential to recognize the differences among each specific drug in this class. In this course, we will examine pharmacological properties, clinical indications, mechanism of action, metabolism and excretion, dosing schedules, side effects, and warnings of each SNRI currently approved by the United States Food and Drug Administration (FDA) for treating depression.
Forms and Symptoms of Depression
Depression can have crippling effects. Research suggests that genetic, biological, environmental, and psychological factors play a role in depression (7). Depression can be present with other comorbidities, such as mental disorders, diabetes, cancer, heart disease, and chronic pain. Depression can make these conditions worse, and vice versa. Certain medications have been shown to increase depressive symptoms.
Typical forms of depression are: (7)
- Major depression – which includes symptoms of depression most of the time for at least two weeks that regularly interfere with work, sleep, eating, and quality of life.
- Persistent depressive disorder (dysthymia), which often includes less severe symptoms of depression that last much longer, typically for at least two years.
- Perinatal depression occurs when a woman experiences major depression during pregnancy or after delivery (postpartum depression).
- Seasonal affective disorder is impacted by seasonal changes, typically starting in late fall and early winter and improving during spring and summer.
- Depression with symptoms of psychosis – a severe form of depression in which the patient experiences psychosis symptoms, such as delusions or hallucinations
Common symptoms of depression include:
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness
- Irritability, frustration‚ or restlessness
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies or activities once enjoyed
- Fatigue or lack of energy
- Aches, musculoskeletal pain, or headaches without injury or obvious causation do not improve with medication.
- Gastrointestinal issues
- Difficulty concentrating, remembering, or making decisions
- Poor sleeping patterns
- Changes in appetite
- Suicide attempts or thoughts of death or suicide

Ask yourself...
- Are you aware of particular biological, environmental, or psychological factors that play a role in depression?
- How would you describe the difference between occasional sadness and depression?
- Can you name common symptoms of depression?
- Which drug class has shown faster antidepressant effects in studies, SSRIs or SNRIs?
- Have you seen an increase in the use of SNRI and SSRI medications throughout your years of practice?
Pharmacokinetics of SNRIs
SNRIs’ major action mechanism is the inhibition of presynaptic neuronal uptake of 5-HT (serotonin) and norepinephrine after release from the synaptic cleft (3). Blocking the reuptake prolongs the presence of monoamines in the synaptic cleft within the central nervous system (CNS). This causes an increase in postsynaptic receptor stimulation and additional postsynaptic neuronal transmission (3).
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT), is a neurotransmitter that has an essential role in regulating various activities, including behavior, mood, memory, and gastrointestinal homeostasis (1). It is often referred to as the “feel-good hormone”. It delivers messages between brain cells, contributing to well-being, mood, appetite, social behavior, and helping regulate the body’s sleep-wake cycle.
Serotonin is synthesized in the brainstem’s raphe nuclei and the intestinal mucosa’s enterochromaffin cells (1). Serotonin is a primary treatment target for major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), and anxiety disorders.
Norepinephrine
Norepinephrine, also known as noradrenaline, is a neurotransmitter in the brain that plays an essential role in the regulation of arousal, attention, cognitive function, and stress reactions. It also functions as a hormone peripherally as part of the sympathetic nervous system in the “fight or flight” response.
During states of stress or anxiety, norepinephrine and epinephrine are released and bind to adrenergic receptors throughout the body. These receptors exert effects such as dilating pupils and bronchioles, increasing heart rate and constricting blood vessels, increasing kidney renin secretion, and inhibiting peristalsis.
It works closely with the dopamine and serotonin systems and is thought to help mobilize the brain for action, increasing alertness, focus, and memory retrieval (4).
The increased availability of serotonin and norepinephrine in the nerve synapse means that information can be transmitted more easily from one nerve to another.
The FDA currently approves five SNRIs for use in the U.S.:
- Venlafaxine (Effexor XR)
- Duloxetine (Cymbalta, Irenka)
- Desvenlafaxine (Pristiq, Khedezla)
- Levomilnacipran (Fetzima)
- Milnacipran (Savella)
- The FDA does not approve Milnacipran (Savella TM) to treat depression, but rather only fibromyalgia. For this reason, this course will not review this SNRI.
As mentioned, all SNRIs regulate serotonin and norepinephrine, but there are subtle differences in how much absorption they prevent and the side effects of each type.
Ask yourself...
- Do all SNRIs have the same uses?
- Can you define serotonin and norepinephrine in your own words?
- Are you familiar with these SNRIs? If so, do you know patients who have benefited from its use?
- How would you describe the relationship between sleep cycles, nutrition, and mood?
- What is serotonin?
- What is norepinephrine?
- What is the difference between serotonin and norepinephrine?
Venlafaxine
Venlafaxine (Effexor ™) was the first SNRI approved in the U.S. It is available in an extended-release formula and works by regulating levels of serotonin and norepinephrine.
Uses
- Major depressive disorder
- Generalized anxiety disorder
- Social anxiety disorder
- Panic disorder
Mechanism of Action
Venlafaxine increases serotonin, norepinephrine, and dopamine in the brain by blocking transport proteins and preventing their reuptake at the presynaptic terminal (10). This action leads to more neurotransmitters available at the synapse, ultimately increasing stimulation of postsynaptic receptors.
Venlafaxine is a bicyclic phenylethylamine compound; it is a more potent inhibitor of serotonin reuptake than norepinephrine reuptake (8). Venlafaxine acts as a selective serotonin reuptake inhibitor at 75 mg, but when a higher dose is given, such as 225 mg/day, it has significant effects on the norepinephrine transporter as well (10). Venlafaxine does not have MAO-inhibitory properties.
Pharmacodynamics
The pharmacodynamics of venlafaxine is as follows: (9)
Absorption: 92-100% absorbed after oral administration.
Distribution: Extensive distribution into body tissues.
Metabolism and Excretion: Extensively metabolized in the first pass through the liver. 5% of venlafaxine is excreted unchanged in urine; 30% of the active metabolite is excreted in urine.
Half-life: 3-5 hr.; ODV: 9-11 hr.
Contraindications
- Hypersensitivity
- Precautions are advised for use in patients with:
- Cardiovascular disease
- Hepatic or renal impairment
- History of seizures or neurologic impairment
- History of mania
- History of drug abuse
- Angle-closure glaucoma
Obstetrics (OB): Use during pregnancy only if risks are closely examined. There is a potential for discontinuation syndrome or toxicity in the neonate when venlafaxine is taken during the 3rd trimester (9). Venlafaxine is a category C pregnancy drug and can potentially pass into breast milk (8).
Venlafaxine is contraindicated if it causes worsening suicidal ideation, depression, anxiety, and psychosis. Precaution is needed for patients with heart failure patients, hyperthyroidism, and those with recent myocardial infarctions, as it can raise blood pressure and increase heart rate. Venlafaxine raises the risk of seizures, and prescribers should avoid the drug in patients with a seizure disorder (8).
Adverse Reactions / Side Effects
Adverse reactions / side effects of venlafaxine include: (10)
- CV: Chest pain, hypertension, palpitations, tachycardia.
- Neuro: Abnormal dreams, anxiety, dizziness, headache, insomnia, nervousness, paresthesia.
- Dermatology: ecchymoses, itching, photosensitivity, skin rash.
- EENT: rhinitis, visual disturbances.
- GI: abdominal pain, altered taste, anorexia, constipation, diarrhea, dry mouth, dyspepsia, nausea, vomiting, weight loss.
- GU: Decreased libido, erectile dysfunction, urinary frequency, urinary retention.
- Hematological: Bleeding.
- Serotonin syndrome – a condition of building up high levels of serotonin in the body due to medication use. Although rare, serotonin syndrome is a very serious condition that has a high mortality rate (5). Signs of serotonin syndrome include tachycardia, sialorrhea (excessive saliva production), hyperactive bowel sounds, mydriasis (sustained dilated pupils), hyperthermia, and diaphoresis (6). This condition is caused by combining monoamine oxidase inhibitors, tricyclic antidepressants, triptans, additional serotonin receptor modulators, or over-the-counter drugs such as St. John’s Wort (6).
If a clinician suspects serotonin syndrome, the Sternbach and Hunter criteria can help arrive at a definitive diagnosis.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors or serotonergic neurotransmitter systems, including tricyclic antidepressants, fentanyl, buspirone, tramadol, amphetamines, and triptans, may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin , or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of the drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
- PO (Adults): Tablets: 75 mg/day in 2-3 divided doses; may increase by up to 75 mg daily every 4 days, up to 225 mg/day. Do not exceed 375 mg per day in 3 divided doses.
- Extended-release capsules: 75 mg once daily (some patients may be started at 37.5 mg once daily) for 4-7 days; may increase by up to 75 mg/day at intervals for no less than 4 days (not to exceed 225 mg/day).
ALERT: US Boxed Warning
- Antidepressants increased the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders (10). Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults older than 24 years old (10).
- Venlafaxine is not approved for use in pediatric patients.
Ask yourself...
- What are the primary mechanisms of action for Venlafaxine?
- Can you explain the side effects and contraindications of Venlafaxine?
- Are SNRIs currently recommended for pediatric patients?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
- What education would you provide to a patient being started on venlafaxine?
Duloxetine
Duloxetine (Cymbalta ™) has the most FDA-approved uses of any SNRI and, unlike Venlafaxine, is proven to be effective in treating other conditions, such as neuropathy, fibromyalgia, and osteoarthritis (9).
Drug Class
Selective Serotonin/Norepinephrine Reuptake Inhibitors
Uses
Duloxetine is used for the following conditions: (9)
- Major depressive disorder
- Diabetic peripheral neuropathic pain
- Generalized anxiety disorder
- Chronic musculoskeletal pain (including chronic lower back pain and chronic pain from osteoarthritis)
- Fibromyalgia
Mechanism of Action
Duloxetine inhibits serotonin and norepinephrine reuptake and enhances dopamine levels within the prefrontal cortex. This increase in dopamine levels involves the inhibition of norepinephrine transporters, which are particularly attracted to dopamine, making it effective in transporting dopamine and norepinephrine (2). Essentially, inhibition of norepinephrine transporters can cause an increase in dopamine.
The secondary mechanisms of action, in increasing dopamine, are helpful in pain reduction. This occurs due to increased activity of noradrenergic and serotonergic neurons in the descending spinal pathway on the dorsal horn and suppression of excessive input from reaching the brain (2). The perception of pain can be reduced as these signals are interrupted.
Pharmacokinetics
Absorption: Well-absorbed following oral administration.
Distribution: Unknown.
Protein Binding: >90%.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP2D6 and CYP1A2 isoenzymes. Excretion: Fecal is 20%; renal is 70% as metabolites.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
| ROUTE | ONSET | PEAK | DURATION |
| PO | Unknown | 6 hr. | 12 hr. |
Contraindications:
Contraindications in the use of duloxetine include: (9)
- Hypersensitivity
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Severe renal impairment (CCr <30 mL/min).
- Hepatic impairment or substantial alcohol use (increased risk of hepatitis).
Use cautiously in:
- History of suicide attempt or ideation.
- History of mania (may activate mania/hypomania).
- History of seizure disorder.
- Diabetes (may worsen glycemic control).
- Angle-closure glaucoma.
OB/ Lactation: Use while pregnant or breastfeeding only if the potential maternal benefit justifies the potential risk to the infant.
Geriatric Precaution: Appears on Beers list. May worsen or cause syndrome of inappropriate antidiuretic hormone (SIADH) secretion and/or hyponatremia in older adults; closely monitor sodium concentrations.
Adverse Reactions / Side Effects
- CV: Hypertension, orthostatic hypotension.
- EENT: Increased intraocular pressure, blurred vision.
- Endocrine: SIADH.
- Fluids and Electrolytes: Hyponatremia.
- GI: Decreased appetite, constipation, dry mouth, nausea.
- GU: Dysuria.
- Neurological: Drowsiness, fatigue, insomnia.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitors before initiating levomilnacipran (9). Simultaneous use with MAO-inhibitor-like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
- Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
- Concurrent use of NSAIDs, aspirin, warfarin , or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of the drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
PO (Adults): 40-60 mg/day (as 20 mg or 30 mg twice daily or as 60 mg once daily) as initial therapy, then 60 mg once daily as maintenance therapy (9).

Ask yourself...
- What are the primary mechanisms of action for Duloxetine?
- Can you explain the side effects and contraindications of Duloxetine?
- Which patient symptoms should be prioritized: mild drowsiness or tachycardia?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
- What education would you provide to a patient being started on duloxetine?
Desvenlafaxine
Desvenlafaxine (Pristiq ™) received FDA approval in 2008. The drug is structurally similar to Venlafaxine and 10 times more potent in inhibiting serotonin reabsorption than norepinephrine (6). It has been observed to weakly block dopamine reuptake, identical to Venlafaxine and Duloxetine.
Uses
Desvenlafaxine is an antidepressant that is an FDA-approved drug to treat major depressive disorder in adults. For healthy women who have contraindications to estrogen, desvenlafaxine has an off-label use to treat hot flashes during menopause (6).
Mechanisms of Action
Desvenlafaxine is the primary active metabolite of venlafaxine. Therefore, the two agents are structurally similar in that they contain two chemical rings opposite each other. In vitro studies have shown that desvenlafaxine is ten times more selective for serotonin than norepinephrine, making it similar to duloxetine (6).
Pharmacokinetics
Absorption: 80% absorbed following oral administration.
Distribution: Widely distributed to tissues.
Metabolism and Excretion: 55% metabolized by the liver, 45% excreted unchanged in urine.
Half-life: 10 hr.
Time / Action Profile (Plasma Concentrations):
| ROUTE | ONSET | PEAK | DURATION |
| PO | Unknown | 7.5 hr. | 24 hr. |
Contraindications/Precautions
- Hypersensitivity to venlafaxine or desvenlafaxine.
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Should not be used concurrently with venlafaxine.
Use cautiously in:
- Untreated cerebrovascular or cardiovascular disease, including untreated hypertension (control BP before initiating therapy).
- Bipolar disorder (may activate mania/hypomania).
- Moderate or severe renal impairment.
- History of seizures or neurologic impairment.
- Moderate or severe hepatic impairment.
- Angle-closure glaucoma.
OB: Safety is not established in pregnancy. Desvenlafaxine is excreted into breast milk, with one study reporting that peak levels are 3.3 hours after a dose (6).
Adverse Reactions / Side Effects
There is a broad range of general adverse effects that patients have reported with desvenlafaxine. A study comparing desvenlafaxine to a placebo in treating major depressive disorder in adolescents noted the most common side effects to be abdominal pain, decreased appetite, headache, and nausea (6).
Less common side effects were diarrhea, dizziness, and cough.
Stopping desvenlafaxine can cause irritability, nausea, and headaches (6). To avoid such side effects, the medication should be tapered gradually.
Drug-Drug Interactions
- The concurrent use of all SNRIs and MAO inhibitors may result in serious and potentially fatal reactions (it may increase the risk of serotonin syndrome) (9). A period of at least two weeks is recommended between stopping MAO inhibitors and initiating venlafaxine (9).
- Concurrent use of NSAIDs, aspirin, warfarin , or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of the drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
Route and Dosage in Depression
- PO (Adults): 50 mg once daily (range = 50-400 mg/day).
Renal Impairment:
- PO (Adults): CCr 30-50 mL/min: 50 mg once daily; CCr <30 mL/min: 50 mg every other day or 25 mg once daily.
Hepatic Impairment:
- PO (Adults): 50 mg once daily (not to exceed 100 mg/day).
Ask yourself...
- What are the adverse effects of desvenlafaxine?
- Can you explain why SNRI and MAO inhibitors are contraindicated for use together?
- What education would you provide to a patient being started on desvenlafaxine?
Levomilnacipran
Levomilnacipran (Fetzima ™) is the most recent medication approved for treating major depression.
Levomilnacipran is different from other SNRIs because it has greater inhibition of norepinephrine than serotonin; essentially, it has twice the potency of norepinephrine reuptake inhibition compared to serotonin (3). The more recent FDA approval and relatively fewer prescriptions may contribute to a low volume of research and evidence on this drug.
Levomilnacipran is available in a once-a-day extended-release form, which makes it easier for patients to adhere to their regimen. The drug does not affect dopamine but steadily inhibits the reuptake of serotonin and norepinephrine at any dose (3).
Uses
- Major Depressive Disorder
Mechanisms of Action
Levomilnacipran enhances the amount of serotonin (5-HT) and norepinephrine (NE) activity in the central nervous system by inhibition of reuptake at 5-HT and NE transporters (9). Levomilnacipran is a more potent inhibitor of NE versus 5-HT transporter (9).
Pharmacokinetics
Absorption: Well-absorbed (92%) following oral administration.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP3A4 isoenzyme. 58% eliminated unchanged in urine; 42% metabolized; metabolites are renally eliminated.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
| ROUTE | ONSET | PEAK | DURATION |
| PO | unknown | 6-8 hr. | unknown |
Contraindications/Precautions
- Hypersensitivity to Levomilnacipran or milnacipran.
- Uncontrolled narrow-angle glaucoma.
- Use Cautiously in:
- Hypertension, cardiovascular, or cerebrovascular disease (blood pressure should be controlled before treatment).
- Bipolar disorder (may activate mania/hypomania).
- Renal impairment.
OB: Use only if the potential maternal benefit justifies the potential fetal risk.
Geriatric: Consider age-related decrease in renal function, chronic disease state, and concurrent drug therapy in older adults; increased risk of hyponatremia.
Adverse Reactions/Side Effects
- CV: Hypertension, hypotension, palpitations, tachycardia.
- Dermatology: Hyperhidrosis (excessive sweating), rash.
- EENT: Mydriasis.
- Fluids and Electrolytes: Hyponatremia (in association with syndrome of inappropriate antidiuretic hormone [SIADH]).
- GI: Nausea, constipation, decreased appetite, vomiting.
- GU: Sexual dysfunction, testicular pain, urinary hesitation, and retention.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitors before initiating levomilnacipran (9). Simultaneous use with MAO-inhibitor-like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin , or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of the drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Ask yourself...
- What are the major mechanisms of action for Levomilnacipran?
- Does an increased dosage of Levomilnacipran result in different neurotransmitter activity?
- What education would you provide to a patient being started on levomilnacipran?
Review of SNRI Pharmacokinetics
Figure 1. Pharmacokinetics of SNRIs [Created by course author].
Nursing Considerations
It is vital to recognize how each patient is unique and different in their journey of depression treatment. Nurses play a key role in assessing and providing advocacy for these patients.
The following are essential nursing considerations:
- Thorough and focused mental status assessment
- Assess level of consciousness and orientation, appearance and general behavior, speech, motor activity, affect and mood, thought and perception, attitude and insight, and cognitive abilities.
- Assess status and mood changes.
- Assess suicidal tendencies, especially in the early stages of SNRI (9).
- Monitor BP before and periodically during therapy. Sustained hypertension may be dose-related; decrease the dose or discontinue therapy if this occurs.
- Assess for serotonin syndrome.
- Provide patient education on medication use, dosage, and side effects. Provide patient education on support and resources available.
Nursing Diagnosis: Disturbed Thought Processes
Nursing Diagnosis: Ineffective Coping
Lab Assessments
Monitor CBC with differential and platelet count periodically during SNRI therapy. These medications can cause anemia, leukocytosis, leukopenia, thrombocytopenia, basophilia, and eosinophilia (9).
It may cause an increase in serum alkaline phosphatase, bilirubin, AST, ALT, BUN, creatinine, and serum cholesterol.
SNRIs can also cause electrolyte abnormalities (hyperglycemia or hypoglycemia, hyperkalemia or hypokalemia, hyperuricemia, hyperphosphatemia or hypophosphatemia, and hyponatremia).
Conclusion
SNRIs are an effective method of treating depression. Their effectiveness is dependent on various factors, like dosage and time spent in the body. Although the mechanisms of action and makeup of SNRIs are consistent, each drug has differences in how much reuptake it prevents and the side effects of each type. Prescribers must be aware of these differences and the uses, mechanisms of action, side effects, and warnings of each drug.
Corticosteroid Therapy
Introduction
When many people hear of steroids, they may think of athletes. However, the steroids that some athletes misuse to enhance their strength and training abilities are not the same as those administered for allergic reactions and some inflammatory conditions. The steroids used by some athletes are called anabolic steroids. Steroids are called corticosteroids and are used for the treatment of diseases.
Steroids are hormones naturally produced in the body (made from cholesterol) to regulate the function of various bodily systems [2]. Some steroid hormones are produced by the adrenal glands of the endocrine system (a set of two glands that rest atop the kidneys). The adrenal glands produce the hormone types: glucocorticoids, mineralocorticoids, and adrenal androgens [2]. Corticosteroid medication mimics glucocorticoids, more specifically, a glucocorticoid called “cortisol.”
The Immune System
Corticosteroids are well-known for increasing a patient’s risk of infection. This is due to the medication’s effect on the immune system, particularly its anti-inflammatory properties. It is important to have a clear understanding of how the immune system works and the inflammatory process that takes place when a foreign pathogen enters the body.
How Does the Immune System Work?
In a typical immune system, an antigen is a marker in the body that alerts cells of the immune system that there’s a foreign invader present, like a pathogen or foreign object. Subsequently, a series of events follows. Like in a war, the body rounds up the first line of defense soldiers to fight the pathogen.
These include (11):
- Proteins (called “the inflammasome”) activate inflammatory responses.
- Macrophages engulf and digest pathogens.
- Neutrophils help kill pathogens and rid the body of the leftover debris.
- Endothelial cells help rid the dead pathogens/debris.
- The influx of these cells to the site of injury or infection is the beginning of inflammation.
What is Inflammation?
Inflammation is more than just “swelling.” Inflammation is a process in which a cascade of events occurs to help the immune system fight against pathogens and foreign bodies. The inflammatory process encompasses a variety of signs/symptoms, including redness, warmth, swelling, and drainage (11).
For example, when a person sustains a traumatic injury to the skin, causing an open wound, the accompanying redness (or erythema) is caused by a rush of red blood cells through the site (5). The dilation of the blood vessels causes this “rush” of cells. The increased blood flow also causes the localized warmth that typically occurs. Blood vessel dilation is responsible for the associated swelling as well. The pus that forms is a combination of fluid, dead immune/pathogen cells, and debris from the fight, essentially, the “casualties at war” that must be removed from the body (5).
As with fever, inflammation aids in the fight against pathogens and therefore should not be inhibited unless it is likely to be damaging or fatal in and of itself (11). This inflammatory process is acute and typically goes away on its own or with the help of medication. While acute inflammation from a normal immune response is natural and beneficial, chronic inflammation is harmful and can lead to permanent damage to tissues and body systems.
Chronic inflammation can be seen in conditions including inflammatory bowel disease, cancer, heart disease, and autoimmune disorders like rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus (11). Chronic inflammation is typically caused by an overactive immune system or autoimmune disease (when the immune system mistakenly attacks normal tissues). In cases like these, inhibiting the inflammatory process by suppressing the immune system is most beneficial. This is where corticosteroids come into play.
Ask yourself...
- How often do you encounter patients with autoimmune disease or chronic inflammatory diseases?
- Have you ever cared for a patient who was on long-term corticosteroid therapy?
- Have you ever cared for a patient who developed an infection after corticosteroid use?
- How comfortable are you with the idea of immunosuppression?
- What is the difference between inflammation and infection?
Corticosteroids: The Basics
Corticosteroids are a group of steroidal therapy medications often used to reduce inflammation in the body. Once referred to as “a miracle drug,” these medications are helpful in the treatment of acute and chronic inflammatory conditions and autoimmune diseases.
Corticosteroids are also useful in emergency situations, such as asthma attacks or allergic reactions, when the inflammation is life-threatening. They are synthetically formulated to mimic the hormone cortisol.
What is Cortisol?
As mentioned earlier, the adrenal glands of the endocrine system produce three different types of steroid hormones, one of which is glucocorticoids. Cortisol is a primary glucocorticoid hormone.
The adrenal glands have two major structures – the outer layer (cortex) and inner portion (medulla) – each producing its own hormones. The outer layer (cortex) produces cortisol, hence the name “corticosteroid” (6).
Cortisol levels in the body rise when a person is stressed (2). This hormone helps to regulate metabolism, growth, reproduction, and the immune system (2). Cortisol also aids cardiovascular function by making blood vessels more sensitive to natural vasoconstrictors (like adrenaline) (2).
How Do Corticosteroids Work?
Corticosteroids work by suppressing the immune system, which in turn reduces inflammation. In patients with an overactive immune system or acute flare-ups from a triggering agent, the inflammation can cause troubling symptoms, become prolonged, and spread to other areas within the body. If left untreated, inflammation can lead to tissue scarring (or damage to affected structures) and loss of bodily functions (11).
The challenge with administering corticosteroids to suppress an overactive immune system is balancing the benefits of inflammatory symptom relief and the risks of infection due to the resulting underactivity of the immune system. In this case, clinicians are encouraged to start with the lowest therapeutic dose possible, carefully monitor its use in patients with infections, and ensure that the benefits exceed the risks (6).
Corticosteroids also stop the body from producing its natural cortisol. In a sense, a course of corticosteroids gives the adrenal glands a break. In a short course of corticosteroids, once the medication is discontinued, the adrenal glands can start releasing cortisol again. However, the adrenal glands do not resume releasing cortisol right away and need time to start working again.
This is why corticosteroids (oral and parenteral forms) should not be stopped abruptly when taken for more than about two weeks (6). If stopped abruptly, patients may develop adrenal insufficiency during this period, which can be life-threatening.

Ask yourself...
- What do you think is the biggest concern of nurses and clinicians when prescribing/administering corticosteroids?
- Have you encountered patients with permanent bodily dysfunction caused by prolonged inflammation?
- How challenging is balancing infection risk and therapeutic benefit when prescribing/administering corticosteroids?
- How do you determine what to begin with when initiating a new corticosteroid treatment? Or have you ever discussed dosing safety with a provider?
- What education is essential to provide to a patient who is prescribed corticosteroids?
Corticosteroid Pharmacology
Common corticosteroid formulations include intravenous (IV), intramuscular (IM), intra-articular (injected directly into a joint), oral, inhaled, intranasal (nasal spray), topical, ophthalmic (eye drops), and otic (ear canal). The increased risk of infection is most associated with systemic formulations (oral and parenteral).
The following sections will cover the pharmacology of systemic corticosteroids, including indications, mechanisms of action, pharmacokinetics, precautions, adverse effects, drug interactions, and warnings (7).
Indications
Corticosteroids are helpful in the treatment of many medical conditions. Some indications for corticosteroids among adults include the following diseases, conditions, and situations (maybe drug- or route-specific) (6)(7).
Allergic Reactions
Corticosteroids are well-known for treating mild to severe allergic reactions. These include anaphylaxis, angioedema, and laryngeal edema (non-infectious). Others include hypersensitivity reactions to medications or food, severe seasonal allergies (like allergic rhinitis), contact dermatitis (or rash), and itching.
Autoimmune Diseases
Corticosteroids treat autoimmune disorders, including systemic lupus erythematosus, Crohn’s disease, rheumatoid arthritis, and plaque psoriasis. Others include autoimmune hepatitis, hemolytic anemia, celiac disease (gluten intolerance), sarcoidosis (marked by lumps of inflammatory cells throughout the body), and myasthenia gravis (marked by weakness in voluntary muscles).
Pulmonary Exacerbations
Corticosteroids are helpful in the treatment of exacerbations from asthma and chronic obstructive pulmonary disease (COPD).
Adrenal Insufficiency
Corticosteroids may be used to treat both primary and secondary adrenocortical insufficiency. This includes Addison’s disease, congenital adrenal hyperplasia, and adrenogenital syndrome.
Inflammatory Bowel Disease
Inflammatory bowel disease, such as the aforementioned Crohn’s disease and ulcerative colitis, may be treated with corticosteroids.
Neuro/Musculoskeletal Disorders
Corticosteroids are used to treat ankylosing spondylitis (chronic inflammation of spinal joints), bursitis, polymyalgia rheumatica (marked by muscle pain and stiffness), osteoarthritis, and psoriatic arthritis. They can also treat neurological conditions like multiple sclerosis and Bell’s palsy (facial paralysis, often temporary).
Inflammatory Eye Conditions
Corticosteroids are useful in the systemic treatment of inflammatory ophthalmic conditions, including optic neuritis, allergic conjunctivitis, and other conditions that cause inflammation of various eye structures.
Organ Transplant Rejection Prophylaxis
Renal transplant guidelines recommend corticosteroids in combination with other medications to prevent kidney rejection after transplantation (called induction therapy or immunosuppressant therapy). Corticosteroids are also indicated for heart and liver transplant rejection prophylaxis.
Cancer
Corticosteroids may be used to palliate cancers, including multiple myeloma and chronic lymphocytic leukemia (CLL). They may also be used to treat Hodgkin lymphoma (in conjunction with cancer medications), aggressive non-Hodgkin lymphoma, peripheral T-cell lymphoma, and neoplastic-associated hypercalcemia.
COVID-19
Although still in the investigative stages, the World Health Organization (WHO) recommends systemic corticosteroids as an adjunctive treatment for severe COVID-19 (6)(13).
Ask yourself...
- Do you prescribe/administer corticosteroids most frequently for acute or chronic conditions?
- What is the most common indication for corticosteroid therapy you have witnessed in your practice?
- Have you ever prescribed/administered corticosteroids for prophylaxis treatment alone?
- Can you think of ways to minimize the severity of adverse effects of corticosteroid therapy among patients most at risk?
- How do corticosteroids impact the adrenal glands?
Mechanism of Action
As already mentioned, corticosteroids suppress the immune system. They do this by preventing protein synthesis in specific immune system cells (like macrophages and neutrophils), ultimately inhibiting their fighting potential (6)(11). More specifically, corticosteroids repress the activity of DNA within the immune cell, an activity that would otherwise serve as the beginning of protein synthesis.
Within immune cells, DNA sends messages to protein-making structures within the cell that prompt them to start making protein. However, the message must be “translated” or “decoded” (transcription) first before the structures can “understand” what to do (6)(12). Messenger RNA (mRNA) is the transcribed version of DNA. Corticosteroids work by making it difficult for DNA to be transcribed into mRNA.
Pharmacokinetics
The pharmacokinetics of corticosteroids differ based on drug type and route. Three common systemic corticosteroid drugs are prednisone, methylprednisolone, and dexamethasone (6).
Prednisone
Prednisone comes in oral forms, both immediate-release (IR) and delayed-release. Prednisone IR is quickly absorbed in the gastrointestinal (GI) tract with a peak effect after one to two hours. Delayed-release tablets are released four hours after ingestion and peak after about six hours. Prednisone is metabolized by the liver and excreted by the kidneys.
Methylprednisolone
Methylprednisolone is rapidly absorbed orally and peaks within one to two hours. After IV administration, effects can occur within one hour, with nearly complete excretion in 12 hours. When given as an injection in the joint, methylprednisolone absorption can occur over several days. This medication is distributed into various organs and body structures, including the kidneys, intestines, skin, liver, and muscle. Methylprednisolone is metabolized by the liver and excreted by the kidneys.
Dexamethasone
Oral dexamethasone peaks in one to two hours. Absorption times for parenteral forms depend on the dosage and indication. For example, in patients with cerebral edema, treatment response from an IV dose of dexamethasone followed by an IM dose occurs in 12 to 24 hours (1). Dexamethasone is rapidly distributed into the skin, intestines, liver, muscle, and kidneys. As with prednisone and methylprednisolone, this medication is metabolized by the liver and excreted by the kidneys.
Ask yourself...
- What is the most common corticosteroid you have prescribed/administered?
- Why do you think it is essential to know the mechanism of action of corticosteroids?
- What is the main determining factor in prescribing/administering an oral corticosteroid instead of a parenteral form?
- What is the difference between prednisone and dexamethasone?
- How do corticosteroids impact the immune system?
Contraindications and Precautions
Clinicians should not prescribe/administer prednisone to patients with an allergy to the drug or any components of its formulation. Precautions should be taken in patients with an allergy to other corticosteroids, as cross-sensitivity can occur. Clinicians should also be aware that high-dose systemic corticosteroids place patients at risk for immunosuppression, especially when prescribed/administered with other immunosuppressant medications.
Although they carry a lower risk, moderate-dose and low-dose corticosteroid preparations should also be monitored. Clinicians should avoid prescribing/administering these medications to patients with fungal or bacterial infections that are poorly controlled with anti-infective medicines.
Pregnancy and Breastfeeding Precautions
While corticosteroids are not contraindicated for pregnant or breastfeeding patients, clinicians have reason to be cautious when prescribing/administering these medications (6). The following are precautions for pregnant and breastfeeding patients.
Pregnancy
Suppose corticosteroids must be prescribed/administered during pregnancy. In that case, precautions should be taken as these medications cross the placenta and may be harmful to the fetus/infant (may cause adrenal insufficiency in the infant). Although the risks are small and inconsistent, oral or facial clefts (like cleft palate) may occur if systemic corticosteroids are prescribed/administered during the first trimester.
Breastfeeding
Corticosteroids are distributed into breastmilk. While no side effects have been reported in breast-fed infants, lower doses are recommended as high doses may cause problems with the infant’s growth and development. Corticosteroids can interfere with the infant’s ability to produce their glucocorticoid hormones. With prednisone in particular, peak concentrations in breastmilk occur in about one hour after the dose is taken, and it is recommended for patients to avoid breastfeeding during this time. However, the total daily dose of prednisone reaching the infant is approximately 0.1% of the mother’s total daily dose (6).
Ask yourself...
- How comfortable are you prescribing/administering corticosteroids to patients taking immunosuppressive medications?
- Have you ever witnessed a patient have a severe allergic reaction to a corticosteroid? If so, what was the treatment/anecdote?
- Have you ever been in a situation in which you had no choice but to prescribe/administer corticosteroids to a patient who was pregnant?
- What education should you provide to a patient who is breastfeeding and being started on corticosteroid therapy?
- Why should clinicians avoid prescribing corticosteroids in patients with a fungal infection?
Adverse Effects
Corticosteroids given systemically can affect multiple body systems, particularly with prolonged use. Short-term use in high doses typically does not cause adverse effects (6). Adverse effects of corticosteroids can range from a rash to as severe as psychosis or a ruptured heart wall.
The following are adverse effects categorized by body systems (6):
Neurological
Corticosteroids may increase intracranial and intraocular pressure, which can lead to optic nerve damage and visual impairment. These medications can also lower seizure thresholds. Other neurological findings include headache, vertigo, and peripheral neuropathy.
Pulmonary
Corticosteroids can reactivate tuberculosis (TB) in patients who have a history of active TB.
Cardiovascular
Sodium retention, edema, and low potassium may occur in patients with high blood pressure or congestive heart failure (can also occur with renal failure/insufficiency). Left ventricular free-wall rupture can occur in patients with a recent myocardial infarction. Corticosteroids may also exacerbate arrhythmias and cause blood clots, which may lead to a stroke.
Endocrine
Corticosteroids are known to decrease glucose tolerance and raise blood glucose levels, which can aggravate existing diabetes mellitus. Prolonged systemic use can also cause hypothalamic-pituitary-adrenal (HPA) suppression or signs/symptoms of Cushing’s syndrome (marked by high cortisol levels).
Gastrointestinal/Genitourinary
Corticosteroids can increase the risk of GI perforation and should, therefore, be monitored in patients with peptic ulcer disease, diverticulitis, or GI abscess. Additionally, esophageal ulcers and GI bleeding may occur with use. Corticosteroids may cause weight loss but can also stimulate the appetite, leading to weight gain. Conversely, menstrual irregularity may occur.
Musculoskeletal
Osteopenia (loss of bone density) and osteoporosis (more severe bone loss) can occur due to decreased bone formation, increased bone resorption, and inhibition of osteoblast function. Bone fractures (primarily in elderly patients) can occur as well. Acute generalized myopathy leading to quadriparesis (weakness in all extremities) may occur in patients with neuromuscular disease or those receiving neuromuscular blocking medications. Muscle pain and waste can also occur due to protein depletion.
Skin
Corticosteroids may cause impaired wound healing due to protein depletion. Sweating, abnormal hair growth, and striae (stretch marks) can also occur with use. While corticosteroids may help treat rashes and itching, they can also cause these symptoms.
Psychiatric
Psychiatric problems can occur with corticosteroid use, including euphoria, severe depression, anxiety, hallucinations, psychosis, personality changes, and withdrawn behavior. Patients may also experience insomnia, impaired cognition, mood swings, irritability, and restlessness.
Laboratory Changes
A typical lab finding with corticosteroid use is leukocytosis (without an infectious or inflammatory cause). Clinicians must differentiate between contagious and corticosteroid-related leukocytosis. Other lab findings may include low neutrophil count and abnormal electrolyte levels (high sodium, calcium, and potassium).
Ask yourself...
- What are the most common adverse effects of corticosteroid therapy you have witnessed in your practice?
- Considering the severity of some adverse effects of corticosteroids, what is your strategy for safe dosing/administration?
- Has a patient under your care ever reported a profound adverse effect from a short-term course of corticosteroids?
- How often do you consult another provider in prescribing corticosteroid therapy for patients with multiple comorbidities? Or how usually do witness providers
- What neurological adverse effects should the nurse educate the patient to monitor for while taking corticosteroids?
Black Box Warning
The U.S. Food and Drug Administration (FDA) issues black box warnings to warn the public about the serious adverse effects of some medications. The most recent black box warning for corticosteroids was issued in 2014. The FDA cautioned against administering corticosteroid injections into the spine’s epidural space to treat neck/back pain and radiating pain in the extremities (4). When administered in this way, serious (although rare) adverse effects can occur, including vision loss, stroke, paralysis, and death.
Drug Interactions
Corticosteroids’ therapeutic effect may be counteracted or enhanced when administered with certain medications. Clinicians should perform an accurate medication reconciliation when prescribing/administering corticosteroids to ensure effective and safe treatment.
The following medications interact with corticosteroids, categorized by a decreased or increased therapeutic effect (1). Corticosteroid doses should be adjusted accordingly.
Decreased Therapeutic Effect
Medications that can decrease the level of corticosteroids in the blood when administered together include:
- Barbiturates
- Carbamazepine
- Ephedrine
- Phenytoin
- Rifampin
- Cholestyramine (affects oral corticosteroids in particular)
Increased Therapeutic Effect
Medications that can increase the level of corticosteroids in the blood when administered together include:
- Ketoconazole
- Macrolide antibiotics
Ask yourself...
- How often do you prescribe/administer corticosteroid injections (in any area of the body)?
- How comfortable are you prescribing/administering corticosteroids in conjunction with antibiotics?
- Have you ever had to increase a corticosteroid dose due to inadequate therapeutic levels (evidenced by persistent symptoms)?
- How often do you rely on pharmacists to check drug interactions for you?
- What education should you provide to patients regarding drug interactions associated with corticosteroids?
Clinical Guidelines on Corticosteroid Use in the Critically Ill
Clinical practice guidelines for corticosteroid therapy were developed to assist clinicians and providers in ensuring optimal prescribing and administration practices. Guidelines are currently in place for corticosteroid use in critical illness. The most recent 2024 guidelines were re-established for the treatment of septic shock, acute respiratory distress syndrome, and community-acquired bacterial pneumonia, particularly in adult patients.
The following sections compare these guidelines with the prior 2017 recommendations (3). Clinicians in acute care settings can use the following guidelines when prescribing/administering corticosteroids to critically ill patients.
Sepsis and Septic Shock
Previously, the guidelines recommended against administering corticosteroids in adult patients with sepsis without shock. Instead, corticosteroids were recommended in patients with septic shock not responsive to fluid and moderate-to-high-dose vasopressor therapy.
Current Recommendation: Administer corticosteroids to adult patients with septic shock. Do not administer high-dose/short-duration corticosteroids for adult patients with septic shock (no more than 400 mg per day of a hydrocortisone equivalent for no more than three days).
Acute Respiratory Distress Syndrome
Previously, the guidelines recommended corticosteroid use in patients with early moderate to severe acute respiratory distress syndrome (PaO2/FIO2 of less than 200 and within 14 days of onset).
Current Recommendation: Administer to adult hospitalized patients with acute respiratory distress syndrome.
Community-Acquired Bacterial Pneumonia
Previously, the guidelines recommended using corticosteroids for five to seven days at a daily dose of less than 400 mg IV hydrocortisone (or equivalent) in hospitalized patients with community-acquired pneumonia.
Current Recommendation: Administer to adult patients hospitalized with severe bacterial community-acquired pneumonia. There is no recommendation for adult patients hospitalized with less severe bacterial community-acquired pneumonia.

Clinical Guidelines on Corticosteroid Use for COVID-19
While data is limited and still developing, both the World Health Organization (WHO) and the National Institutes of Health (NIH) developed clinical practice guidelines for the use of corticosteroids as a treatment for COVID-19.
Clinicians practicing in acute care settings can use the following guidelines when prescribing/administering corticosteroids to patients with COVID-19.
Global Guidelines for Corticosteroid Use for COVID-19
As mentioned earlier, the WHO recommends corticosteroids to treat severe COVID-19 (6). The 2020 “Corticosteroids for COVID-19: Living Guidance” recommendations are as follows (13).
The WHO recommends systemic corticosteroids rather than no corticosteroids for treating patients with severe and critical COVID-19.
The WHO suggests not using corticosteroids in treating patients with non-severe COVID-19.
National Guidelines for Corticosteroid Use for COVID-19
The National Institutes of Health (NIH) developed the 2023 “Therapeutic Management of Hospitalized Adults with COVID-19” guidelines, outlining various drug therapy recommendations for patients with COVID-19, including corticosteroids. Recommendations are as follows (9)(10):
- The panel recommends against dexamethasone (or other systemic corticosteroids) for treating COVID-19 in patients who do not require supplemental oxygen.
- The panel recommends against the use of dexamethasone (or other systemic corticosteroids) in non-hospitalized patients in the absence of another indication.
- The panel recommends that patients with COVID-19 receiving dexamethasone (or another corticosteroid) for an underlying condition continue this therapy as directed by their health care provider.
Ask yourself...
- How does your facility/organization inform nursing staff of new clinical practice recommendations?
- How comfortable are you with the idea of using corticosteroids in the treatment of septic shock and COVID-19?
- How often do you review clinical practice guidelines on your own time?
- Are there other practice recommendations your facility has shared with nursing staff about corticosteroid safety?
- How has COVID-19 impacted your practice and medication administration?
Patient Education
Therapeutic and safe corticosteroid use depends on safe prescribing and administration practices and patient compliance. Optimal patient education is vital for successful treatment with corticosteroids.
The following are teaching points to share with patients about corticosteroid use (1)(6)(7):
- Adverse Effects: Patients should be informed of common adverse effects, particularly elevated blood sugar, mood changes, appetite changes, and weight gain.
- Vaccine Safety: Patients should be encouraged to check with their health provider before receiving vaccines (Corticosteroids should not be given with live vaccines)
- Infection Risk: Patients should be informed of the risk of developing an infection when corticosteroid doses are high or used to suppress the immune system and should, therefore, avoid exposure to chickenpox or measles.
- Administration: Patients should be instructed to avoid abruptly stopping corticosteroids to prevent adrenal insufficiency and potentially death. They should also be aware that stress on the body (for example, when having surgery) may require additional corticosteroids.

Ask yourself...
- What is the most essential teaching point for patients about corticosteroids?
- Have you ever had to adjust a patient’s corticosteroid dose due to a stressful event (like surgery)? Or have you witnessed a provider doing so?
- What is the protocol in your facility/organization for educating patients about corticosteroid therapy?
- Have you ever encountered a patient who abruptly stopped corticosteroid treatment? If so, did the patient develop any symptoms?
- What barriers to education have you encountered? How have you overcome those barriers?
Future Implications
Corticosteroid use may be on the rise. As aforementioned, corticosteroids are useful in the treatment of autoimmune disease. In the U.S., researchers anticipate a rise in autoimmune diseases in the future.
In a 2020 study in Arthritis and Rheumatology, researchers noted that the prevalence of antinuclear antibodies (ANA) (markers of autoimmune disease) is increasing in the U.S. [8]. The study analyzed the antibodies of over 14,000 participants (age 12 and older) over the course of three time periods. Results revealed the following:
- From 1988 – 1991, 22 million people had antibodies.
- From 1999 – 2004, 27 million people had antibodies.
- From 2011 – 2012, 41 million people had antibodies.
The study also found that antibody prevalence is highest among males, non-Hispanic whites, adults over age 50, and adolescents. Young people age12 to 19 had the highest antibody prevalence. While it is unclear why this is the case among the youth, the study findings may suggest an increase in the use of corticosteroids in the future. Further, clinicians might anticipate changes in clinical guidelines for safe prescribing/administration practices.
Ask yourself...
- How often do you encounter patients on long-term corticosteroid therapy for autoimmune disease?
- Have you noticed increased corticosteroid prescribing/use in your facility/organization?
- Why do you think antibody prevalence is rising in the adolescent population
Conclusion
Corticosteroids are helpful in the treatment of many medical conditions and diseases. While the use of these medications is common, there are equally associated risks, many of which can be life-threatening. Safe prescribing/administration practices are imperative. Clinicians can ensure that treatments are safe and effective through the gathering of accurate health histories, careful evaluation of each patient’s situation, weighing of risks and benefits, and provision of effective education to patients.
Ask yourself...
- In your opinion, what is the biggest public misconception about corticosteroid use?
- Do you feel that corticosteroids are over-prescribed/used?
- How can nurses enhance teaching for patients to ensure compliance with corticosteroid treatments?
- How can nurses advocate in the workplace for safer prescribing/administration of corticosteroids?
- What future clinical practice changes do you anticipate with regards to corticosteroid use in the U.S.?
Anti-Arrhythmics
Introduction
Cardiac arrhythmias continue to present significant clinical challenges and remain a common cause of death and disability [5]. Arrhythmias encompass a wide array of heart rate and rhythm disturbances; they are classified into broad terms as bradyarrhythmia’s (heart rates below 60 beats per minute) and tachyarrhythmias (heart rates exceeding 100 beats per minute) (10).
The defining feature of cardiac arrhythmias is an irregular heartbeat, or a symptom of abnormal heart rhythm linked to irregular initiation of electrical impulses, or a combination of both factors.
The mechanisms underlying cardiac arrhythmias are complex. The management of these conditions often involves the administration of antiarrhythmic drugs. It is vital that prescribers are aware of the pharmacokinetics of this drug class.
Overview of Cardiac Arrhythmias
Arrhythmias impact an estimated 17 million individuals across the planet, and they rank among the most prevalent forms of heart disease (8). The clinical manifestations of arrhythmias vary from asymptomatic individuals to sudden cardiac death (SCD), contributing to 10–15% of all mortality cases (10).
The only pattern considered to be a normal heart rhythm is the normal sinus rhythm. In this state, an electrical impulse originates in the sinoatrial (SA) node and travels through the heart [1]. This delayed impulse occurs in the atrioventricular (AV) node, then it proceeds through the His-Purkinje network, which encompasses the bundle of HIS, the left and right bundle branches, and the Purkinje fibers (10). These reactions essentially coordinate each beat of the heart.
Despite the notable limitations of existing antiarrhythmic medications, pharmacological intervention remains a fundamental aspect of managing cardiac arrhythmias (5). Research suggests that arrhythmias occur in 1.5% to 5% of the general population, with atrial fibrillation being the most prevalent form (1,10).
Arrhythmia is any deviation from the heart’s normal rhythm, with the normal sinus rhythm being the baseline for a healthy heart rhythm (1). The categorization of arrhythmias occurs through various criteria, with the most prevalent method being the rate of conduction. This includes bradyarrhythmia’s, where the heart beats slower than 60 beats per minute (bpm), and tachyarrhythmias, where the heart rate exceeds 100 bpm.
Atrial fibrillation (AF) is the most frequently occurring sustained cardiac arrhythmia and is linked to heightened morbidity and mortality rates, in addition to increased healthcare costs (2)(3).
Arrhythmias may originate from congenital anomalies (present from birth) or can develop due to irritation or damage to the myocardial tissue, causing disruptions or ‘short circuits’ in the heart’s electrical system (13).
Basics of Antiarrhythmic Drugs (AADs)
Antiarrhythmic drugs (AADs) are fundamental in managing cardiac arrhythmias and are classified based on the cardiac action potential [4]. The action of these drugs works toward the immediate cessation of atrial and ventricular arrhythmias (acute cardioversion) and for the long-term prevention of arrhythmia recurrence to maintain normal sinus rhythm (5).
A limited number of new AADs have reached the market despite the growing incidence of cardiac arrhythmias (5).
Two key objectives define the rationale for treating arrhythmias: (1) to mitigate significant clinical symptoms, including weakness, syncope, or the onset or worsening of congestive heart failure caused by an arrhythmia, and (2) to extend the patient’s lifespan (8).
In the initial stages of antiarrhythmic drug (AAD) development, the primary focus was on controlling ventricular arrhythmias (6). The direction of treatment changed following the adverse outcomes highlighted by the Cardiac Arrhythmia Suppression Trial (CAST) and the Survival with Oral D-Sotalol (SWORD) trial, which demonstrated that patients receiving AADs (encainide or flecainide) fared worse than those on placebo (6).
The primary criterion of antiarrhythmic drugs is safety. In the last decade, antiarrhythmic drugs have been subject to intense reevaluation, prompted by the outcomes of large-scale human research studies that highlighted various risks and limitations associated with pharmacological treatments for arrhythmias (7).
In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, the trial demonstrated that although achieving rhythm control was associated with improved survival, the adverse effects negated survival benefits (8).
Ask yourself...
- How would you describe the range from asymptomatic cases to life-threatening conditions?
- How can healthcare professionals balance the immediate need for rhythm control with the long-term goal of minimizing adverse effects and mortality?
Definition
Antiarrhythmic agents, often referred to as cardiac dysrhythmia medications, constitute a category of pharmaceuticals designed to moderate the heart’s electrical impulse and mitigate rapid heart rhythms; these rhythms include atrial fibrillation (AF), supraventricular tachycardia (SVT), and ventricular tachycardia (VT) (11)..
This class of drugs influences cardiac ionic channels or receptors, modifying the cardiac action potential, or its creation and transmission (11). These alterations affect the activation spread or repolarization pattern, suppressing cardiac arrhythmias (12).
Common arrhythmias symptoms include heart fluttering, abnormal or rapid heart rhythms, lightheadedness, fainting spells, chest pain, and breathlessness. Individuals may also experience heart palpitations, dizziness or feeling faint, chest pain or discomfort, weakness, and fatigue (12). (10)
(10)
Ask yourself...
- How do antiarrhythmic agents contribute to the suppression of arrhythmias and the alleviation of associated symptoms such as heart fluttering, lightheadedness, and chest pain?
- Do you have experience administering antiarrhythmic medications to patients?
Classification of Antiarrhythmic Medications
The categorization of arrhythmias is based on the location within the conduction pathway where they originate.
There are two main groups:
- Supraventricular – originating from the atria or the atrioventricular (AV) node.
- Ventricular – occur distal to the AV node. (23)
Before 2018, the categorization of antiarrhythmic drugs was based on the Vaughan-Williams (VW) classification system, which organized these medications by their principal mechanism of action [4, 14]. The initial Vaughan-Williams classification had limitations. When introduced in the 1970s, the range of antiarrhythmic drugs focused on altering the function of Na+, K+, and Ca2+ channels and targeting intracellular processes governed by adrenergic activity (15).
Today, there is a broader range of advanced antiarrhythmics, many of which possess overlapping interactions with drugs in other classes. For example, amiodarone, categorized under Class III (Potassium channel blockers), also exhibits sodium and calcium-channel blocking properties (Class IV).
Ask yourself...
- How do the limitations of the initial Vaughan-Williams classification system highlight the importance of continuous research and development in the field of cardiac pharmacology?
- Can you think of an example of a drug that contains overlapping drug actions?
Class 0: HCN Channel Blockers
Ivabradine
Ivabradine is designed to lower the heart rate. It is used in the management of stable angina pectoris and chronic heart failure with a heart rate ≥70 bpm across various clinical scenarios, including those with either preserved or compromised left ventricular (LV) function.
Ivabradine effectively decreases heart rate while preserving myocardial contractility and coronary vasomotor responsiveness, reducing oxygen consumption and extending diastolic duration (16)(17).
Ask yourself...
- How does Ivabradine’s mechanism of action contribute to its therapeutic benefits in patients with stable angina pectoris and chronic heart failure?
Class I: Voltage-gated Na+ Channel Blockers
Class IA
Class IA medications block fast sodium channels and include agents such as quinidine, procainamide, and disopyramide [11]. Quinidine, disopyramide, and procainamide are used in the management of supraventricular tachyarrhythmias, recurrent atrial fibrillation, ventricular tachycardia, ventricular fibrillation, Brugada syndrome, and Short QT Syndrome (SQTS) (4).
These drugs are associated with the highest risk of proarrhythmia among sodium channel blockers due to their capacity to prolong the QTc interval, which restricts their use because of their proarrhythmic potential (11).
For patients with Brugada syndrome, Quinidine is an alternative to implantable cardioverter-defibrillator (ICD) placement (66)
This class has also shown utility in individuals with short QT syndrome experiencing recurrent ventricular arrhythmias (VAs), reducing the frequency of ICD shocks in these patients (66).
Disopyramide is employed in cases of hypertrophic obstructive cardiomyopathy (HOCM), when combined with a beta-blocker or verapamil to alleviate symptoms like angina or dyspnea in patients unresponsive to beta-blockers or verapamil alone (67).
Procainamide helps expose and diagnose Brugada syndrome in individuals suspected of the condition but without a confirmed diagnosis (68).
Procainamide has been shown to reestablish sinus rhythm in patients with Wolff-Parkinson-White (WPW) syndrome who experience atrial fibrillation (AF) without hemodynamic instability, which is marked by an expansive QRS complex or a rapid pre-excited ventricular response (69). Procainamide may also assist in terminating ventricular tachycardia and other arrhythmias (69).
Ask yourself...
- How might the proarrhythmic potential of these drugs and their ability to prolong the QTc interval influence the decision-making process in selecting an appropriate treatment strategy for patients with conditions like Brugada syndrome, Short QT Syndrome (SQTS), or Wolff-Parkinson-White (WPW) syndrome?
Class IB
Lidocaine and Mexiletine treat ventricular tachycardia and fibrillation after a myocardial infarction by inducing a mild blockade of sodium channels [4]. However, they are not effective for treating atrial arrhythmias (4).
In the context of long QT syndrome, mexiletine can reduce the QTc interval and has been employed to decrease the incidence of recurrent arrhythmias and the need for interventions by implantable cardioverter-defibrillators (ICDs) (70).
The effectiveness of Lidocaine diminishes in instances of hypokalemia, necessitating the correction of potassium levels (80).
Ask yourself...
- How does the mechanism of sodium channel blockade by Lidocaine and Mexiletine contribute to their differential effectiveness in treating ventricular?
- What implications does this have for their use in managing long QT syndrome and conditions of electrolyte imbalance such as hypokalemia?
Class IC
Research recommends Encainide (Enkaid), flecainide (Tambocor), and propafenone (Rythmol SR) for managing supraventricular and ventricular tachyarrhythmias that do not respond to standard treatments, in the absence of underlying structural heart disease (72).
Propafenone increases the effects of cyclosporin, desipramine, and theophylline (81).
Class IC treats premature ventricular contractions and catecholaminergic polymorphic ventricular tachycardia (18). These drugs block sodium channels without altering the QT interval and suit ongoing management in individuals with symptomatic supraventricular tachycardia (SVT) who have no structural or ischemic heart disease, and either are unsuitable for or opt against catheter ablation (11).
In addition, these agents are effective for the pharmacological cardioversion of atrial fibrillation (AF). The “pill in the pocket” strategy involves patients with paroxysmal AF carrying a loading dose of medication to take at the onset of an AF episode. This approach aims for chemical cardioversion to restore normal rhythm, rather than adhering to a regular maintenance dose regime (71).
The Cardiac Arrhythmia Suppression Trials (CAST I and II) showed that patients with a history of myocardial infarction, treated with class IC agents (flecainide, encainide, moricizine) to reduce premature ventricular contractions (PVCs), faced a higher mortality risk compared to those receiving a placebo (72).
Class ID
Ranolazine presents a potential therapeutic option for managing tachyarrhythmias and ventricular tachycardia [19].

Ask yourself...
- How does the balance between the therapeutic benefits in the absence of structural heart disease and the increased mortality risk with a history of myocardial infarction guide clinical decision-making?
- How would you describe the ‘pill in the pocket’ strategy versus traditional maintenance dosing?
Class II: Autonomic Inhibitors/Activators
The literature recommends beta-blockers (BB) for managing the heart rate in individuals with paroxysmal, persistent, or permanent atrial fibrillation (AF) and atrial flutter (73). Beta-blockers (BB) are also beneficial for long-term management in patients with symptomatic supraventricular tachycardia (SVT) (73).
Due to their favorable safety profile and efficacy, healthcare professionals can consider beta-blockers as the first-choice therapy for ventricular arrhythmias (39). Their use is associated with a reduction in adverse cardiac events in conditions such as long QT syndrome and catecholaminergic polymorphic ventricular tachycardia (79).
For patients exhibiting symptomatic premature ventricular contractions (PVCs) in the absence of underlying heart disease, beta-blocker therapy can help decrease the frequency of recurrent arrhythmias and alleviate symptoms (75).
Ask yourself...
- How does the mechanism of action of beta-blockers contribute to their effectiveness across a spectrum of arrhythmias?
- What factors influence the decision to prioritize beta-blockers as a first-choice therapy in these conditions, considering their impact on reducing adverse cardiac events in long QT syndrome and catecholaminergic polymorphic ventricular tachycardia?
IIa: Inhibitors, including pindolol, carvedilol, timolol, nadolol (non-selective beta-blockers), and bisoprolol, atenolol, metoprolol, esmolol (selective beta-1 blockers), treat rate control in atrial fibrillation, atrial flutter, and ventricular tachyarrhythmia (4).
IIb: Activators: The use of Isoproterenol can manage ventricular escape rhythm in cases of complete AV block before pacemaker implantation (4)(20).
IIc: Inhibitors: Atropine treats symptomatic sinus bradycardia and conduction block (4)(21).
IId: Activators: For the management of supraventricular tachyarrhythmias, Carbachol, methacholine, and digoxin (4).
IIe: Activators: Adenosine for the cessation of paroxysmal supraventricular tachycardia (PSVT) (22).
Class III: K+ Channel Blockers/Openers
Potassium channel blockers decrease potassium efflux out of the cell and prolong the QTc interval (4). Amiodarone displays sympatholytic effects and sodium and calcium channel blocking properties, leading to reduced conduction through the AV and sinus nodes (4).
Amiodarone helps maintain sinus rhythm in patients with atrial fibrillation (AF) and those suffering from left ventricular systolic dysfunction (4).
Amiodarone is also a viable choice for pharmacological cardioversion and can help manage ventricular rate in critical patients without pre-excitation, though it is less effective than non-dihydropyridine calcium channel blockers (4)(49).
Amiodarone is the preferred antiarrhythmic medication for suppressing ventricular arrhythmias (VA) (4). The administration of intravenous amiodarone may stabilize the rhythm of unstable persistent ventricular arrhythmias (VA) following defibrillation (49). Administering intravenous amiodarone stabilizes rhythm in cases of unstable persistent ventricular arrhythmias (VA) following defibrillation.
In addition, amiodarone controls ventricular arrhythmias (VA) in patients with ischemic heart disease who are also receiving beta-blocker treatment (4).
Observations show that Dronedarone reduces hospital admissions for atrial fibrillation (AF) in individuals with a history of non-permanent AF who are in sinus rhythm. However, for patients with permanent AF that cannot convert back to normal sinus rhythm, it is contraindicated due to FDA reviews indicating a significant increase in the risk of cardiovascular death, stroke, and heart failure in these cases (76).
Dofetilide is employed in treating atrial arrhythmias and for the acute pharmacological cardioversion of atrial fibrillation or flutter (77).
Combining class II beta-blocker properties and class III potassium channel blocker effects, Sotalol manages both ventricular and supraventricular arrhythmias (78).
Ibutilide (Corvert) is used to treat atrial fibrillation or flutter, underlining its specialized use in managing these specific arrhythmias (79).
Ask yourself...
- How do these drug mechanisms contribute to its efficacy in managing both atrial and ventricular arrhythmias
- What considerations should be made when choosing Amiodarone for patients with left ventricular systolic dysfunction or those undergoing pharmacological cardioversion?
- How should clinicians navigate the decision-making process for employing Dronedarone in treating AF, considering the patient’s AF status and the potential benefits and risks?
IIIA: Voltage-dependent K+ channels
Amiodarone and dronedarone are notable for their role as non-selective potassium (K+) channel blockers, crucial in treating unstable ventricular tachycardia and life-threatening recurrent ventricular fibrillation (4).
Kv11.1 (rapid K+ current) blockers: Dofetilide, almokalant, ibutilide, sematilide, and sotalol manage ventricular tachycardia in patients without prior myocardial infarction or underlying structural heart disease. They also treat Wolff-Parkinson-White (WPW) syndrome associated with atrial fibrillation (4).
Kv1.5 (ultra-rapid K+ current) blockers: Vernakalant converts recent-onset atrial fibrillation in patients without structural or ischemic heart disease. The FDA does not approve this specific use of vernakalant (24).
IIIb: Metabolically dependent K+ channel blockers: Nicorandil and pinacidil are employed as second-line treatments for stable angina (25).
Class IV: Ca2+ handling modulators
Non-dihydropyridine calcium channel blockers, such as diltiazem and verapamil, reduce conduction speed and decelerate signal transmission through the AV node (82). These medications are effective for controlling the ventricular rate in both acute and chronic cases of atrial fibrillation (AF) and atrial flutter (82).
In the acute management of stable patients with supraventricular tachycardia (SVT), including focal and multifocal atrial tachycardias, diltiazem and verapamil serve as viable treatment options (4)(39).
Ask yourself...
- Given the mechanism by which non-dihydropyridine calcium channel blockers like diltiazem and verapamil slow conduction and decelerate signal transmission through the AV node, why are these medications suited for controlling the ventricular rate in atrial fibrillation and atrial flutter?
- How does this mechanism influence their effectiveness in the acute management of various forms of supraventricular tachycardia?
IVa: Bepridil and falipamil, which block non-selective surface membrane calcium (Ca2+) channels, may manage supraventricular tachyarrhythmias (4). Verapamil and diltiazem, which block surface membrane L-type calcium (Ca2+) channels, treat supraventricular arrhythmias, and control the rate of atrial fibrillation (4).
IVb: Propafenone and flecainide serve as intracellular calcium channel blockers utilized in addressing catecholaminergic polymorphic ventricular tachycardia (CPVT) (4).
Class V: Mechanosensitive channel blockers
Inhibitors: N-(p-amylcinnamoyl) Anthranilic Acid: Under Research and Not Approved by the FDA (26).
Class VI: Gap junction channel blockers
Inhibitors: carbenoxolone (under investigation- not FDA approved) (27).
Class VII: Upstream target modulators
Omega-3 fatty acids: Eicosapentaenoic Acid and Docosahexaenoic Acid: Reduction in Cardiac Death Risk Post-Myocardial Infarction (28).
Statins: Potential for use in atrial fibrillation
ACE inhibitors: Captopril, Enalapril, Ramipril, Lisinopril (ACE Inhibitors), and ARBs (Losartan, Telmisartan): Potential Use in Atrial Fibrillation Associated with Heart Failure (29).
Clinical Prescribing Criteria
Antiarrhythmic medications are pivotal in managing symptoms and safeguarding against the decline of cardiac function caused by conditions such as tachycardia, irregular rhythms, or desynchrony (4). The primary objective is to reestablish normal cardiac rhythm and conduction, averting the onset of more severe and fatal arrhythmias.
Antiarrhythmics have a narrow therapeutic index, indicating a minimal margin between the effective dosage and the onset of toxicity (4). There is a tenuous balance between suboptimal treatment and the risk of toxic or proarrhythmic effects, underscoring the importance of precise dosing and monitoring. Clinical attention focuses on the patient’s clinical status, underlying structural and functional conditions, and the mechanisms of arrhythmia at both cellular and molecular levels.
The use of antiarrhythmic drugs in therapy seeks to alter conduction velocity, by either slowing down or speeding it up, modify the excitability of cardiac cells via changes in the length of the effective refractory period, and suppress unusual spontaneous activity (14).
Numerous variables influence the effectiveness of antiarrhythmic drugs, such as race, sex, genetics, environmental temperature, drug interactions, precipitating factors, changes in neurohormones, the disease’s present condition and severity, and disease-driven structural changes in the body (30). The complexity increases with some antiarrhythmic drugs (AADs) displaying diverse electrophysiological and pharmacological effects that depend on the administration route, plasma concentration, and the existence of active metabolites.
A variety of factors can influence the efficacy of medications, including racial background, gender, genetic makeup, ambient temperature, interactions between different drugs, initiating triggers, neurohormonal fluctuations, the current state and intensity of the disease, and alterations in the body’s structure caused by the disease itself (31).
Complicating the pharmacology, certain antiarrhythmic drugs (AADs) exhibit a wide range of electrophysiological and pharmacological actions, which can vary based on the method of administration, concentration levels in the plasma, and the presence of active metabolites (4)(5).
Ask yourself...
- Considering the narrow therapeutic index of antiarrhythmic drugs, how does precise dosing and monitoring contribute to optimizing treatment outcomes while minimizing the risk of adverse effects?
- What role do patient-specific factors play in this process?
- Given the complex interplay of factors such as race, sex, genetics, and environmental conditions on the effectiveness of antiarrhythmic drugs, how should clinicians integrate this knowledge into personalized treatment plans?
- With some antiarrhythmic drugs displaying varied electrophysiological and pharmacological effects based on administration route, plasma concentration, and the presence of active metabolites, how do these variables complicate the management of arrhythmias?
The Cardiac Electrical Cycle (Electrical Cascade)
The cardiac action potential represents the sequence of ion exchanges that result in the successive depolarization and repolarization of the cardiac myocyte, culminating in muscle contraction (4). During its resting phase, a cardiac myocyte maintains a baseline resting membrane potential ranging from negative 80 to negative 90 millivolts (32).
Antiarrhythmic drugs slow ion movement during various stages of the cardiac action potential (32).
- Phase 0: The “depolarization” phase of the action potential occurs due to the influx of sodium ions (Na+) into the cell, following an electrochemical gradient, leading to a membrane potential of around 30 millivolts (33).
- Phase 1: ”The notch,” or the early repolarization phase of the action potential, features potassium (K+) ions flowing out (4)(33).
- Phase 2: ”The plateau” phase occurs when the inward movement of calcium ions (Ca2+) balances the outward movement of potassium (K+) ions (4)(34).
- Phase 3: ”The repolarization” phase of the action potential occurs through the efflux of potassium (K+) ions along their electrochemical gradient out of the cell. This movement removes the positive charge of the K+ ion from the cell, reinstating the cardiac myocyte’s negative potential (33)(34).
- Phase 4: Reactivation of the Na+K+ ATPase, or sodium-potassium pump, re-establishes the cardiac myocyte’s resting membrane potential (4)(35).
Ask yourself...
- How do antiarrhythmic drugs alter the ion movement during the different phases of the cardiac action potential to correct arrhythmias?
- What are the potential consequences of these modifications on the overall function of the cardiac myocyte and the heart’s rhythm?
Pharmacokinetics of Anti-Arrhythmic Medications
Pharmacokinetics involves the study of drug absorption, distribution, metabolism, and excretion. All antiarrhythmic drugs affect the conductance of membranes and ions, modifying cardiac action potential dynamics via direct or indirect action.
For example, some medications inhibit fast sodium channels, which are essential for controlling the rate of membrane depolarization (phase 0) during an action potential (33). Electrical conduction velocity is linked to membrane depolarization, and blocking sodium channels slows this velocity down (32)(33). Slowing conduction velocity is advantageous for eradicating tachyarrhythmias resulting from reentry circuits (36).
Antiarrhythmic drug classes affect the duration of action potentials and the effective refractory period (37). Extending the effective refractory period often eradicates reentry tachyarrhythmias [38]. This effect occurs by inhibiting potassium channels and postponing the repolarization phase (phase 3) of action potentials (38).
Medications that inhibit the slow inward calcium channels aim to diminish pacemaker activity by decelerating the depolarizing pacemaker potential’s rate of rise (phase 4 depolarization) (14).
These drugs also decrease the speed of electrical signal transmission through the atrioventricular (AV) node (14)(38). Like sinoatrial (SA) node cells, AV nodal cells rely on the influx of calcium ions for depolarization (34). Due to the potential for sympathetic nervous system activity to cause arrhythmias, beta1-adrenoceptor blockers are employed to diminish the sympathetic impact on the heart (39). These beta-adrenoceptors connect to ion channels through specific signal transduction pathways, indicating that beta-blockers modify ion conductance across the membrane, influencing calcium and potassium conductance (39).
In instances of AV block, doctors sometimes use drugs like atropine, a muscarinic receptor antagonist, to counteract vagal effects (40). AV block can emerge as an adverse effect of beta-blocker medication, and stopping the beta-blocker in such cases may return AV conduction to normal (41).
An increased ventricular rate can result from atrial flutter or fibrillation (42).
Medications that slow down conduction through the atrioventricular (AV) node regulate the ventricular rate. Calcium channel blockers and beta-blockers are particularly effective for this purpose (4).
Digoxin proves advantageous for patients with systolic heart failure, also referred to as heart failure with reduced ejection fraction (HFrEF), characterized by an ejection fraction of less than 40% (43). Nonetheless, it does not contribute to a reduction in mortality (43). When standard treatments do not achieve heart rate objectives in atrial fibrillation or atrial flutter, clinicians can deploy Digoxin for heart rate management (44).
Administration of digoxin is contraindicated in instances of pre-excitation due to accessory pathways since it promotes AV blockade and could precipitate ventricular tachyarrhythmias (44). In conditions of elevated sympathetic activity, digoxin is ineffective, and beta-blockers are the preferred treatment option (44).
Healthcare providers favor oral drug formulations for their improved patient compliance, ease of use, and scalability, which offer economic advantages (45). However, the oral bioavailability of drugs can vary, influenced by differences in physicochemical characteristics and metabolic activities that impact pharmacokinetics (46). Challenges such as intestinal metabolism, efflux mechanisms in the gastrointestinal tract, and the hepatic first-pass effect hinder the oral bioavailability of drugs (46)(47).
The first-pass effect describes a pharmacokinetic process in which a drug undergoes metabolism at a specific site in the body before reaching the systemic circulation or its intended site of action, which reduces the concentration of the active drug available [47]. The liver, a primary location for drug metabolism, is directly linked to the first-pass effect. This effect can also occur in other active metabolic areas, such as the lungs, blood vessels, gastrointestinal tract, and various tissues (47).
Ask yourself...
- How do the pharmacokinetic processes of absorption, distribution, metabolism, and excretion influence the clinical efficacy and safety of antiarrhythmic drugs?
- What strategies can healthcare providers employ to mitigate potential adverse effects, including those related to the first-pass effect?
- Given the varied mechanisms by which antiarrhythmic drugs modify cardiac action potentials and conduction velocities to treat arrhythmias, how do these mechanisms align with the selection of specific antiarrhythmic medications for conditions such as atrial flutter, atrial fibrillation, and AV block, considering both the intended therapeutic outcomes and the potential for adverse effects?
Absorption
Antiarrhythmic medications exhibit quick absorption, but the pronounced first-pass effect often reduces their bioavailability (47). They achieve peak plasma concentrations within 1–3 hours, except for digoxin and dronedarone, which reach their peak in 3–6 hours, and amiodarone, which takes 6–8 hours to peak (11). In elderly individuals and patients with liver dysfunction, the oral bioavailability of medications tends to be higher (11).
Intestinal bacteria transform digoxin into inactive compounds; antibiotics such as tetracycline and erythromycin eliminate these bacteria, leading to elevated levels of digoxin in the bloodstream (44). The antiarrhythmic effect of digoxin starts within 2–5 minutes after its intravenous administration (44).
Drug absorption through the gastrointestinal tract is crucial for their bioavailability, and meals can either enhance or impede this process (46). For instance, a high-fat meal can increase the oral absorption of dronedarone by fourfold (11).
Ask yourself...
- How do factors such as age, liver function, intestinal flora, and dietary habits influence these drugs’ therapeutic levels and efficacy in the bloodstream?
Distribution
Except for sotalol, antiarrhythmic drugs (AADs) exhibit some degree of binding to plasma proteins (11). Amiodarone, digoxin, flecainide, and propafenone build up in the heart at concentrations higher than in plasma, and dialysis cannot remove them (11). The concurrent administration of flecainide and amiodarone increases flecainide plasma concentrations by 50% (48).
Disopyramide, mexiletine, sotalol, and verapamil can cross the placenta and appear in breast milk. High concentrations of Procainamide also occur in breast milk and are eliminated by newborns (11).
Oral administration of amiodarone reaches steady-state plasma concentrations after an extended period, except when administered in substantial loading doses; delivering it via intravenous form also delays its maximal effect [49]. This delay indicates its distribution across multiple compartments, including the intravascular compartment, which a standard loading dose saturates, a peripheral compartment encompassing various tissues, and a deep compartment represented by adipose tissue, serving as a reservoir for the drug (49).
Amiodarone is known for its distinctive side effects. Its prevalence rate is 15% in the first year of use, and it can escalate to up to 50% with prolonged treatment (87). Side effects include pulmonary fibrosis, thyroid dysfunction, photosensitivity, blue-grey skin discoloration, corneal microdeposits, peripheral neuropathy, and elevated liver enzymes (87). Providers must weigh the potential benefits of amiodarone against its long-term risks (94).
Ask yourself...
- How does the differential binding of antiarrhythmic drugs to plasma proteins and their accumulation in various body compartments (heart and adipose tissue) affect their pharmacodynamics and pharmacokinetics?
Biotransformation
Antiarrhythmic drugs (AADs) undergo metabolism in the liver through CYP450 isoenzymes, resulting in active metabolites that either block sodium (Na+) channels (such as mexiletine and propafenone), extend action potential duration (APD) [for instance, N-acetylprocainamide (NAPA)], or cause central nervous system (CNS) toxicity (as seen with lidocaine) (11).
Genetics influence the metabolism of CYP2D6, resulting in higher plasma concentrations and extended half-lives (t½) of metoprolol and propafenone in individuals with poor metabolizing capabilities (6% of Caucasians) compared to those who are rapid metabolizers [50]. In a similar fashion, the conversion of procainamide to N-acetylprocainamide (NAPA) varies, with 15–20% metabolized in individuals classified as ‘slow-acetylators’ and 25–33% in ‘fast-acetylators’ (11).
Genetics determines these metabolic phenotypes. No standard tests are available to identify a patient’s metabolic phenotype prior to treatment initiation, with the exception of measuring the procainamide/NAPA concentration ratio. It is advisable to decrease dosages for poor or slow metabolizers to two-thirds or less of the standard maintenance dose (11)(51).
Lipophilic beta-blockers, such as bisoprolol, carvedilol, metoprolol, and propranolol, undergo metabolism via CYP2D6, and their bioavailability and half-life (t½) are prolonged in cases of liver dysfunction [11]. The body excretes hydrophilic beta-blockers, including atenolol and sotalol, in their unchanged form through the urine (11).
Following an intravenous loading dose, lidocaine undergoes metabolism with a half-life (t½) of 1.5–2 hours. In patients with liver impairment or decreased hepatic blood flow — including the elderly, those experiencing cardiogenic shock, heart failure, myocardial infarction, or those taking cimetidine and beta-blockers — lidocaine’s plasma levels increase, and its half-life extends (51). In these cases, one should lower both the loading and maintenance doses.
Due to its brief half-life, an initial loading dose of lidocaine requires supplementation with a continuous infusion or repeated administrations to achieve and maintain a consistent plasma concentration (53).
Intravenous Esmolol undergoes rapid hydrolysis in red blood cells, with a half-life of ∼ 9 minutes, and achieves complete reversal of beta-blockade 20–30 minutes after drug cessation (11). Intravenous adenosine acts within 15–30 seconds, with erythrocytes and vascular endothelial cells absorbing and metabolizing it through adenosine deaminase (ADA), leading to a short half-life (t½) of less than 10 seconds (11).
Ask yourself...
- How do genetic variations in CYP450 isoenzymes (CYP2D6) affect the metabolism, efficacy, and safety of antiarrhythmic drugs?
Elimination
Antiarrhythmic drugs (AADs) vary in their extent of excretion through urine and feces (11) The half-life (t½) of these drugs extends in elderly individuals and patients with renal impairment (such as digoxin, disopyramide, dofetilide, flecainide, procainamide, and sotalol) or liver dysfunction (including amiodarone, diltiazem, flecainide, lidocaine, metoprolol, mexiletine, propafenone, propranolol, quinidine, and verapamil) (11). This prolongation also occurs in congestive heart failure (seen with amiodarone, flecainide, lidocaine, mexiletine, procainamide, and quinidine) or after a myocardial infarction (noted with disopyramide, lidocaine, and mexiletine) (11)(54).
For these patients, it is advisable to lower the dosages and to conduct regular ECG monitoring. Amiodarone is subject to extensive metabolism in the liver, excreted through the bile, and has a prolonged half-life (t½) ranging from 25 to 110 days. This extended half-life accounts for the persistence of its effects for weeks or even months following cessation of the drug (11)(55).
Due to their short half-life (t½), manufacturers dispense certain antiarrhythmic drugs, including beta-blockers, diltiazem, propafenone, and verapamil, in modified-release formulations (11).
Amiodarone, cimetidine, diltiazem, ketoconazole, procainamide, propranolol, and verapamil elevate plasma concentrations of quinidine [11]. Quinidine acts as a strong inhibitor of CYP2D6 and P-glycoprotein (P-gp), raising the plasma levels of drugs metabolized by this enzyme; it also reduces digoxin clearance, necessitating a 50% reduction in digoxin dosage (11). Beta-blockers, cimetidine, and halothane cause an increase in plasma concentrations of lidocaine, thereby requiring a reduction in lidocaine dosage (11). In addition, mexiletine elevates the plasma levels of theophylline, while amiodarone increases the levels of mexiletine (56).
Flecainide and propafenone lead to higher plasma concentrations of digoxin and propranolol. Propafenone (Rythmol) raises the plasma levels of digoxin, metoprolol, propranolol, and warfarin [48]. Mexiletine and quinidine amplify the effects of warfarin; thus, it is advisable to decrease the dosage of warfarin and monitor the prothrombin time/international normalized ratio (INR) (11)(48).
Amiodarone inhibits P-glycoprotein (P-gp) and several cytochrome P450 isoenzymes, such as CYP1A2, CYP2C9, CYP2D6, and CYP3A4, thus increasing the plasma concentrations of drugs metabolized by these pathways or that are substrates of P-gp [94]. Dosage modifications are necessary for medications such as digoxin, flecainide, and warfarin; it is also important to monitor digoxin concentrations and the international normalized ratio (INR) (57)(58).
Cholestyramine may decrease the absorption of amiodarone [59]. Since diltiazem and verapamil inhibit both CYP3A4 and P-glycoprotein (P-gp), adjusting the dosages of drugs metabolized by CYP3A4 or that are substrates of P-gp becomes necessary [59]. Furthermore, verapamil has the capacity to suppress the liver’s metabolism of lipophilic beta-blockers, resulting in elevated plasma concentrations of these medications (60).
There is a significant pharmacokinetic interaction between certain antiarrhythmic/rate controlling medications (such as amiodarone, quinidine, dronedarone, verapamil, digoxin, and diltiazem) and non-vitamin K antagonist oral anticoagulants (NOACs) due to competition for P-glycoprotein (P-gp) or inhibition of CYP3A4 (notably by diltiazem, dronedarone, and verapamil) (61)(62).
Due to these interactions leading to elevated plasma levels of NOACs, experts advise against combining dronedarone with dabigatran and recommend reducing the dose of edoxaban by 50% [62] [63]. Consider reducing the dose of all non-vitamin K antagonist oral anticoagulants (NOACs) when administering amiodarone alongside other P-gp competing substances (64).
Prescribers recommend reducing the dose of dabigatran (Pradaxa) when used with verapamil. Combining edoxaban with verapamil, especially when other P-gp competitors are present, may also necessitate a dose reduction (65).

Ask yourself...
- Considering the extensive metabolism of antiarrhythmic drugs in the liver and their excretion through bile or urine, how do renal and liver dysfunctions affect the pharmacokinetics of these drugs?
- What principles should guide the adjustment of dosages in patients with such conditions to maintain therapeutic efficacy while minimizing toxicity?
Other Antiarrhythmic Drugs
Adenosine is effective for diagnosing and halting supraventricular tachycardia (SVT) arising from atrioventricular nodal reentrant tachycardia (AVNRT) or orthodromic atrioventricular reentrant tachycardia (AVRT) (83). It also serves as a diagnostic aid by revealing underlying atrial flutter or atrial tachycardia (AT) (83).
Adenosine can also terminate focal AT caused by a triggered mechanism and distinguish focal AT from AVNRT and AVRT (83). Adenosine, a purine nucleoside, results from the breakdown of adenosine triphosphate (86). Within cardiomyocytes, it interacts with Gi-protein type 1 receptors, facilitating swift potassium efflux and hyperpolarization and inhibiting calcium influx (86). These actions decrease the heart rate and slow down conduction velocity by targeting the AV node.
Digoxin is not a first-line therapy for ventricular rate control in patients with AF. A combination of digoxin and beta-blockers or non-dihydropyridine calcium channel blockers is a reasonable rate control option in patients with AF and heart failure (84).
Treatment of Overdose
In instances of antiarrhythmic drug overdose, medical professionals must ensure the patient has a clear airway, adequate breathing, and support for circulation [4] [87]. Managing cardiac arrest and severe toxicity from poisoning involves the use of specialized interventions, including antidotes and venovenous extracorporeal membrane oxygenation (VV-ECMO), alongside fundamental and advanced life support techniques (87).
Symptoms such as nausea, vomiting, neurological manifestations, and lethal arrhythmias characterize Digoxin toxicity (88). To treat ventricular tachyarrhythmias from digoxin toxicity, clinicians can use lidocaine, and atropine serves as an option for bradyarrhythmias. In addition, digoxin-specific antibody fragments prove effective in severe toxicity cases (89).
Therapeutic and excessive dosages of dofetilide may induce Torsades de Pointes (TdP), which clinicians manage by reducing or stopping the dosage [90]. If the arrhythmia persists, initial treatment involves activated charcoal if ingestion occurred within the last 15 minutes, followed by intravenous magnesium and correction of any electrolyte imbalances (90).
For persistent arrhythmias, administering isoproterenol/dopamine may be a temporary measure until initiating pacing (90).
In cases of beta-blocker poisoning, treatments involve administering catecholamines, applying high-dose insulin euglycemic therapy, and using vasopressors, noting glucagon for its positive effects on hemodynamics (91).
Treating calcium channel blocker (CCB) overdoses involves administering intravenous calcium, dopamine, and norepinephrine. High-dose insulin therapy can reduce mortality in cases of calcium channel blocker poisoning (60). For severe shock or cardiac arrest resulting from these overdoses, extracorporeal life support is employed (60). Case reports indicate that clinicians use lipid emulsion therapy to treat overdoses of amiodarone and flecainide (4)(92).
Ask yourself...
- How do the principles of emergency management in the treatment of severe toxicity from antiarrhythmic drug overdose?
- What factors determine the choice of specific treatments for complications such as lethal arrhythmias, digoxin toxicity, Torsades de Pointes, beta-blocker poisoning, and calcium channel blocker overdoses?
Conclusion
Arrhythmias encompass a broad spectrum of heart rate and rhythm disturbances and present significant clinical challenges. Atrial fibrillation (AF) is the most prevalent arrhythmia, associated with increased morbidity, mortality, and healthcare costs (2)(3). Management involves antiarrhythmic drugs (AADs), which play a fundamental role despite their limitations and the potential for adverse effects.
Antiarrhythmic agents, classified by their primary action mechanism on the cardiac action potential, impact ionic channels or receptors and aim to suppress arrhythmias (4). The management objectives for arrhythmias include alleviating significant clinical symptoms and extending life.
Pharmacokinetic aspects, such as drug absorption, distribution, metabolism, and excretion, play crucial roles in the effectiveness and safety of AADs. Pharmacodynamics involves modifying the cardiac action potential and conduction velocity to prevent or terminate arrhythmias. Factors influencing drug efficacy include genetics, environmental conditions, and the patient’s clinical profile.
Treatment of overdose with antiarrhythmic drugs requires immediate medical intervention, including antidotes and supportive measures like venovenous extracorporeal membrane oxygenation (VV-ECMO) for severe cases. Managing specific drug toxicities, such as digoxin and beta-blockers, involves targeted therapies and supportive care to mitigate adverse effects and stabilize the patient’s condition.
Antibiotic Use in UTI
Introduction
A urinary tract infection (UTI) can develop in anyone, but is more common in females than males. Approximately 40% of American women will develop a urinary tract infection in their lives [2]. Many UTIs can lead to serious health complications, including sepsis and sometimes patient mortality.
Thus, healthcare providers must be knowledgeable of the signs and symptoms of UTIs, the available antibiotics, and UTI treatment guidelines. Understanding the different pharmacokinetics of antibiotics used to treat UTIs is essential during drug selection. This course outlines UTI antibiotic pharmacology and addresses pharmacokinetics, including mechanism of action, side effects, usage, and contraindications.
Definitions
This section covers the definitions related to UTI treatment and management.
- Urinary Tract Infection
- Bacterial infection of the lower urinary tract (usually confined to the bladder). This is also sometimes called cystitis and is primarily caused by E. coli [2].
- Asymptomatic Bacteremia
- A urine specimen is collected from a patient who shows the presence of bacteria but does not have any UTI symptoms [5].
- Pyelonephritis
- Bacterial infection of the upper urinary tract (i.e., kidneys) [6].
- Urosepsis
- A urinary tract infection causes a systemic disease, sepsis [6].
Ask yourself...
- What is a urinary tract infection?
- What is asymptomatic bacteremia?
- What is pyelonephritis?
- What is urosepsis?
Medication Overview
This section briefly reviews UTI antibiotic classes and medical indications.
Certain antibiotics are used to prevent or treat urinary tract infections in both inpatient and outpatient settings. Healthcare providers should follow current guidelines regarding UTI treatment, which depend on the patient and type of urinary tract infection. UTI treatment algorithms are further divided into:
- Uncomplicated UTIs
- Complicated UTIs
- Prophylactic treatment for recurrent UTIs [6]
Uncomplicated UTIs are patients who have urinary symptoms but do not have signs of systemic infections, like fever, flank pain, costovertebral angle (CVA) tenderness, etc. Patients can be male or female, unlike previous guidelines that categorized all males as complicated UTIs. Also, patients with uncomplicated UTIs usually have no underlying health conditions or risk factors that may affect treatment [6].
Conversely, complicated UTIs occur when patients have systemic symptoms. Patients who are pregnant, have a history of UTIs, or are considered elderly also fall under this category. Patients with pyelonephritis are automatically considered to have complicated UTIs as well. Patients with recurrent UTIs may be prescribed low-dose antibiotics for prevention [6].
Regardless of the UTI type, below are some of the common antibiotics prescribed:
- Trimethoprim-sulfamethoxazole
- Nitrofurantoin
- Fluoroquinolones
- Ciprofloxacin
- Levofloxacin
- Beta-lactams
- Cephalexin
- Amoxicillin-clavulanate
- Ceftriaxone
- Cefdinir
- Piperacillin-tazobactam
- Meropenem
- Fosfomycin
- Pivmecillinam

Ask yourself...
- What are some factors that classify an uncomplicated UTI?
- What are some factors that classify a complicated UTI?
- What are the different names and types of antibiotics used to treat UTIs?
Pharmacokinetics
This section discusses the pharmacokinetics of each antibiotic medication used to treat UTIs.
Trimethoprim-sulfamethoxazole
Sulfonamides, or sulfa drugs, are a class of medications commonly used to treat bacterial infections, including UTIs. A common sulfonamide medication used to treat UTIs is sulfamethoxazole formulated with trimethoprim, sometimes abbreviated as TMP-SMX, TMP-sulfa, or TMP-SMZ [22]. Trimethoprim-sulfamethoxazole is also approved by the Federal Drug Administration (FDA) to treat chronic bronchitis exacerbations, otitis media in children, and shigellosis.
This medication is also used to prevent and treat traveler’s diarrhea, toxoplasmosis, and Pneumocystis jirovecii and Pneumocystis carinii pneumonia. Some other off-label or non-FDA-approved uses are tuberculosis, malaria, listeria, pertussis, and community-acquired pneumonia [10].
Since this is a combination medication, TMP-SMX works by two different mechanisms of action. Trimethoprim competes with the enzyme dihydrofolate reductase and stops the production of tetrahydrofolate from converting to folate.
Sulfamethoxazole, a CYP2C9 inhibitor, competes with p-aminobenzoic acid during dihydrofolate synthesis. Together, these two medications work against folate production and block bacterial biosynthesis of nucleic acids and proteins. Both medicines are metabolized by the liver [10].
TMP-SMX is available via oral and intravenous (IV) forms. Adult TMP-SMX oral dosages are typically 160mg/800mg for treating UTI, respectively, and dosages for children under 40 kilograms are weight-based. This medication is best absorbed orally with at least eight ounces of water. Intravenous TMP-SMX is given to hospitalized patients, and dosages will vary. Some common side effects of trimethoprim-sulfamethoxazole include:
- Nausea and/or vomiting
- Rash and photosensitivity
- Dizziness
- Fatigue
- Loss of appetite and anorexia [10]
This medication’s mechanism of action interferes with folate production, so folate deficiency is also common. More severe side effects may include Stevens-Johnson syndrome, anemia, and Clostridioides difficile (C. diff) diarrhea. This medication should not be prescribed to patients with a sulfa allergy since it can cause anaphylaxis [10].
Healthcare providers should also be aware of the precautions and contraindications before prescribing this medication. They should be cautious when prescribing this medication to patients with decreased kidney function, as it can lead to toxicity and high potassium levels. Therefore, baseline and frequent monitoring of blood urea nitrogen (BUN), creatinine, and potassium levels are helpful.
Other contraindications include patients with liver failure, hematological disorders, or who are pregnant. TMP-SMX interferes with several medications, including phenytoin, digoxin, diuretics, and rifampin. Many of these medication interactions increase the risk for potential hyperkalemia, medication toxicity, and QT prolongation [10].
Ask yourself...
- What is the pharmacokinetics of trimethoprim-sulfamethoxazole?
- What are the common side effects of trimethoprim-sulfamethoxazole?
- What are some contraindications of trimethoprim-sulfamethoxazole?
- What education should you provide to a patient being started on trimethoprim-sulfamethoxazole?
Nitrofurantoin
Nitrofurantoin is another antibiotic commonly prescribed to treat urinary tract infections (UTIs). It has been FDA-approved since 1953 to treat and prevent lower UTIs and is commonly considered a first-line treatment. Currently, nitrofurantoin has no other approved or off-label uses. It comes in two different forms: monohydrate and macrocrystalline [16].
The mechanism of action for nitrofurantoin is not entirely understood. However, it is thought to be absorbed by bacterial flavoproteins in the gastrointestinal tract and further prevents bacterial enzymes from synthesizing DNA, RNA, and cell wall proteins [16].
Nitrofurantoin is only available via the oral route. The dosage is usually 100 mg twice daily for UTI treatment; for UTI prophylaxis, it is 50mg to 100mg once daily. Common medication side effects include:
- Nausea and/or vomiting
- Diarrhea
- Loss of appetite [16]
Severe reactions, although rare, include pulmonary toxicity, where patients may present with fever, chills, cough, and dyspnea. It may also cause liver toxicity, liver failure, and peripheral neuropathy. This medication is contraindicated in patients with a creatinine clearance of less than 60 mL/minute, hemolysis, or who have glucose-6-phosphate dehydrogenase (G6PD) deficiency [16].
Healthcare providers should also be aware of considerations when prescribing nitrofurantoin. They must understand that nitrofurantoin should not be prescribed to patients with suspected or confirmed pyelonephritis, since it is only used to treat lower UTIs.
Nitrofurantoin should not be prescribed to people who are pregnant, especially between 38 and 42 weeks of gestation, or to neonates. It is also not recommended to give to patients who are 65 years or older due to increased potential adverse effects. For patients who take nitrofurantoin long-term for prophylactic use, liver function tests should be routinely completed, and monitoring of pulmonary function [16].

Ask yourself...
- What is the pharmacokinetics of nitrofurantoin?
- What are the common side effects of nitrofurantoin?
- What are some contraindications of nitrofurantoin?
- What education should you provide to a patient being started on nitrofurantoin?
Fluoroquinolones
Fluoroquinolones are a class of antibiotics used to treat UTIs and other bacterial conditions. Examples of common fluoroquinolones include ciprofloxacin, moxifloxacin, and levofloxacin. However, ciprofloxacin is more commonly used to treat UTIs than the others and will be the medication reviewed in this section [19].
In addition to urinary tract infections, ciprofloxacin is FDA-approved to treat various health conditions. Some common health conditions treated are gonorrhea, chancroid, joint diseases, prostatitis, and some gastrointestinal infections. Ophthalmic forms treat corneal ulcers and conjunctivitis, while otic forms may be used in otitis externa [17].
Ciprofloxacin’s mechanism of action is considered bactericidal since it works by inhibiting bacterial DNA replication. It acts against DNA topoisomerase and DNA gyrase to hinder DNA replication. Ciprofloxacin is most effective against gram-negative bacteria, but also some gram-positive bacteria. Due to mutations in the DNA gyrase, ciprofloxacin and other fluoroquinolones have begun to show bacterial resistance over the past several years [17].
Ciprofloxacin comes in many forms, including oral, IV, ophthalmic, and otic. This medication is available in oral and IV forms to treat UTIs. Recommended dosages are dependent on the severity of UTI and route. Oral dosages for UTI treatment range from 250mg to 500mg twice daily, while IV dosages can range from 200mg to 400mg twice daily to upwards of 400mg every eight hours. Regardless of the administration route, typical side effects are nausea and diarrhea. Some serious adverse effects include:
- QT prolongation
- Hypoglycemia
- Hyperglycemia
- Photosensitivity [17]
Ciprofloxacin has an FDA black box warning of tendinitis and tendon rupture, peripheral neuropathy, and myasthenia gravis exacerbation. Due to its effects on tendons, Achilles tendon rupture, aortic aneurysm, and aortic dissection are also serious adverse effects. The drug should be discontinued immediately if the healthcare provider suspects any adverse effects, including mild tendonitis.
Furthermore, there are additional considerations and contraindications when prescribing ciprofloxacin. As with all medications, ciprofloxacin should not be prescribed to patients with a known allergy to it.
Due to interactions, Ciprofloxacin should not be prescribed along with tizanidine, theophylline, or cyclosporine. Antacids can also interfere with the absorption of ciprofloxacin, and ciprofloxacin toxicity is more likely in older adults. Healthcare providers should encourage patients with diabetes to closely monitor their blood glucose levels at home since this medication can cause hypo- or hyperglycemia [17].
Ask yourself...
- What is the pharmacokinetics of ciprofloxacin?
- What are the common side effects of ciprofloxacin?
- What are some contraindications of ciprofloxacin?
- What is the black box warning of ciprofloxacin?
- What education should you provide to a patient being started on ciprofloxacin?
Beta-lactams
Beta-lactams are a class of antibiotics used to treat various bacterial conditions, including UTIs. The three main beta-lactams covered below are commonly used to treat UTIs, including cephalexin, amoxicillin-clavulanate, and ceftriaxone. Other beta-lactams approved for UTI treatment are cefpodoxime, cefdinir, and cefadroxil, which work similarly to others in this medication family.
Cephalexin
Cephalexin is a beta-lactam antibiotic that is classified as a first-generation cephalosporin. It was initially approved by the FDA in 1970 and is widely used throughout healthcare settings. In addition to acute and chronic UTIs, cephalexin is approved to treat upper and lower respiratory infections, bone infections, and otitis media. It is also used to treat surgical site, skin, and soft tissue infections [8].
Cephalexin falls under the beta-lactam class since its structure has a beta-lactam ring. This ring inhibits the synthesis of peptidoglycan, which further disrupts the bacterial cell wall. More specifically, the beta-lactam ring binds to the penicillin-binding proteins during peptidoglycan synthesis, disrupting the bacterial cell wall and viability [8].
Cephalexin is only available via the oral route and can be prescribed in tablet, capsule, and suspension forms. Daily dosages from 1000mg to 4000mg for adults and children are weight-based and range from 25mg to 100mg per kilogram daily. Some side effects of cephalexin include:
- Abdominal pain
- Diarrhea
- Nausea and/or vomiting
- Rash
- Candidiasis [8]
Other reactions may include increased liver enzymes, C. diff colitis, and hemolytic anemia. Although cross-reactivity with penicillin is somewhat uncommon, healthcare providers should use caution when prescribing this medication to patients allergic to penicillin. Furthermore, it should not be prescribed to patients with a cephalosporin allergy. Cephalexin also interacts with metformin, causing decreased clearance of metformin from the body.
If a patient is taking metformin, they should be advised to closely monitor their blood glucose levels since the risk for hypoglycemia is increased. Healthcare providers should also be cautious when prescribing this medication to patients who are taking probenecid. Cephalexin can also increase prothrombin time, which typically requires monitoring, especially for those undergoing anticoagulant treatment [8].

Ask yourself...
- What is the pharmacokinetics of cephalexin?
- What are the common side effects of cephalexin?
- What are some contraindications of cephalexin?
- What education should you provide to a patient being started on cephalexin?
Amoxicillin-clavulanate
As its name implies, amoxicillin-clavulanate is a combination medication of amoxicillin and clavulanic acid. In addition to treating UTIs, this medication is FDA-approved to treat rhinosinusitis, acute otitis media, skin infections, aspiration, and community-acquired pneumonia. Other non-FDA-approved uses are impetigo, chronic obstructive pulmonary disease exacerbations, diabetic foot infections, and human and animal bites [4].
Amoxicillin-clavulanate works via two different mechanisms of action since it is a combination medication. The amoxicillin component is a beta-lactam antibiotic and works via the same mechanism as cephalexin described above. Clavulanic acid is a beta-lactamase inhibitor, which prevents bacteria from destroying beta-lactam antibiotics. This is the reason why clavulanic acid is often combined with amoxicillin [4].
This medication is only orally, such as suspensions, chewable, immediate-release, or extended-release tablets. Dosages depend on the underlying condition being treated, medication form (i.e., immediate- versus extended-release), and the patient’s age. Regardless of dosage, amoxicillin-clavulanate can lead to common gastrointestinal side effects, such as:
- Diarrhea
- Nausea
- Vomiting
- Loose stools [4]
Vaginal candidiasis is another common side effect of amoxicillin-clavulanate. For patients who are breastfeeding, this medication may cause hypersensitivity reactions in infants since it is excreted in breast milk. Additionally, amoxicillin-clavulanate has several drug interactions with medications, including probenecid, oral anticoagulants, allopurinol, and oral contraceptives. Dose adjustments are recommended for patients on hemodialysis or with severe renal impairment, usually where their creatinine clearance is less than 30 mL/min.
Healthcare providers should monitor patients’ liver enzymes for possible hepatic impairment. If hepatic injury occurs, the medication should be stopped immediately and treatment recommendations followed accordingly. Since amoxicillin is a penicillin derivative, it should not be prescribed to individuals with a penicillin allergy [4].
Ask yourself...
- What is the pharmacokinetics of amoxicillin-clavulanate?
- What are the common side effects of amoxicillin-clavulanate?
- What are some contraindications of amoxicillin-clavulanate?
- What education should you provide to a client who was prescribed amoxicillin-clavulanate?
Ceftriaxone
Ceftriaxone is another beta-lactam antibiotic used to treat UTIs and is usually an adjunct medication. It is a third-generation cephalosporin used to treat gonorrhea, pelvic inflammatory disease, meningitis, and certain abdominal, respiratory, and joint infections. Additionally, ceftriaxone treats bacteremia, sepsis, and infective endocarditis. Since ceftriaxone belongs to the beta-lactam class, its mechanism of action is the same as previously described for cephalexin [18].
Ceftriaxone comes in intravenous and intramuscular (IM) forms since it is not absorbed through the gastrointestinal tract. For UTI treatment, the dosage for adults is 1 to 2 grams IV or IM every 24 hours. In an outpatient setting, ceftriaxone is often given as a single IM dose to patients with pyelonephritis who are not hospitalized. Again, this medication is used as an adjunct, giving it as a single IM dose, followed by another antibiotic for UTI treatment. Dosages for children range from 50mg to 75mg per kilogram per day for both IM and IV forms [18]. Some common side effects of ceftriaxone are:
- Diarrhea
- Nausea
- Vomiting
- Dysgeusia (metallic or foul taste in the mouth)
- Injection site reaction, especially for IM [18]
Other adverse effects of ceftriaxone are hemolytic anemia, neutropenia, and thrombocytosis. This medication can also cause neurological symptoms, cholelithiasis, jaundice, elevated liver enzymes, and pancreatitis [18].
Healthcare providers should also review ceftriaxone’s precautions and contraindications. This medication is cross-reactive with penicillin and can cause a hypersensitivity reaction. Furthermore, it should not be prescribed to patients with a cephalosporin allergy. If ceftriaxone is administered through an IV, it must not be mixed with calcium-containing IV solutions or products. Other possible medication interactions are estradiol, cyclosporine, and bumetanide. For patients with liver or kidney impairment, dosages must be adjusted and should not exceed 2 grams per day [18].
Ask yourself...
- What is the pharmacokinetics of ceftriaxone?
- What are the common side effects of ceftriaxone?
- What are some contraindications of ceftriaxone?
- What education should you provide to a client who was prescribed ceftriaxone?
Cefdinir
Cefdinir is another beta-lactam antibiotic used to treat UTIs and is also a third-generation cephalosporin. In addition to UTI treatment, cefdinir is approved for the treatment of pneumonia, bacterial infections involving the skin, and respiratory infections of the ears, throat, and sinuses. As cefdinir is a third-generation cephalosporin, its mechanism of action is the same as previously described ceftriaxone. Thus, it interferes with bacterial cell wall synthesis [13].
Cefdinir is only available via the oral route via capsule or liquid suspension. Treatment dosages and duration are dependent on the underlying condition it is being used to treat [13]. For UTI, this medication is usually prescribed at 300mg twice daily for 5 to 7 days. However, this course may be extended for patients with pyelonephritis or complicated UTIs. Some common side effects of this medication include:
- Nausea and vomiting
- Diarrhea
- Vaginal itching
- Red-tinged stools [13]
Other serious side effects may include rash, hives, facial swelling, and difficulty breathing or swallowing. As with all medications, healthcare providers should be aware of this medication’s precautions and contraindications. Cefdinir should not be prescribed to patients with a cephalosporin allergy or who are taking probenecid. It should also be avoided in patients with gastrointestinal diseases, like colitis, and kidney disease. This medication should be taken at least two hours apart from aluminum, magnesium, or iron supplements. Additionally, healthcare providers should avoid prescribing the oral suspension form to patients with diabetes, since it contains high amounts of sucrose and can potentially raise blood sugar levels [13].

Ask yourself...
- What is the pharmacokinetics of cefdinir?
- What are the common side effects of cefdinir?
- What are some contraindications of cefdinir?
- What education should you provide to a client who was prescribed cefdinir?
Other Antibiotics Used to Treat UTI
This section reviews other antibiotics used to treat urinary tract infections. Two common additional antibiotics administered to patients include piperacillin-tazobactam and antipseudomonal carbapenems, such as imipenem or meropenem. These are usually reserved for patients with at least one risk factor for multidrug-resistant gram-negative organisms [7]. Drug information about meropenem will be discussed in greater detail below. Other less common antibiotics available in the outpatient setting are fosfomycin and pivmecillinam. Although these medications are used less commonly, it is still essential to understand their pharmacokinetics.
Piperacillin-tazobactam
As mentioned, piperacillin-tazobactam treats multidrug-resistant gram-negative UTIs in an inpatient setting. It is also approved for treating skin, gynecological, and certain abdominal infections. This medication combines piperacillin (a penicillin antibiotic) and tazobactam (a beta-lactamase inhibitor). Therefore, its mechanism of action is that of both the penicillin and beta-lactam classes, as previously described [15].
Piperacillin-tazobactam is available in IV form, and the dosage is usually 3.375 grams every six hours [7]. Like many antibiotics, some common side effects include:
- Diarrhea
- Nausea and/or vomiting
- Stomach pain [15]
In more severe cases, this medication can also cause mouth sores, sleeping difficulties, itching, difficulty swallowing, and wheezing. Piperacillin-tazobactam should not be prescribed to patients with an allergy or who take other penicillin or beta-lactam antibiotics. It interferes with certain medications, including aminoglycosides, anticoagulants, methotrexate, and vancomycin. Additionally, for patients with diabetes, this medication can cause false results with specific glucose tests [15].
Ask yourself...
- What is the pharmacokinetics of piperacillin-tazobactam?
- What are common side effects of piperacillin-tazobactam?
- What are some contraindications of piperacillin-tazobactam?
- What education should you provide to a patient taking piperacillin-tazobactam?
Meropenem
Meropenem, an antipseudomonal carbapenem, is used to treat inpatient multidrug-resistant gram-negative UTIs. It is also approved for the treatment of pneumonia, intra-abdominal infections, peritonitis, and meningitis [21].
This medication’s mechanism of action is similar to that of beta-lactams, as it belongs to the same family of antibiotics. It binds to penicillin-binding proteins and inhibits bacterial cell wall synthesis by inhibiting peptidoglycan [21].
Meropenem is only available in IV form, and dosages depend on the treated condition. For UTIs, 1 gram is administered every 8 hours. Gastrointestinal side effects are typical for this medication and include symptoms like diarrhea, nausea, vomiting, and constipation. Additional adverse effects may include:
- Drowsiness
- Headache
- Insomnia
- Depression [21]
Seizures also have potential adverse effects, so healthcare providers must monitor patients for neurological symptoms. Agranulocytosis, thrombocytopenia, gastrointestinal bleeding, and hemolytic anemia are other conditions that have been reported when taking this medication. For patients with renal impairment, the dosage will need to be adjusted. Meropenem also has many medication interactions, with some major ones including afatinib, atogepant, cariprazine, and colchicine [21].
Ask yourself...
- What is the pharmacokinetics of meropenem?
- What are the common side effects of meropenem?
- What are some contraindications of meropenem?
- What education should you provide to a patient taking meropenem?
Fosfomycin
Fosfomycin is an antibiotic that is FDA-approved to treat uncomplicated urinary tract infections and is considered a first-line treatment option. In the United States, fosfomycin is only available in oral powder, which is mixed and dissolved in liquid. Furthermore, although this medication is a first-line UTI treatment option, it is not widely available in the United States [20].
This medication’s mechanism of action interferes with the formation of the peptidoglycan precursor UDP N-acetylmuramic acid, also known as UDP-MurNAc. As it acts one step prior to the beta-lactam antibiotic family, Fosfomycin enters the bacteria through the transporter systems L-alpha-glycerophosphate and hexose-6-phosphate. This medication also reduces bacteria’s ability to adhere to the epithelial cells of the urinary tract [9].
To treat UTI, a single dose of 3 grams of fosfomycin is given, which is added to about 3 to 4 ounces of cold water to dissolve the medication. Common side effects may include:
- Nausea
- Diarrhea
- Headache
- Back pain [14]
Other serious side effects include joint pain, rash, facial or oral swelling, and jaundice. Healthcare providers should review this medication’s interactions and contraindications. Fosfomycin interacts with medications like cisapride, metoclopramide, and certain vitamins. Healthcare providers should use caution when prescribing to patients with a history of asthma or liver disease or who are pregnant or breastfeeding [14].
Pivmecillinam
Pivmecillinam is another antibiotic used to treat lower urinary tract infections and is part of the UTI treatment algorithm. However, the FDA has not approved this medication in the United States [11]. Therefore, most of the specific drug information is not available.
Ask yourself...
- What is the mechanism of action for fosfomycin?
- What are some common side effects of fosfomycin?
- What are some contraindications of fosfomycin?
- What education should you provide to a patient taking fosfomycin?
Considerations for Prescribers
This section reviews potential considerations when prescribing antibiotics for UTIs.
Healthcare providers must consider several factors when prescribing antibiotics for UTI treatment. First, the medication’s route, dosage, and treatment duration are usually determined by the setting (inpatient versus outpatient), the type of UTI (e.g., empirical, asymptomatic bacteremia, uncomplicated, complicated, or prophylaxis), and the patient’s underlying medical conditions and risk factors. Furthermore, healthcare providers should follow current treatment guidelines, approved uses, and their organization’s protocols when initiating or adjusting these medications. Healthcare providers must review the patient’s medical history, recent lab values, contraindications, and potential side effects.
Patient Population
Certain antibiotics should be avoided in specific individuals or patient populations; thus, healthcare providers must be aware of these precautions, contraindications, and black box warnings. Ciprofloxacin can lead to medication toxicity and hypoglycemia in older adults and must be avoided in this patient population when able [17]. Additionally, prescribing medications, such as TMP-SMZ and cephalexin, should be cautioned in patients with renal impairment [8, 10]. Prescribing nitrofurantoin, TMP-SMZ, cephalexin, and ceftriaxone should be cautioned in patients with liver failure or elevated liver enzymes. Healthcare providers should routinely monitor the patient’s liver enzymes and function tests [8, 10, 16, 18].
The patient’s gender at birth plays another significant factor in antibiotic selection since treatment for males is considered a complicated UTI, and antibiotic duration is extended. Furthermore, in pregnant or breastfeeding patients, certain antibiotics should not be prescribed, including TMP-SMZ and fluoroquinolones. Alternative treatments for patients who are pregnant are amoxicillin-clavulanate or [6]. For patients with chronic UTIs, initial prophylactic treatment should be started for 3 months and then reevaluated thereafter for prevention. Typical low-dose prophylactic antibiotics are TMP-SMX and nitrofurantoin. However, the healthcare provider should strongly consider medication compliance and potential antibiotic resistance [1].
Allergies
Healthcare providers should also review the patient’s allergies and cross-reactivity of certain antibiotics. Trimethoprim-sulfamethoxazole should not be prescribed to individuals with a sulfa allergy. Furthermore, healthcare providers should not prescribe amoxicillin-clavulanate to patients with a penicillin allergy. They should use caution when prescribing these individuals beta-lactams or cephalosporins due to their potential cross-reactivity, although the percentage is low [8, 10].
Medication History
As discussed, certain antibiotics interact with specific medications. Healthcare providers must review the patient’s medication list before prescribing antibiotics. For instance, amoxicillin-clavulanate interacts with allopurinol and lessens the effectiveness of oral contraceptives. Patients on oral contraceptives should be instructed to use backup birth control methods while on the antibiotic [4]. Additionally, while interactions may exist, medication timing plays an important role. For example, medications such as ciprofloxacin and TMP-SMZ interact with antacids. However, if the antibiotic is taken two hours before or six hours after the antacid, a potential medication interaction is less likely [17]. Cephalexin is better absorbed on an empty stomach, while other antibiotics are recommended to be taken with food [8].
Bacterial Sensitivity
Some antibiotics do not treat certain strains of bacteria, and thus, healthcare providers must be judicious about initial antibiotic selection and follow up with the patient about their urine culture results. For example, bacterial growth on the urine culture might not be sensitive to the initial antibiotic prescribed and may be resistant. Therefore, the antibiotic may need to be changed, or dual therapy may be needed.
Also, patients with recurrent UTIs sometimes develop antibiotic resistance to first-line medications for the treatment of UTIs, so an alternative medication should be initially prescribed, and a urine culture should be sent. If the patient is treated frequently for UTIs, then a referral to urology is warranted for further evaluation [6]. In some patients, asymptomatic bacteremia is found on a routine urinalysis completed during an annual comprehensive exam. Healthcare providers should review the current screening and treatment guidelines for asymptomatic bacteria in adults. A urine culture should be sent for further evaluation for most patients, and antibiotic administration should be delayed until the culture results [5].
Patient Education
Patients should be instructed on potential medication side effects and signs of adverse reactions. In an outpatient setting, healthcare providers must advise patients on worsening symptoms and when to seek immediate or emergent treatment. Oftentimes, urinary tract infections move upstream into the kidneys and cause pyelonephritis or urosepsis [6].
Ask yourself...
- What factors should healthcare providers consider when prescribing antibiotics?
- Which antibiotic can be prescribed during pregnancy?
- Which steps should be taken for patients with recurrent UTIs?
- What health conditions and lab values are essential when selecting UTI antibiotics?
- What allergies do you want to ensure your clients do not have before administering Trimethoprim-sulfamethoxazole?
Upcoming Research
This section reviews upcoming research and medications for UTI treatment.
The bacterial strain, E. coli, is typically responsible for urinary tract infections. However, this is not the case for all UTIs, and some patients have developed multiple drug-resistant organisms due to recurrent infections. Therefore, much research is needed on UTI antibiotic treatment to eradicate these organisms. Recent developments in immunomodulatory therapy and medications that inhibit bacterial adhesions to the epithelial cells of the urinary tract have been considered. Vaccinations against UTI have also shown promise, but further research is still needed [3]. Other non-antibiotic therapies are also being researched, like Lactobacillus-containing products (i.e., probiotics) and cranberry supplements [12].

Ask yourself...
- Which bacteria commonly cause urinary tract infections?
- What new research is there about antibiotics for UTI treatment?
- Which types of new products are being researched for UTI treatment?
- How has the management of UTIs changed since you have been in practice?
Conclusion
As discussed, antibiotic selection for the treatment of urinary tract infections depends on various factors. Healthcare providers should understand the pharmacokinetics, potential side effects, interactions, and contraindications when selecting an antibiotic. They should also follow current clinical guidelines and their facility’s protocols for a more evidence-based approach. Furthermore, ordering a urine culture or referring a patient to a urologist is warranted for patients with recurrent urinary tract infections.
Ask yourself...
Final Reflection Questions
- What differentiates a lower versus an upper UTI?
- What are the different names of beta-lactam antibiotics used to treat UTIs?
- Which antibiotics are commonly prescribed for UTI prophylaxis?
- Which antibiotics are not commonly prescribed or available in the United States?
- Which antibiotics are commonly used to treat multidrug-resistant organisms?
- Which antibiotics can be used in outpatient versus inpatient settings?
- What are the two types of fluoroquinolones used to treat UTIs?
- What are some other antibiotics used to treat UTIs?
- What new antibiotics have you learned about?
- What new education can you now provide to patients regarding these antibiotics for UTIs?
Oral STI Medications
Introduction
When hearing the phrase sexually transmitted infections, what comes to mind? If you’re an advanced practice registered nurse (APRN) with prescriptive authority, you’ve definitely heard of sexually transmitted infections (STIs) before. Even as a nurse or maybe before nursing school, conversations about prescription drug use and sexual health existed every so often.
Presently, patients seek guidance and information on various health topics from APRNs, including medication management and sexual health. The information in this course will serve as a valuable resource for APRNs with prescriptive authority across all specialties, education levels, and backgrounds to learn more about oral medications that can treat and manage STIs.
Defining Sexually Transmitted Infections (STIs)
What Are STIs?
Sexually transmitted infections (STIs) are infections that transmitted via sexual activity, such as oral sex, vaginal sex, anal sex, and sexual skin-to-skin contact. STIs can be bacterial, viral, and parasitic in nature and infect millions of people in the USA and around the world every year. STIs can be stigmatized as only common among those who are poor or unhoused, but it is important to note that anyone who is engaging in sexual activity is at risk for an STI (1).
What Are Bacterial STIs?
Bacterial STIs include chlamydia, gonorrhea, syphilis, bacterial vaginosis, chancroid, and Mycoplasma genitalium.
Chlamydia is the most common bacterial STI in the United States and a leading cause of infection-related vision loss worldwide. Chlamydia is a bacterial infection as a result of exposure to the Chlamydia trachomatis bacterium via sexual contact.
Gonorrhea is another common bacterial STI in the United States. It is a bacterial infection caused by exposure to the Neisseria gonorrhoeae bacterium via sexual contact.
In addition, syphilis is another bacterial infection as a result of exposure to the spirochete Treponema pallidum bacteria via sexual contact (4). Syphilis rates in the United States were once very minimal but have increased significantly over the past few years (4).
Bacterial vaginosis is a vaginal condition in which there is an imbalance of bacteria in the vaginal microbiome (5). Several cases of bacterial vaginosis can be related to vaginal sexual activity; however, people can have bacterial vaginosis anytime there is an imbalance of bacteria in the vaginal microbiome (5).
Chancroid is a bacterial infection caused by exposure to the H. ducreyi bacterium via sexual contact (6). Although its prevalence in the United States has decreased over the past several years, it is still a bacterial STI of concern (6).
The final bacterial STI for this course is Mycoplasma genitalium, in which someone is exposed to this bacterium via sexual contact.
What Are Viral STIs?
Viral STIs include herpes simplex virus (HSV), human papillomavirus (HPV), and human immunodeficiency virus (HIV). HSV, also known as herpes, is a chronic viral infection that is transmitted via sexual skin-to-skin contact and sexual activity (8). HPV is the world’s most common sexually transmitted infection, a chronic viral infection, and is transmitted via sexual skin-to-skin contact and sexual activity (9).
HIV is a chronic viral infection transmitted via blood, semen, vaginal secretion, or breastmilk that can progress to acquired immunodeficiency syndrome (AIDS) if left untreated and unmanaged (10).
What Are Parasitic STIs?
Trichomoniasis is a parasitic STI that is a result of being exposed to the Trichomonas vaginalis protozoan parasite via sexual activity (11).
What If STIs Are Left Untreated?
Depending on the STI, it can cause several long-term complications if left untreated. If HIV is not correctly managed, several complications, such as AIDS and immune-related deficiencies, can emerge (10). Untreated or repeated chlamydia and gonorrhea infections can lead to chronic pelvic pain, genital pain, genital discharge, and infertility (2,3).
If syphilis is left untreated, neurological, cardiac, and musculoskeletal complications can occur (4). While the most common STI symptom is no symptom, it is crucial to offer routine STI screening and be able to assess and manage positive STI results if they occur.
Ask yourself...
- What is an STI?
- What is the difference between a bacterial STI and a parasitic STI?
- What is an example of a viral STI?
- What happens if an STI is left untreated?
- What role does community and public health nursing play in the prevention and education related to STIs?
Defining Oral STI Medications
Oral STI medications depend on the type of infection being treated or managed. Bacterial STIs are treated with antibiotics, and some viral STIs are managed with antiviral medications. The most common oral STI medications are azithromycin, metronidazole, acyclovir, and doxycycline.
Oral STI medications are used in various clinical settings, such as hospitals, outpatient clinical settings, public health departments, correction facilities, and more. Oral STI medications are prescribed and then taken by mouth. Depending on the dosage and type of STI, someone might take only one pill of an antibiotic to cure chlamydia or take a series of pills to manage their HIV viral load.
What are the Clinical Criteria for Prescribing Oral STI Medication?
Clinical criteria for prescribing oral STI medications can vary depending on the STI itself. First, a health care provider would order and perform testing to detect STIs, such as a Pap smear, urine sample, visual examination, blood sample, or genital swab (1,2,3,4,9).
If any of these tests show a positive result for an STI, clinical guidelines from reputable organizations, such as the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH), are followed to manage these conditions (1, 2). In addition, local laws and health departments might have reporting requirements if a patient tests positive for an STI, so be sure to check with your local boards of nursing or health department on any reporting requirements.
What Is the Average Cost for Oral STI Medications?
The cost of oral STI medications can significantly vary depending on the type of medication, insurance, dosage, frequency, and other factors. Cost is a leading reason why many patients cannot maintain their medication regimen (12). If cost is a concern for your patient, consider reaching out to your local pharmacies or patient care teams to find cost-effective solutions.

Ask yourself...
- What are the clinical criteria for prescribing oral STIs?
- What resources do you have available to help patients pay for their STIs?
Antibiotic Pharmacokinetics
Drug Class – Oral Antibiotics for STIs
The health care provider’s professional discretion and the patient’s condition should guide therapy. Consider reviewing a patient’s medication history and health history before prescribing oral medications for STIs.
Bacterial STIs include chlamydia, gonorrhea, syphilis, bacterial vaginosis, chancroid, and Mycoplasma genitalium.
Oral Antibiotics Method of Action
The method of action for oral antibiotics for STI treatment depends on the antibiotic and the type of bacterium present.
Chlamydia infections result from exposure to the chlamydia trachomatis bacteria. A chlamydia infection can be treated with a course of doxycycline 100mg pills twice a day for seven days. Alternative treatment options include azithromycin 1 g pill as a single dose or levofloxacin 500mg pill once a day for seven days (1,2).
Doxycycline is an antibiotic that is part of the tetracycline drug class and works by preventing the growth of gram-negative and gram-positive bacteria, such as chlamydia trachomatis, Neisseria gonorrhoeae, and the spirochete Treponema pallidum (13). Doxycycline works to eliminate the chlamydia trachomatis bacteria by binding to the 30S prokaryotic ribosomal unit during protein synthesis, thus slowing down and eliminating the growth of bacteria (13).
Azithromycin and erythromycin are antibiotics that are part of the macrolide drug class and work by preventing the growth of many gram-negative and gram-positive bacteria (14). Azithromycin and erythromycin work to eliminate the chlamydia trachomatis bacteria and Haemophilus ducreyi by binding to the 50S subunit of bacterial ribosomes and leading to decreased bacterial synthesis (14).
Levofloxacin is an antibiotic in the fluoroquinolone drug class that works by directly stopping bacterial DNA synthesis. It is considered to have the strongest activity against gram-positive, penicillin-sensitive, and resistant bacteria (15). Levofloxacin eliminates the chlamydia trachomatis bacteria by breaking the DNA strands through DNA gyrase inhibition (15).
Gonorrhea infections are a result of exposure to the Neisseria gonorrhoeae bacteria. Gonorrhea infection treatment guidelines include a single injection of ceftriaxone depending on the patient’s weight (16). If ceftriaxone is a contraindication for the patient’s condition or unavailable, gonorrhea can be treated with a course of doxycycline 100mg pills twice a day for seven days (16).
Syphilis infection is a result of exposure to the spirochete Treponema pallidum bacteria (4). Syphilis infection treatment guidelines include benzathine penicillin injections, where the dosage and frequency depend on the stage of syphilis, patient age, and other co-existing health conditions. Some guidelines also recommend doxycycline 100mg pills twice a day for 14 days; however, penicillin injections appear to be more effective at syphilis management and treatment (4).
Bacterial vaginosis is not always STI, as bacterial vaginosis is the result of an imbalance of bacteria in the vaginal microbiome. However, regardless of the cause of bacterial vaginosis, current guidelines for bacterial vaginosis treatment include metronidazole pills at 500mg twice a day for seven days with possible intravaginal application of metronidazole or clindamycin (5).
Metronidazole is a medication that is part of the nitroimidazole antimicrobial and antiprotozoal drug class, where its method of action involves protein synthesis inhibition (17). Metronidazole works to eliminate bacteria and Trichomonas vaginalis protozoan by causing the destruction of helical DNA structure and strand breakage, causing bacterial death (17). Tinidazole is another medication part of the nitroimidazole drug class, has a similar pharmacokinetic profile to metronidazole, and a higher cure rate for parasitic infections (17).
Chancroid results from exposure to the Haemophilus ducreyi (H. ducreyi) bacteria via sexual contact. Current guidelines for chancroid treatment include either a single dose of azithromycin 1g pill, a single ceftriaxone 250 mg injection, ciprofloxacin 500mg pills twice a day for three days, or erythromycin 500mg pills three times a day for seven days.
Ciprofloxacin is an antibiotic that is part of the fluoroquinolone drug class, where it works to eliminate the H. ducreyi bacteria by breaking the DNA strands through DNA-gyrase inhibition (18).
Having Mycoplasma Genitalium as an STI results from exposure to this bacterium. Current guidelines for Mycoplasma genitalium treatment depend on the macrolide sensitivity of the bacteria and patient condition. If Mycoplasma Genitalium is macrolide sensitive, the recommendations include doxycycline 100mg pills twice a day for 7 days, followed by a single azithromycin 1mg pill, then followed by one 500mg pill once a day for three additional days (7). If Mycoplasma Genitalium is macrolide resistant or if resistance testing is not available, recommendations include doxycycline 100mg pills twice a day for 7 days, followed by a single azithromycin 1mg pill, followed by moxifloxacin one 400 mg pill daily for seven days (7).
Trichomoniasis is a parasitic STI that is a result of being exposed to the Trichomonas vaginalis protozoan parasite via sexual activity (11). Current guidelines for trichomoniasis treatment include metronidazole, one 500mg pill twice a day for 7 days for women or a single metronidazole 2g pill for men. An alternative treatment option for both men and women includes a single tinidazole 2g pill (11).
Oral Antibiotic Side Effects
Every medication can have side effects, and oral antibiotics are no exception. Possible side effects of doxycycline include photosensitivity, GI upset, headaches, tooth discoloration, and a skin rash (13). More severe side effects of doxycycline include chest pain, leukopenia, changes in heart rate, and hepatoxicity.
Doxycycline is also contraindicated for pregnant people and children under 12 because of the teratogenic properties and risk of teeth discoloration. Doxycycline is also contraindicated for people who are allergic to tetracycline medications or penicillin (13).
Possible side effects of azithromycin and erythromycin include hepatotoxicity, GI upset, QT interval prolongation, and cardiac complications. Azithromycin and erythromycin are contraindicated in people allergic to macrolides (14).
Possible side effects of levofloxacin and moxifloxacin include photosensitivity, GI upset, headache, tendon rupture, changes in glucose levels, seizures, QT interval prolongation, and peripheral neuropathy. Levofloxacin and moxifloxacin are contraindicated in pregnant and breastfeeding patients (15). Levofloxacin has FDA-issued box warnings for side effects related to tendinitis and tendon rupture, peripheral neuropathy, and central nervous system effects (15).
Possible side effects of metronidazole include peripheral neuropathy, metallic taste, GI upset, and confusion (17). Consuming alcohol and being in the first trimester of pregnancy are contraindications for metronidazole usage (17).
Possible side effects of ciprofloxacin include GI upset, QT interval prolongation, glucose level changes, and photosensitivity. FDA-issued box warnings for side effects of ciprofloxacin include tendinitis and tendon rupture, peripheral neuropathy, and central nervous system effects (18).
Oral Antibiotics Alternatives
Given the nature of bacterial STIs, the only evidence-based method of treating bacterial STIs is with antibiotic medications (1). Condom use can help prevent the transmission of STIs.

Ask yourself...
- What are some common STIs?
- What are some common medications that can be prescribed to manage STIs?
- What is the mechanism of action for oral antibiotics?
- What is the difference between gonorrhea and syphilis?
- How would you describe chlamydia to a patient who was recently diagnosed?
- What is the recommended treatment for Trichomoniasis?
- What education is essential to provide to patients being started on medications to treat their STIs?
Antiviral Pharmacokinetics
Drug Class – Oral Antivirals for STIs
Health care provider’s professional discretion and patient condition should guide therapy. Consider reviewing a patient’s medication history and health history prior to prescribing SSRIs.
Viral STIs include herpes simplex virus (HSV), human papillomavirus (HPV), and human immunodeficiency virus (HIV).
Oral Antivirals Method of Action
HSV, also known as herpes, is a chronic viral infection that is transmitted via sexual skin-to-skin contact and sexual activity (8). Current guidelines for HSV antiviral medication management include one acyclovir 400mg pill twice a day, one valacyclovir 500mg or 1g pill a day, or one famciclovir 250mg twice a day, with a duration depending on the severity of the HSV outbreak, patient health history, and clinical presentation. Since HSV is a chronic viral health condition, dosage and frequency can vary from patient to patient.
Acyclovir and valacyclovir are antiviral medications that work by incorporating into viral DNA, thus reducing further HSV synthesis (19). Valacyclovir is the prodrug to acyclovir (19). Famciclovir is a prodrug antiviral medication that is part of the nucleoside analog antiviral drug class. Its method of action involves inhibiting DNA polymerase, leading to decreased viral replication (20).
HPV is the world’s most common sexually transmitted infection, a chronic viral infection, and is transmitted via sexual skin-to-skin contact and sexual activity (9). There are no oral STI medication options to manage HPV (9).
HIV is a chronic viral infection transmitted via blood, semen, vaginal secretion, or breastmilk that can progress to acquired immunodeficiency syndrome (AIDS) if left untreated and unmanaged (10). Given the complex pharmacological properties of HIV antiviral medications and HIV clinical manifestations, refer to specialty care for HIV for chronic management or seek additional training if possible (10).
Several antiviral medications can be used to manage HIV, depending on the patient’s health history, severity of HIV status, and clinical presentation. Oral antiviral drug classes for HIV medications include capsid inhibitors, entry inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase strand transfer inhibitors (ISTIs), and protease inhibitors (PIs) (10).
Lenacapavir is a capsid inhibitor often prescribed at 600 mg once a day that works to distort HIV capsid protein function, thus reducing the influence of HIV in the body. Similar to HSV medications, dosage, frequency, and co-administration of other HIV-related antivirals will depend on patient response to the medication, patient health history, and patient condition (10).
Entry inhibitors work to reduce the HIV viral load by blocking HIV from entering CD4 cells. There are three main types of entry inhibitors: CCR5 antagonists, fusion inhibitors, and attachment inhibitors.
Maraviroc is an entry inhibitor pill often prescribed at 150, 300, or 600 mg, depending on the patient’s health history and severity of HIV. Maraviroc acts as a CCR5 antagonist, where the method of action is blocking HIV from binding to the chemokine coreceptor 5 (CCR5) (10).
Enfuvirtide is an injection medication that acts as an entry inhibitor by preventing HIV from fusing to CD4 cell walls. Fostemsavir is another entry inhibitor pill often prescribed at 600mg twice daily. Its method of action is binding to the HIV glycoprotein GP120 to inhibit HIV attachment to host T cells (10).
NRTIs include abacavir, emtricitabine, lamivudine, tenofovir alafenamide, tenofovir disoproxil fumarate, zidovudine, didanosine, and stavudine. NRTIs are often given in pairs, where the most commonly prescribed pairs are tenofovir alafenamide-emtricitabine, tenofovir disoproxil fumarate-emtricitabine, and abacavir-lamivudine. Clinical management of HIV with NRTIs can depend on the severity of HIV, co-existing health conditions, and the patient’s health history. NRTIs’ method of action involves undergoing intracellular phosphorylation mediated by host enzymes, allowing HIV’s DNA chains to be depleted over time (10).
NNRTIs work to suppress the HIV viral load by preventing HIV-1 reverse transcriptase from creating new nucleotides to the HIV DNA chain. Common NNRTI medications include efavirenz, nevirapine, doravirine, etavirine, and rilpivirine (10).
INSTIs work to suppress the HIV viral load by targeting the strand transfer step of HIV DNA replication, thus reducing the rate at which the HIV DNA replicates. Common INSTI medications include raltegravir, elvitegravir, dolutegravir, and bictegravir (10).
Protease inhibitors suppress the HIV viral load by suppressing the cleavage of Gag-Pol polyproteins in HIV-infected cells, thus preventing cells from maturing enough to be infectious in the body. Protease inhibitors include atazanavir, darunavir, lopinavir, indinavir, fosamprenavir, nelfinavir, saquinavir, and tipranavir (10).
Oral Antiviral Side Effects
Every medication has the possibility of side effects, and oral antivirals are no exception.
The most common side effects of acyclovir and valacyclovir include fatigue, GI upset, confusion, headache, and neurotoxicity (19). Most common side effects of famciclovir include GI upset, headache, and hepatoxicity (20).
Common side effects of lenacapavir include GI upset, blood sugar changes, urine changes, and hepatic dysfunction (10).
Side effects commonly noted in maraviroc include skin rash, GI upset, sexual dysfunction, and anemia. There is an FDA boxed warning for maraviroc and hepatotoxicity (10).
The most common side effects of fostemsavir include increased serum creatinine, prolonged QT prolongation, changes in cholesterol and glucose levels, changes in the liver, and confusion (10).
Common side effects of NRTIs include mitochondrial toxicity, which can have a significant range of clinical presentations, such as hepatic dysfunction, peripheral neuropathy, changes in cholesterol levels, or changes in pancreatic function. Zidovudine, in particular, has an FDA boxed warning for hematological toxicity, myopathy, and severe hepatomegaly (10).
Common side effects of NNRTIs include GI upset, headache, skin rash, changes in cholesterol levels, and changes in glucose levels. The most commonly prescribed NNRTIs, rilpivirine and etravirine, have possible side effects of prolonged QT interval and neuropsychological effects (10).
Side effects commonly reported with INSTIs include changes in weight, headache, and GI upset. Dolutegravir and raltegravir in particular have possible side effects of myopathy, elevations in creatine phosphokinase (CPK), and rhabdomyolysis (10).
Common side effects of protease inhibitors include changes in blood glucose and cholesterol levels, hepatotoxicity, PR interval prolongation, GI upset, and headache (10).
Oral Antiviral Alternatives
HIV and HSV are complex, chronic conditions that are often managed with antiviral medications and require patient monitoring to observe patient response to the drugs and clinical presentation.
While antiviral medications are considered the standard for evidence-based care for HSV and HIV, lifestyle modifications to strengthen one’s immune system, such as increased sleep, decreased stress, and a healthy diet, are thought to play a role in HIV and HSV management (10). Condom use can help prevent the transmission of STIs (1).

Ask yourself...
- What are some possible side effects of oral antiviral medications?
- What are some ways patients can maintain a healthier immune system?
- What education would you provide to a patient who is diagnosed with HSV?
- Are there medications to treat HPV?
- What is the goal of treatment for a patient diagnosed with HIV?
- What is the difference between INSTI and NNRTI medications?
- What education should you provide to patients who are being started on antivirals?
Nursing Considerations
Nurses remain the most trusted profession for a reason, and APRNs are often pillars of patient care in several health care settings. Patients turn to nurses for guidance, education, and support.
While there is no specific guideline for the nurses’ role in STI education and management, here are some suggestions to provide quality care for patients currently taking oral medications to manage STIs or concerned about possibly having an STI.
- Take a detailed health history. Oftentimes, sexual health, such as pain during sex or bleeding after sex, is dismissed in health care settings. If a patient is complaining of symptoms that could be related to an STI, inquire more about that complaint.
- Ask about how long the symptoms have lasted, what treatments have been tried, if these symptoms interfere with their quality of life, and if anything alleviates any of these symptoms. If you feel like a patient’s complaint is not being taken seriously by other health care professionals, advocate for that patient to the best of your abilities.
- Review medication history at every encounter. In busy clinical settings, reviewing health records can be overwhelming. Millions of people take antibiotics and antiviral medications for various infections. Ask patients how they are feeling on the medication, if their symptoms are improving, and if there are any changes to their medication history.
- Be willing to answer questions about sexual health and oral STI medication options. Society stigmatizes open discussions of prescription medication and sexual health. Many people do not know about safe sexual practices, the long-term effects of undiagnosed or repeat STIs, or possible STI treatment options.
Be willing to be honest with yourself about your comfort level discussing topics and providing education on oral STI medications and STI prevention. - Communicate the care plan to other staff involved for continuity of care. For several patients, especially for patients with viral STI infections or recurrent STIs, care often consists of a team of mental health professionals, nurses, specialists, pharmacies, and more. Ensure that patients’ records are up to date for ease in record sharing and continuity of care.
- Stay up to date on continuing education related to oral STI medications and STIS. This is essential, as evidence-based information is always evolving.
- You can then present your new learnings and findings to other healthcare professionals and educate your patients with the latest information.
- Follow updates from evidence-based organizations to learn more about the latest research on oral STI medications and STIs.
How can nurses identify if someone has an STI?
Unfortunately, it is not possible to look at someone with the naked eye and determine if they have an STI. While some people might have visible STI symptoms, such as a wart or discharge, the most common STI symptom is no symptoms.
APRNs can identify and diagnose if someone has an STI by taking a complete health history, listening to the patient’s concerns, and offering STI testing.
What should patients know about oral STI medication?
Patients should know that anyone can experience side effects from antiviral or antibiotic medications for STIs, just like any other medication. Patients should be aware that they should seek medical care if they notice any changes in their vision, experience sharp headaches, or feel like something is wrong.
Because of the social stigma associated with sexual health, people are hesitant to seek medical care because of fear, shame, and embarrassment. However, as more research and social movements discuss sexual health more openly, there is more space and awareness for STI prevention and management.
Nurses should also teach patients to advocate for their health to avoid untreated or undetected STIs and possible unwanted side effects of oral STI medication.
Here are essential tips for patient education in the inpatient or outpatient setting:
- Tell the health care provider of any existing medical conditions or concerns (need to identify risk factors)
- Tell the health care provider of any existing lifestyle concerns, such as alcohol use, other drug use, sleeping habits, diet, and menstrual cycle changes (need to identify lifestyle factors that can influence SSRI use and major depressive disorder management)
- Tell the health care provider if you have any changes in your pelvis, such as pain with urination, pain during sex, or bleeding during or after sex (potential STI symptoms)
- Tell the nurse or health care provider if you experience any pain that increasingly worsens or interferes with your quality of life.
- Keep track of your sexual health, medication use, and health concerns via an app, diary, or journal (self-monitoring for any changes)
- Tell the health care provider right away if you are having thoughts of hurting yourself or others, as a possible increased risk of suicidality is a potential side effect.
- Take all prescribed medications as indicated and ask questions about medications and possible other treatment options, such as non-pharmacological options or surgeries.
- Tell your health care provider if you notice any changes while taking medications or receiving other treatments to manage STIs (potential worsening or improvement of your health situation).
Ask yourself...
- What are some problems that can occur if oral medications are not managing STIs adequately?
- What are some possible ways you can obtain a detailed, patient-centric health history?
- What are some possible ways APRNs can educate patients on STIs and oral STI medication options?
- What assessments are needed to help diagnose STIs in patients?
- What barriers to providing adequate education to your patients have you encountered? What have you done to overcome those barriers?
- What obstacles have you seen in practice to getting patients to comply with their treatment? What interventions have you done to help circumvent those?
- What education can you provide to a patient in the prevention of STI transmission?
Research Findings
There is extensive publicly available literature on antibiotics and antiviral medications via the National Institutes of Health and other evidence-based journals.
If a patient is interested in participating in clinical trial research, they can seek more information on clinical trials from local universities and health care organizations.

Ask yourself...
- What are some reasons someone would want to enroll in clinical trials?
- What practices have you seen changed over the years?
- What community resources are available to assist with the education and prevention of STIs?
Case Study
Sabrina is a 26-year-old Latina woman working as a teacher. She arrives for her annual exam at the local health department next to her place of work. She reports nothing new in her health, but she says she’s been having some pain when she has sex and sometimes bleeds after sex.
Sabrina said she’s never felt this way before, and she denies having any major changes in her life that could be affecting her pelvic health. She heard one of her friends talk about STIs, but she doesn’t think she has any because she tested negative for STIs a few years ago. She wants to know if she could have an STI and what else could be causing pain and bleeding during sex.
Ask yourself...
- What are some specific questions you would ask about her sexual health?
- What are some questions on health history you would want to highlight?
- What lab work would you suggest performing?
Case Study (Continued)
Sabrina agrees to provide a urine sample, complete a Pap smear, and complete bloodwork later this week. She said that no health care provider talked to her about sexual health, but she heard about different STI symptoms from her friends recently.
She’s back in the office a few weeks later to discuss her lab results. Her Pap smear shows that she is HPV negative, her bloodwork is unremarkable, and her urine culture is positive for trichomoniasis and chlamydia. Sabrina states that she is shocked about the results since she is in a monogamous relationship with her boyfriend. She would like to know how she contracted two STIs.
Ask yourself...
- How would you discuss Susan’s sexual health concerns and her diagnosis?
- How would you discuss STI transmission routes?
Case Study (Continued)
Sabrina is willing to take antibiotics for her STI infections. She has questions about how to take these medications since she read online that antibiotics can interfere with the birth control pill. She reports taking a birth control pill called Sprintec. She also wants to know if there is a way to have treatment for her boyfriend as well, and if he can get tested for STIs. Sabrina also wants to be pregnant in the future, and she would like to know if these STIs can affect her fertility.
Ask yourself...
- Knowing Susan’s concerns, how can you check for drug-drug interactions between her antibiotics and Sprintec?
- What patient education talking points would you discuss with Sabrina about STIs and future fertility concerns?
- What are some side effects of antibiotics that Susan should be educated on?
Conclusion
STIs affect millions of people nationwide and can affect anyone who is having sex. Oral STI medication is often a first-line pharmacological option for managing several STIs. However, clinical presentation and symptom management for STIs can vary widely. Education and awareness of different STIs and different oral STI medications can influence the health of many people.
References + Disclaimer
- Abi-Aad, K., & Derain, A. (2023, August 17). Hydromorphone. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK470393/
- Adler, J., Ballantyne, J., Chou, R., Davies, P., Fanciullo, G., & Fine, P. (2009). Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic NonCancer Pain. The Journal of Pain, 113-130.
- Ahmad, F., Rossen, L., Sutton, P., & & Talih, M. (2018). Provisional drug overdose. National Vital Statistics Reports, pp. 1-12.
- Altman, R., Clark, M., Huddart, R., & Klein, T. (2018, October 28). Pharm GKB Summary: Oxycodone Pathway, Pharmacokinetics. Retrieved from Pharmacogenet Genomics: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6602093/#:~:text=Approximately%2072%25%20of%20an%20oxycodone,oxycodone%20is%20unconjugated%20%5B70%5D
- Atrux-Tallau, N., naimi, Z., & Jaudinot, E. (2022, November 4). Pharmocokinetics of Morphine Sulfate Orodispersible tablets and Bioequivalence with Immediate-Release Oral Morphine Sulfate Formulations in Healthy Adult SubjectsUnder fastin Conditions. Retrieved from Clinical Drig Investigation: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9705466/
- Baldwin, G., Compton, W. M., Han, B., Houry, D., Jones, C. M., & Vivolo-Kantor, A. (2021, September 24). Methamphetamine use in the United States: epidemiological update and implocations for prevention, treatment and harm reduction. Annual New York Academy of Science. New York: National Institute of Health.
- Berg, K. A., & Clarke, W. P. (2018). Making Sense of Pharmacology: Inverse Agonism and Functional Selectivtiy. International Journal of Neuropsychopharmacology, 962-977.
- Bistas, K., Lopez-Ojeda, W., & Ramos-Matos, C. (2023, May 29). Fentanyl. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK459275/
- Blyth, F., Briggs, A., Hoy, D. G., March, L. M., & Schneider, C. H. (2019). The Global Burden of Musculoskeletal Pain-Where to From Here? American Journal of Public Health, 35-40.
- Bramanti, P., Cavalli, E., Mammana, S., Mazzon, E., & Nicoletti, F. (2019). The neuropathic pain: An Overview of the current treatment and future therapeutic approaches. International Journal of Immunopathology and Pharmacology, 33.
- Brennan, F., Carr, D., Cousins, M., & Pain, D. (2013). Pain Management: A Fundamental Human Right. Anesthesia and Analgesia, 224-227.
- Carver, A. C., & Foley, K. (2003). Types of Pain. In D. Kufke, & R. W. Pollock, Cancer Medicine. Hamilton: Holland Frei Cancer Medicine.
- Centers for Disease Control and Prevention. (2022). CDCs Clinical Practice Guidelines for Prescribing Opioids. Retrieved from Opioids: https://www.cdc.gov/opioids/healthcare-professionals/prescribing/guideline/index.html?s_cid=DOP_Clinician_Search_Paid_001&gclid=CjwKCAjwrranBhAEEiwAzbhNtVKOCMzLLnwhfj7B2D_aBCZM_SryNcEyXl0GRigmorFhKve2ekCVrhoCNYQQAvD_BwE
- Centers for Disease Control and Prevention. (2023, August 8). Opioids. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/opioids/basics/epidemic.html#print
- Chiang, N., Gyaw, S., & Krieter, P. A. (2019). Comparison of the Pharmacokinetic Properties of Naloxone Following the Use of FDA-Approved Intranasal and Intramuscular Devices Versus a Common Improvised Nasal Naloxone Device. Journal of Clinical Pharmacology, 1078-1084.
- Cicero, T., & Ellis, M. &. (2017). The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA, 673-681.
- Cofano, S., & Yellon, R. (2022, October 24). Stat Pearls: Hydrocodone. Retrieved from National Institutes of Health: https://www.ncbi.nlm.nih.gov/books/NBK537288/
- Dart, R., Hoyte, C., & Nakhee, S. (2021). A Review on Tramadol Toxicity: Mechanism of Action, Clinical Presentation, and Treatment. Forensic Toxicology, 293-310. Retrieved from Forensic Toxicology: https://link.springer.com/article/10.1007/s11419-020-00569-0
- Delsignor, R. e. (2001). Lofexidine versus clonidine in rapid opiate detoxification. Journal of Substance Abuse Treatment, 11-17.
- Department of Defense Veterans Association. (2023). Defense and Veterans Pain Rating Scale. Retrieved from VA.gov: https://www.va.gov/PAINMANAGEMENT/docs/DVPRS_2slides_and_references.pdf
- Department of Health and Aged Care. (2013, January 20). FLACC Pain Scale. Retrieved from health.gov: https://www1.health.gov.au/internet/publications/publishing.nsf/Content/triageqrg~triageqrg-pain~triageqrg-FLACC
- Donaldson, S. L. (2017). Provider-Based Interventions to Mitigate Risk for Opioid Pain Medication Abuse Among Adult Patients in a Primary Care Setting.
- Dydyk, A. M., & Grandhe, S. (2023, January 29). Pain Assessment. Retrieved from STAT PEARLS: https://www.ncbi.nlm.nih.gov/books/NBK556098/#:~:text=Pain%20is%20the%20most%20common,pain%20in%20the%20United%20States.
- Fan, M., Hamilton, M., Hyland, B., & Tscheng, D. (2019). Diversion of Controlled Drugs in Hospitals: A Scoping review of Contributors and Safeguards. Journal of Hospital Medicine.
- GeriatricPain.org. (2022, January). Fast Facts: Selected Pain Assessment Tools. Retrieved from Geriatricpain.org: Geriatricpain.org https://geriatricpain.org/selected-pain-assessment-tools-fc
- Haffajee, R., Jena, A., Weiner, S., & & Kesselheim, A. (2019). Mandatory use of prescription drug monitoring programs. Journal of the American Medical Association, 1667-1668.
- International Association for the Study of Pain . (2020, July 16). IASP Announces Revised Definition of Pain. Retrieved from International Association for the Study of Pain: https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/
- JAMA. (2023). Substance Use and Addiction Medicine. Retrieved from JAMA Network: https://jamanetwork.com/collections/5921/substance-use-and-addiction-medicine
- John, W. S., Schwartz, R. P., Subramanian, G., & Wu, L.-T. (2018). Prevalence, Patterns and correlates of multiple substance use disorders among adults. Drug and Alcohol Dependency, 79-87.
- Jones, C., Einstein, E., & Compton, W. (2018). Changes in synthetic opioid involvement in drug overdose deaths in the United States. JAMA, 1819-1821.
- Kalava, A., King, K. C., & Preuss, C. V. (2023, April 29). Perscription of Controlled Substances: Benefits and Risks. Retrieved from STAT PEARLS: https://www.ncbi.nlm.nih.gov/books/NBK537318/
- Khalid, N., Patel, P., & Singh, A. (2023, January). Cannabis Versus Opioids for Pain. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK573080/
- Kreutzwiser, D., & Tawfic, Q. (2020). Mathadone for Pain Management. A Pharmacotherapeutic Review, 827-839.
- Kroenke, K., Spiter, R., Williams, J., & Lowe, B. (2011, Nov). An ultra-brief screening scale for anxiety and depression: the PHQ-4. From Principles of Neuropathic Pain Assessment and Management, 613-21. Psychosomatics 2009.
- Manchikanti, L., Kaye, A., Knezevic, N., McAnally, H., Slavin, K., Trescot, A., & Hirsch, J. (2018). Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians guidelines. Pain Physician, S3-S92.
- Marquez, C. B. (2020). Changing the outlook toward the problem of opioid addiction: From “War on Drug” to “Public Health Emergency”. Seton Hall: Law Student Scholarship.
- Marsch, L., Borodovsky, J., & Dallery, J. (2019). Advances in the psychosocial treatment of addiction: The role of technology in the delivery of evidence-based psychosocial treatment. The Psyciatric Clinics of North America, 585-596.
- McLellan, T., & Volkow, N. (2016). Opioid Abuse in Chronic Pain-Misconceptions and Mitigation Strategies. New England Journal of Medicine, 1253-1263.
- National Institute of Drug Abuse (NIDA). (2023, August 30). Prescription Opioid Drug Facts. Retrieved from National Institute on Drug Abuse: https://nida.nih.gov/publications/drugfacts/prescription-opioids
- National Institute of Health. (2023, August 25). Medications for Opioid Overdose, Withdrawal, & Addiction. Retrieved from National Institute of Drug Addiction: https://nida.nih.gov/research-topics/opioids/medications-opioid-overdose-withdrawal-addiction-infographic
- Pain Physician. (2008). Opioid Special Issue. Pain Physician, S133-S153.
- Ransford, e. a. (2019, February 08). The Ransford Pain Drawing . Retrieved from compassionandsupport.org: https://compassionandsupport.org/wp-content/uploads/sites/2/2019/08/PainScaleRansford.pdf
- Rudd, R. A., Zibbell, J., & Gladden, M. (2016). Increases in drug and opioid overdose deaths-Untied States. Morbidity and Mortlity Weekly Report, 1378-1382.
- SAMHSA. (2020). Key Substance Use and Mental Health Indicators in the United States: Ressults from the 2019 National Survey on Drug Use and Health. SAMHSA.
- Scalpel, S. (2016, November). The Joint Commission Deserves Some Blame for the Opioid Crisis. Retrieved from Missouri Medicine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6139759/
- Scholl, L., Seth, P., Kariisa, M., Wilson, N., & Baldwin, G. (2019). Drug and opioid-involved overdose deaths-Unioted States, 2013-2017. Morbidity and Mortatlity Weekly Report 67, 1419-1427.
- Stauffer, L., & Gibson, S. (2017). The Nurse’s Role in Identifying and Educating Patients At Risk for Opioid Abuse. AAACN Viewpoint, 1.
- The Joint Commission. (2023, January). The Joint Commission History Timeline. Retrieved from JointCommission.org: https://www.jointcommission.org/-/media/tjc/documents/tjc-history-timeline-through-2022.pdf
- Toombs, K., Kral, L., Colleran, C., & & Varney, S. (2018). Nurse practitioner-pharmacist collaboration in pain management: Strategies for success. . Journal of the American Association of Nurse Practitioners, 141-148.
- U.S. Food and Drug Administration. (2023, May 23). FDA. Retrieved from Information about Medication Assisted Treatment (MAT): https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat
- Volkow, N., Jones, E., Einstein, E., & Wargo, E. (2019). Prevention and Treatment of Opioid mIsuse and Addiction: A Review. JAMA Psychiatry, 208-216.
- Katie Kerwin McCrimmon, Uch. (2023, May 15). What is Mounjaro? and does it work better for weight loss than Ozempic and Wegovy?. UCHealth Today. https://www.uchealth.org/today/what-is-mounjaro-and-how-does-it-work-for-weight-loss/
- National Institutes of Health. (2023, July 28). DailyMed – mounjaro- tirzepatide injection, solution. U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d2d7da5d-ad07-4228-955f-cf7e355c8cc0
- Type 2 Diabetes Treatment to Lower A1C | Mounjaro® (tirzepatide). (2023). https://www.mounjaro.com/?utm_id=go_cmp-16966473997_adg-133042365262_ad-626584063672_kwd-1651326559888_dev-c_ext-_prd-_mca-_sig-EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE&utm_source=google&utm_medium=ppc&campaign=16966473997&adgroup=133042365262&ad=626584063672&utm_keyword=kwd-1651326559888&gclid=EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE
- GoodRX – error. (2023, July 14). https://www.goodrx.com/mounjaro/how-make-you-feel
- Chen, S. (2023, July 28). Diabetes drug Mounjaro shown to have extraordinary weight loss for people without diabetes. diaTribe. https://diatribe.org/diabetes-drug-mounjaro-shown-have-extraordinary-weight-loss-people-without-diabetes
- VA Pharmacy Benefits Management Services. (2022, August). Tirzepatide Injection Criteria for Use. https://www.va.gov/formularyadvisor/DOC_PDF/CFU_Tirzepatide_MOUNJARO_Criteria_Aug_2022.pdf.
- Allied PR. (2023 B.C.E., May 6). Mounjaro Weight Loss: Newly Launch Over The Counter Alternative Pills To Tirzepatide & Mounjaro Weight Loss. https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569. Retrieved August 30, 2023, from https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569
- Mounjaro (2023). Savings and support for Mounjaro. Retrieved on August 31st 2023 from https://www.mounjaro.com/savings-resources?gclid=Cj0KCQjw9MCnBhCYARIsAB1WQVWAcINPKFwiHl4SbZQiGC8E_o9wHVOkDJDDUuphW3OlsKBagVTF1rQaApLJEALw_wcB
- Global diabetes cases to soar from 529 million to 1.3 billion by 2050. (n.d.). The Institute for Health Metrics and Evaluation. https://www.healthdata.org/news-events/newsroom/news-releases/global-diabetes-cases-soar-529-million-13-billion-2050.
- Diabetes Research Institute Foundation. (2023, March 31). Diabetes Statistics – DRIF. DRIF. https://diabetesresearch.org/diabetes-statistics/#:~:text=37.3%20million%20people%2C%20or%2011.3,yet%20been%20diagnosed%20(2022)
- Manage blood sugar. (2021, April 28). Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/managing/manage-blood-sugar.html
- About the National Diabetes Prevention Program | CDC. (n.d.). https://www.cdc.gov/diabetes/prevention/about.htm#:~:text=The%20National%20Diabetes%20Prevention%20Program%E2%80%94or%20National%20DPP%E2%80%94was%20created,diabetes%20in%20
- Ms, M. G. R. (2023, February 16). All about Ozempic. Healthline. https://www.healthline.com/health/drugs/ozempic#_noHeaderPrefixedContent
- Professional, C. C. M. (n.d.). Ghrelin. Cleveland Clinic. https://my.clevelandclinic.org/health/body/22804-ghrelin#:~:text=Ghrelin%20and%20leptin%20are%20two,brain%20when%20you’re%20hungry
- Prelipcean, M., MD. (2020, June 22). Behind the counter: Glucagon-like peptide-1 receptor agonists for type 2 diabetes. https://www.medicalnewstoday.com/articles/glp-1-receptor-agonists-type-2-diabetes
- Mba, A. B. P. (2023, May 1). Ozempic side effects: What you should know. https://www.medicalnewstoday.com/articles/drugs-ozempic-side-effects
- Delong, C. (2023, February 11). Black box warning. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538521/
- MNT Medical Network. (2023, February 16). Ozempic (semaglutide). https://www.medicalnewstoday.com/articles/326252
- Ozempic® vs. Other Type 2 Diabetes Treatments | Ozempic® (semaglutide) injection 0.5 mg or 1 mg. (n.d.). https://www.ozempic.com/why-ozempic/diabetes-medicines-comparison.html
- Northrop, A. (2023, August 3). Ozempic For Weight Loss: Side Effects, Risks And More. Forbes Health. https://www.forbes.com/health/body/ozempic-for-weight-loss/%20)
- Kerwin McCrimmon, UCHealth, K. (2023, April 5). Wegovy vs. Ozempic: The truth about new ‘weight-loss’ drugs. Uchealth. https://www.uchealth.org/today/wegovy-vs-ozempic-the-truth-about-new-weight-loss-drugs/#:~:text=If%20you%20take%20Wegovy%20or,to%20keep%20benefiting%20from%20them
- Stott, R. (2023, August 4). Lawsuit claims Ozempic, Mounjaro labels ‘downplayed severity’ of gastroparesis. Healio Gastroenterology. Retrieved August 11, 2023, from https://www.healio.com/news/gastroenterology/20230804/lawsuit-claims-ozempic-mounjaro-labels-downplayed-severity-of-gastroparesis#:~:text=While%20the%20drug%20label%20for,not%20recommended%20in%20these%20patients.%E2%80%9D
- How Does Gastroparesis Affect People with Diabetes? (2022, August 30). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/professionals/diabetes-discoveries-practice/how-gastroparesis-affect-people-with-diabetes
- Living Healthy with Diabetes. (2023, March 29). www.heart.org. https://www.heart.org/en/health-topics/diabetes/prevention–treatment-of-diabetes/living-healthy-with-diabetes
- Move more; sit less. (2023, June 22). Centers for Disease Control and Prevention. https://www.cdc.gov/physicalactivity/basics/adults/index.htm#:~:text=Each%20week%20adults%20need%20150,Physical%20Activity%20Guidelines%20for%20Americans.&text=We%20know%20150%20minutes%20of,do%20it%20all%20at%20once
- Good sleep habits. (2022, September 13). Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
- Centers for Disease Control and Prevention. (2022, August 1). Food and nutrition insecurity and diabetes. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/library/features/diabetes-and-food-insecurity.htm
- American Psychiatric Association. (2020, December). What is a substance use disorder? https://www.psychiatry.org/patients-families/addiction-substance-use-disorders/what-is-a-substance-use-disorder
- Durrani M, Bansal K. Methadone. [Updated 2023 Apr 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562216/
- Kumar R, Viswanath O, Saadabadi A. Buprenorphine. [Updated 2023 Apr 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459126/
- National Center for Drug Abuse Statistics. (2023). Drug abuse statistics. https://drugabusestatistics.org/
- National Institute of Health. (2018, June). Understanding drug use and addiction. https://nida.nih.gov/publications/drugfacts/understanding-drug-use-addiction
- Singh D, Saadabadi A. Naltrexone. [Updated 2023 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534811/
- Substance Abuse and Mental Health Services Administration. (2023, August). Methadone take-home flexibilities extension guidance. https://www.samhsa.gov/medications-substance-use-disorders/statutes-regulations-guidelines/methadone-guidance#:~:text=In%20treatment%200%2D14%20days,be%20provided%20to%20the%20patient
- S. Food and Drug Administration. (2023, May). Information about medication assisted treatment (MAT). https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat
- Akbari, P., Khorasani-Zadeh, A. (Updated 2023, January 23). Thiazide Diuretics. In Stat Pearls. Stat Pearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK532918/
- Alley, W.D., & Schick, M.A. (Updated 2023, July 24). Hypertensive Emergency. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470371/
- American Heart Association. (2023 June 7). Types of Blood Pressure Medications. Retrieved from https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/types-of-blood-pressure-medications
- Arumugham, V.B., & Shahin, M.H. (Updated 2023, May 29). Therapeutic Uses of Diuretic Agents. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557838/
- Brater, D.C., & Ellison, D.H. (Updated 2022, November 30). Mechanism of action of diuretics. UpToDate. Retrieved from https://www.uptodate.com/contents/mechanism-of-action-of-diuretics#H5
- Centers for Disease Control and Prevention. (2023, July 6). Facts about Hypertension. Retrieved from https://www.cdc.gov/bloodpressure/facts.htm
- Centers for Disease Control and Prevention. (2021, May 18). High Blood Pressure Symptoms and Causes. Retrieved from https://www.cdc.gov/bloodpressure/about.htm
- Farzam, K., & Jan, A. (Updated 2023, August 22). Beta Blockers. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Goyal, A., Cusick, A.S., & Thielemier, B. (Updated 2023, June 26). ACE Inhibitors. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK430896/
- Hariri, L., & Patel, J.B. (Updated 2023, August 14). Vasodilators. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554423
- Hill, R.D., & Vaidya, P. (Updated 2023, March 27). Angiotensin II Receptors Blockers (ARBs). In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537027/
- Huxel, C., Raja, A., Ollivierre-Lawrence, M.D. (Updated 2023, May 22). Loop Diuretics. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK546656/
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Loop Diuretics. [Updated 2021 Oct 13]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548619/
- Mann, J.F.E, &Flack, J.M. (Updated 2023, June 22). Choice of Drug Therapy in Primary (Essential) Hypertension. UpToDate. Retrieved from https://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension
- McKeever, R.G., & Hamilton, R.J. (Updated 2022, August 5). Calcium Channel Blockers. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK482473/
- MedlinePlus. (2023). Hydrochlorothiazide. National Institutes of Health. Retrieved from https://medlineplus.gov/druginfo/meds/a682571.html
- MedlinePlus. (2023). Metoprolol. National Institutes of Health. Retrieved from https://medlineplus.gov/druginfo/meds/a682864.html
- Nachawati D, Patel JB. (Updated 2023, July 3). Alpha-Blockers. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK556066/
- Naranjo, M., Chauhan, S., & Paul, M. (Updated 2023, June 19). Malignant Hypertension. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK507701
- Taylor, B.N., & Cassagnol, M. (Updated 2023, July 10). Alpha-Adrenergic Receptors. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539830/
- Verdecchia, P., Cavallini, C., & Angeli, F. (2022). Advances in the Treatment Strategies in Hypertension: Present and Future. Journal of cardiovascular development and disease, 9(3), 72. https://doi.org/10.3390/jcdd9030072
- American Academy of Neurology. (2019). AAN Updates Guideline on Valproate for Women with Epilepsy. Neurology Today, 19(5), 46-47.
- American Headache Society. (2020). The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache: The Journal of Head and Face Pain, 60(3), 415-423.
- American Nurses Association. (2017). Nursing: Scope and standards of practice (3rd ed.). Silver Spring, MD: American Nurses Association.
- Ashina, M., Goadsby, P. J., Reuter, U., Silberstein, S., Dodick, D. W., Xue, F., … & Trugman, J. M. (2020). Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. European Journal of Neurology, 27(6), 1058-1067.
- Buse, D. C., Silberstein, S. D., Manack, A. N., Papapetropoulos, S., & Lipton, R. B. (2019). Psychiatric comorbidities of episodic and chronic migraine. Journal of Neurology, 266(3), 634-646.
- Davies, N. M., & Anderson, K. E. (2020). Clinical pharmacokinetics of diclofenac: therapeutic implications and applications in pain and inflammatory disorders. Clinical Pharmacokinetics, 59(2), 153-162.
- Dodick, D. W. (2018). A Phase-by-Phase Review of Migraine Biology, Burden, and Pharmacotherapy. Headache: The Journal of Head and Face Pain, 58(S1), 4-16.
- Dodick, D. W., Lipton, R. B., Ailani, J., Lu, K., Finnegan, M., Trugman, J. M., & Szegedi, A. (2019). Ubrogepant for the treatment of migraine. New England Journal of Medicine, 381(23), 2230-2241.
- Dodgson, S. J., Shank, R. P., & Maryanoff, B. E. (2018). Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia, 39(12), 16-22.
- D’Souza, P., Himme, A., & Herndon, C. M. (2020). Migraine headache prophylaxis. American Family Physician, 101(5), 297-298.
- Edvinsson, L., & Warfvinge, K. (2019). Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia, 39(3), 366-373.
- Gomez, M., & Turner, D. (2020). Tailoring Migraine Medications: A Comprehensive Approach. Journal of Neuropharmacology, 15(4), 201-215.
- Gormley, P., Anttila, V., Winsvold, B. S., Palta, P., Esko, T., Pers, T. H., … & Chasman, D. I. (2016). Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nature Genetics, 48(8), 856-866.
- Gupta, R., & Silberstein, S. D. (2020). Migraine and its impact on daily functioning. Headache: The Journal of Head and Face Pain, 60(3), 572-585.
- Headache Classification Committee of the International Headache Society (IHS). (2018). The International Classification of Headache Disorders, 3rd edition. Cephalalgia, 38(1), 1-211.
- Johnson, A., & Davis, R. (2022). Triptans: Mechanisms of Action and Clinical Applications. Headache Medicine Review, 18(2), 87-104.
- Johnson, M., et al. (2022). Unraveling the Neurological Complexity of Migraines: A Comprehensive Review. Neurological Insights, 12(3), 112-129.
- Jones, A. B., & Brown, C. D. (2019). Contemporary pharmacotherapy for migraine prevention in adults: A review of current and emerging treatment options. Drugs, 79(11), 1223-1238.
- Jones, B., & Miller, S. (2022). Tailoring Migraine Medications Based on Attack Characteristics: A Practical Guide. Neurological Insights, 17(3), 211-228.
- Jones, B., et al. (2021). Emerging Trends in Migraine Pharmacotherapy: A Comprehensive Review. Neuropharmacological Insights, 25(3), 312-328.
- Lee, C. R., Bottiglieri, T., Fisher, W. G., La Du, B. N., & Stamer, W. D. (2019). Relative importance of genetic determinants for naproxen and ibuprofen hydroxylation in human liver microsomes. Clinical Pharmacology & Therapeutics, 105(1), 131-140.
- Linde, K., Allais, G., Brinkhaus, B., Manheimer, E., Vickers, A., & White, A. R. (2016). Acupuncture for the prevention of tension‐type headache. Cochrane Database of Systematic Reviews, 2016(4).
- Lipton, R. B., Goadsby, P. J., Smith, J., Schaeffler, B. A., Biondi, D. M., Hirman, J., … & Tepper, S. J. (2019). Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology, 92(15), e1705-e1716.
- Lipton, R. B., Hutchinson, S., Ailani, J., Reed, M. L., Fanning, K. M., Manack Adams, A., … & Aurora, S. K. (2017). Discontinuation of acute prescription medication for migraine: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache: The Journal of Head and Face Pain, 57(10), 1655-1668.
- MaassenVanDenBrink, A., De Vries, T., Danser, A. H., & Headache Expert Panel of the European Academy of Neurology. (2019). Pathophysiology of migraine: a disorder of sensory processing. Physiological Reviews, 99(2), 901-968.
- Miller, S., Johnson, J., & Patel, J. (2021). Ensuring Patient Safety in Migraine Medication Administration: A Comprehensive Guide. Journal of Neurological Nursing, 43(2), 67-78.
- Mims, M. (2021). Mims Medical Microbiology and Immunology. Elsevier Health Sciences.
- Ong, C. K., Seymour, R. A., Lirk, P., Merry, A. F., & Combet, E. (2017). An evidence‐based update on nonsteroidal anti‐inflammatory drugs. Clinical Medicine & Research, 15(2), 73-100.
- Ong, J. J., De Felice, M., & Migraine Pathophysiology, Treatment, and Prevention. (2020). Migraine: Mechanism of action and treatment. The Scientific World Journal, 2020.
- Perucca, P. (2018). Pharmacokinetic variability of new antiepileptic drugs at different ages. Therapeutic Drug Monitoring, 40(6), 723-733.
- Serrano, D., Lipton, R. B., Scher, A. I., Reed, M. L., Stewart, W. F., & Adams, A. M. (2015). Fluctuations in episodic and chronic migraine status over the course of 1 year: Implications for diagnosis, treatment and clinical trial design. Journal of Headache and Pain, 16(1), 1-12.
- Silberstein, S. D., Holland, S., Freitag, F., Dodick, D. W., Argoff, C., Ashman, E., … & Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. (2019). Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults. Neurology, 92(18), 1-12.
- Smith, J., & Patel, S. (2023). Advances in Migraine Pharmacotherapy: Current Landscape and Future Directions. Neurology Today, 19(1), 45-62.
- Smith, R. H., et al. (2020). Neurobiological Insights into Migraine Mechanisms: A Comprehensive Review. Neurology Research International, 2020, 8743521.
- Smith, J., et al. (2021). Cardiovascular Considerations in Migraine Medication Prescribing: A Current Perspective. Current Neurology and Neuroscience Reports, 21(5), 15.
- Smith, S. M., Gums, J. G., & Potti, A. (2021). Cardiovascular safety of Triptans in patients with cardiovascular disease: A review. The American Journal of Medicine, 134(2), 146-154.
- Tepper, S. J., & Ashina, M. (2020). Reuter U. Fremanezumab in the prevention of high-frequency episodic and chronic migraine: Efficacy in the phase 2b study according to patient characteristics. The Journal of Headache and Pain, 21(1), 23.
- Tepper, S. J., & Cady, R. K. (2019). Diagnosis and Management of Acute Migraine. Disease-a-Month, 65(4), 100856.
- Tepper, S. J., & Diener, H. C. (2020). The Place of Triptans in the Management of Migraine in Clinical Practice. Cephalalgia, 40(2), 155-166.
- Tiseo, C., Vacca, G., & Pinzi, C. (2020). Predictors of triptan use in patients with migraine: Results from a cross-sectional study in Italy. PLoS ONE, 15(7), e0235568.
- Siynor, B. and Perez, L. C. (2023). Pathophysiology of Asthma. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK551579/
- Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI), National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), et al. (2020). 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 146(6):1217-1270. Retrieved from https://www.nhlbi.nih.gov/resources/2020-focused-updates-asthma-management-guidelines
- Rohatgi, K. W., Humble, S., McQueen, A., Hunleth, J. M., Chang, S. H., Herrick, C. J., & James, A. S. (2021). Medication Adherence and Characteristics of Patients Who Spend Less on Basic Needs to Afford Medications. Journal of the American Board of Family Medicine: JABFM, 34(3), 561–570. https://doi.org/10.3122/jabfm.2021.03.200361
- Hiday, R., and Abbott, R. D. (2022). Asthma Guideline Updates. In: Irons BK, Meredith AH, eds. Ambulatory Care Self-Assessment Program, 2022 Book 1. Pulmonary Care. Lenexa, KS: American College of Clinical Pharmacy, 2022:7-36. Retrieved from https://www.accp.com/docs/bookstore/acsap/ac2022b1_sample.pdf
- Ocejo, A. and Correa, R. (2022). Methylprednisolone. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK544340/
- Puckett, Y., Gabbar, A., and Bokhari, A. (2023). Prednisone. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK534809
- Hsu, E. and Bajaj, T. (2023). Beta2-Agonists. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK542249/
- Jilani, T. N., Preuss, C. V., and Sharma, S. (2023). Theophylline. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK519024/
- Choi, J. and Azmat, C. E. (2023). Leukotriene Receptor Antagonists. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554445/
- Dhaliwal, A. and Bajaj, T. (2023). Zafirlukast. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557844/
- Bouchette, D. and Preuss, D. (2023). Zileuton. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK448202/
- Minutello, K. and Gupta, V. (2023). Cromolyn Sodium. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557473/
- Kumar, C. and Zito, P. (2023). Omalizumab. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK545183
- Gade, A., Ghani, H., and Rubenstein, R. (2023). Dupilumab. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK585114/
- Lange-Vaidya, N. and Hartert, T. (2023). Initiating Asthma Therapy and Monitoring in Adolescents and Adults. UpToDate. Retrieved 29 Jan 2024 from https://www.uptodate.com/contents/initiating-asthma-therapy-and-monitoring-in-adolescents-and-adults
- Chu A. and Wadhwa R. Selective Serotonin Reuptake Inhibitors. (2023). In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK55440
- Woodall, A. and Walker, L. (2022). A guide to prescribing antidepressants in primary care. Prescriber, 33: 11-18. https://doi.org/10.1002/psb.2006
- Bains N. and Abdijadid S. Major Depressive Disorder. (2023). In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK559078/
- Rohatgi, K. W., Humble, S., McQueen, A., Hunleth, J. M., Chang, S. H., Herrick, C. J., & James, A. S. (2021). Medication Adherence and Characteristics of Patients Who Spend Less on Basic Needs to Afford Medications. Journal of the American Board of Family Medicine: JABFM, 34(3), 561–570. https://doi.org/10.3122/jabfm.2021.03.200361
- Lam, M. K., Lam, L. T., Butler-Henderson, K., King, J., Clark, T., Slocombe, P., Dimarco, K., & Cockshaw, W. (2022). Prescribing behavior of antidepressants for depressive disorders: A systematic review. Frontiers in Psychiatry, 13, 918040. https://doi.org/10.3389/fpsyt.2022.918040
- Moncrieff, J., Cooper, R. E., Stockmann, T., Amendola, S., Hengartner, M. P., & Horowitz, M. A. (2023). The serotonin theory of depression: a systemic umbrella review of the evidence. Molecular Psychiatry, 28, 3243-3256. https://doi.org/10.1038/s41380-022-01661-0
- Boschloo, L., Hieronymus, F., Lisinski, A., Cuijpers, P., & Erikkson, E. (2023). The complex clinical response to selective serotonin reuptake inhibitors in depression: a network perspective. Translational Psychiatry, 13, 2-25. https://doi.org/10.1038/s41398-022-02285-2
- Hirsch, M., Birnbaum, R. J. (2023). Selective serotonin reuptake inhibitors: Pharmacology, administration, and side effects. UptoDate. Retrieved 12 Jan 2024 from https://www.uptodate.com/contents/selective-serotonin-reuptake-inhibitors-pharmacology-administration-and-side-effects
- Bains, N. & Abdijadid, S. (2023). Major Depressive Disorder. In: StatPearls: Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK559078/
- Agrawal, A., Kerndt, C.C., & Manna, B. (Updated 2023, April 19). Apixaban. In Stat Pearls. Stat Pearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK507910/
- American Heart Association. (2023, November 12). New Anti-clotting Medication Reduces Bleeding Among People with Atrial Fibrillation. Retrieved from https://newsroom.heart.org/news/new-anti-clotting-medication-reduces-bleeding-among-people-with-atrial-fibrillation
- Bentounes, N. K., Melicine, S., Martin, A. C., Smadja, D. M., & Gendron, N. (2023). Development of new anticoagulant in 2023: Prime time for anti-factor XI and XIa inhibitors. Journal de medecine vasculaire, 48(2), 69–80. https://doi.org/10.1016/j.jdmv.2023.04.002
- Centers for Disease Control and Prevention. (2023, June 28). Data and Statistics on Venous Thromboembolism. Retrieved from https://www.cdc.gov/ncbddd/dvt/data.html
- Centers for Disease Control and Prevention. (2023, June 28). Diagnosis and Treatment of Venous Thromboembolism. Retrieved from https://www.cdc.gov/ncbddd/diagnosis-treatment.html
- Chen, A., Stecker, E., & A Warden, B. (2020). Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. Journal of the American Heart Association, 9(13), e017559. https://doi.org/10.1161/JAHA.120.017559
- Hull, R.D., Garcia, D.A., Vazquez, S.R. (Updated 2023, January 24). Biology of Warfarin and Modulators of INR Control. UpToDate. Retrieved from https://www.uptodate.com/contents/biology-of-warfarin-and-modulators-of-inr-control
- Iqbal, A.M., Lopez, R.A., & Hai, O. (Updated 2022, November 7). Antiplatelet Medications. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537062/
- Leung, L.LK. (Updated 2023, July 27). Direct oral anticoagulants (DOACs) and parenteral direct-acting anticoagulants: Dosing and adverse effects. UpToDate. Retrieved from https://www.uptodate.com/contents/direct-oral-anticoagulants-doacs-and-parenteral-direct-acting-anticoagulants-dosing-and-adverse-effects/print
- Mahat, K.C., Sedhair, Y.R., Krishnan, P. (Updated 2023, August 4). Argatroban. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK555971/
- Patel, S., Singh, R., Preuss, C.V., and Patel, N. (Updated 2023, March 24). Warfarin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470313/
- Singh, R., & Emmady, P.D. (Updated 2023, April 17). Rivaroxaban. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557502/
- Solari, F., & Varacallo, M. (Updated 2023, August 4). Low-Molecular-Weight Heparin (LMWH). In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK525957/
- Umerah, C.O., & Momodu, I.I. (Updated 2023, July 17). Anticoagulation. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Warnock, L.B., & Huang, D. (Updated 2023, July 10). Heparin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK538247/
- Weitz, J.I. (Updated 2023, August 17). Investigational Anticoagulants. UpToDate. Retrieved from https://www.uptodate.com/contents/investigational-anticoagulants
- Bamalan, O.A., Moore, M.J., Al Khalili Y. (2024). Physiology, serotonin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK545168/
- Dhaliwal JS, Spurling BC, Molla M. (2023). Duloxetine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Fanelli, D., Weller, G., & Liu, H. (2021). New Serotonin-Norepinephrine Reuptake Inhibitors and Their Anesthetic and Analgesic Considerations. Neurology international, 13(4), 497–509. https://doi.org/10.3390/neurolint13040049
- Hussain LS, Reddy V, Maani CV. (2023) Physiology, Noradrenergic Synapse. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK540977/
- Li, J., Lu, C., Gao, Z, Feng, Y., Luo, H., Lu, T., Sun, X., Hu, J., & Luo, Y. (2020). SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology, 177, 108237, ISSN 0028-3908. https://doi.org/10.1016/j.neuropharm.2020.108237.
- Naseeruddin, R., Rosani, A., & Marwaha, R. (2023). Desvenlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534829/
- National Institute of Mental Health (NIH). (2022). Depression. Retrieved from https://www.nimh.nih.gov/health/publications/depression.
- Singh, D., & Saadabadi, A. (2022). Venlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Vallerand, A. H., & Sanoski, C. A. (2023). Davis’s drug guide for nurses (Eighteenth edition.). Philadelphia, PA. F. A. Davis Company. ISBN-10: 1-7196-4640-6. eISBN-10: 1-7196-4811-5.
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Venlafaxine. Access Pharmacy. Retrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426975.
- American Society of Health-System Pharmacists (ASHP). (2023). Dexamethasone (monograph). https://www.drugs.com/monograph/dexamethasone.html
- Dutt, M., Wehrle, C.J., & Jialal, I. (2024). Physiology, adrenal gland. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK537260/
- Chaudhuri, D., Nei, A. M., Rochwerg, B. et at. (2024). 2024 Focused update: Guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Critical Care Medicine ():10.1097/CCM.0000000000006172 https://journals.lww.com/ccmjournal/fulltext/9900/2024_focused_update__guidelines_on_use_of.275.aspx
- U.S. Food and Drug Administration. (2016). FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-label-changes-warn-rare-serious-neurologic-problems-after
- Hannoodee, S., & Nasuruddin, D.N. (2024). Acute inflammatory response. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK556083/
- Merck Manual. (2024). Drug information. Elsevier. https://www.merckmanuals.com/professional/drug-names-generic-and-brand
- Merck Manual. (2024). Corticosteroids: Uses and side effects. Elsevier. https://www.merckmanuals.com/home/multimedia/table/corticosteroids-uses-and-side-effects
- National Institutes of Health, U.S. Department of Health and Human Services. (2020). Autoimmunity may be rising in the United States. https://www.nih.gov/news-events/news-releases/autoimmunity-may-be-rising-united-states
- National Institutes of Health, U.S. Department of Health and Human Services. (2023). Therapeutic management of hospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults–therapeutic-management/
- National Institutes of Health, U.S. Department of Health and Human Services. (2023). Therapeutic management of nonhospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults–therapeutic-management/
- Oronsky, B., Caroen, S., & Reid, T. (2022). What exactly is inflammation (and what is it not?). International Journal of Molecular Sciences, 23(23), 14905. https://doi.org/10.3390/ijms232314905
- Sen, S. K. (2024). Messenger RNA (MRNA). National Human Genome Research Institute. https://www.genome.gov/genetics-glossary/messenger-rna
- World Health Organization. (2020). Corticosteroids for COVID-19: Living guidance. https://iris.who.int/bitstream/handle/10665/334125/WHO-2019-nCoV-Corticosteroids-2020.1-eng.pdf?sequence=1%26isAllowed=y
- Desai, D. S. (2023, June 5). Arrhythmias. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK558923/
- Benjamin, E. J., Wolf, P. A., D’Agostino, R. B., Silbershatz, H., Kannel, W. B., & Levy, D. (1998). Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation, 98(10), 946-952.
- Atrial fibrillation | Cdc.gov. (2022, October 14). Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- King, G. S. (2023, February 19). Antiarrhythmic medications. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482322/
- Saljic, A., Heijman, J., & Dobrev, D. (2023). Recent Advances in Antiarrhythmic Drug Therapy. Drugs, 83(13), 1147–1160. https://doi.org/10.1007/s40265-023-01923-3
- Kowey, P. R., & Robinson, V. M. (2020). The relentless pursuit of new drugs to treat cardiac arrhythmias. Circulation, 141(19), 1507–1509. https://doi.org/10.1161/circulationaha.119.045149
- Wright, K. N. (2009). Antiarrhythmic agents. In Elsevier eBooks (pp. 807–810). https://doi.org/10.1016/b978-1-4160-2591-7.10190-0
- Geng, M., Lin, A., & Nguyen, T. P. (2020). Revisiting Antiarrhythmic Drug Therapy for Atrial Fibrillation: Reviewing Lessons Learned and Redefining Therapeutic Paradigms. Frontiers in pharmacology, 11, 581837. https://doi.org/10.3389/fphar.2020.581837
- Sharma, A.K., Singh, S., Bhat, M. et al. new drug discovery of cardiac anti-arrhythmic drugs: insights in animal models. Sci Rep 13, 16420 (2023). https://doi.org/10.1038/s41598-023-41942-4
- Heart arrhythmias. (2024). Physiopedia. https://www.physio-pedia.com/Heart_Arrhythmias
- Dan, G., Martínez‐Rubio, A., Agewall, S., Boriani, G., Borggrefe, M., Gaïta, F., Van Gelder, I. C., Görenek, B., Kaski, J. C., Kjeldsen, K., Lip, G. Y., Merkely, B., Okumura, K., Piccini, J. P., Potpara, T., Poulsen, B. K., Saba, M., Savelieva, I., Tamargo, J., & Wolpert, C. (2018). Antiarrhythmic drugs–clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace, 20(5), 731–732an. https://doi.org/10.1093/europace/eux373
- Heart arrhythmia – Symptoms and causes – Mayo Clinic. (2023, October 13). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/heart-arrhythmia/symptoms-causes/syc-20350668
- Arrhythmias and congenital defects. (2023, June 15). www.heart.org. https://www.heart.org/en/health-topics/congenital-heart-defects/the-impact-of-congenital-heart-defects/arrhythmias-and-congenital-defects
- CV Pharmacology | Vaughan-Williams Classification of Antiarrhythmic Drugs. (2024). https://cvpharmacology.com/antiarrhy/vaughan-williams
- Lei, M., Wu, L., Terrar, D. A., & Huang, C. L. (2018). Modernized classification of cardiac antiarrhythmic drugs. Circulation, 138(17), 1879–1896. https://doi.org/10.1161/circulationaha.118.035455
- Custodis F, Schirmer SH, Baumhäkel M, et al. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 2010; 56:1973–83. 10.1016/j.jacc.2010.09.014
- Kaski, J. C., Gloekler, S., Ferrari, R., Fox, K., Lévy, B., Komajda, M., Vardas, P., & Camici, P. G. (2018). Role of ivabradine in management of stable angina in patients with different clinical profiles. Open Heart, 5(1), e000725. https://doi.org/10.1136/openhrt-2017-000725
- Salvage, S. C., Chandrasekharan, K. H., Jeevaratnam, K., Dulhunty, A. F., Thompson, A. J., Jackson, A. P., & Huang, C. L. (2018). Multiple targets for flecainide action: implications for cardiac arrhythmogenesis. British journal of pharmacology, 175(8), 1260–1278. https://doi.org/10.1111/bph.13807
- Bazoukis, G., Tse, G., Letsas, K. P., Thomopoulos, C., Naka, K. K., Korantzopoulos, P., Bazoukis, X., Michelongona, P., Papadatos, S. S., Vlachos, K., Liu, T., Efremidis, M., Baranchuk, A., Stavrakis, S., & Tsioufis, C. (2018). Impact of ranolazine on ventricular arrhythmias – A systematic review. Journal of arrhythmia, 34(2), 124–128. https://doi.org/10.1002/joa3.12031
- Rahman, M. F. F. (2023, July 24). Atrioventricular dissociation. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK563205/
- Perera, R. K., Fischer, T. H., Wagner, M., Dewenter, M., Vettel, C., Bork, N. I., Maier, L. S., Conti, M., Wess, J., El-Armouche, A., Hasenfuß, G., & Nikolaev, V. O. (2017). Atropine augments cardiac contractility by inhibiting cAMP-specific phosphodiesterase type 4. Scientific reports, 7(1), 15222. https://doi.org/10.1038/s41598-017-15632-x
- Singh, S. (2023, August 28). Adenosine. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK519049/
- Patti, L. (2023, August 7). Supraventricular tachycardia. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK441972/
- McIntyre, W. F., Healey, J. S., Bhatnagar, A. K., Wang, P., Gordon, J. A., Baranchuk, A., Deif, B., Whitlock, R. P., & Belley-Côté, É. P. (2019). Vernakalant for cardioversion of recent-onset atrial fibrillation: a systematic review and meta-analysis. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 21(8), 1159–1166. https://doi.org/10.1093/europace/euz175
- Ahmed L. A. (2019). Nicorandil: A drug with ongoing benefits and different mechanisms in various diseased conditions. Indian journal of pharmacology, 51(5), 296–301. https://doi.org/10.4103/ijp.IJP_298_19
- PubChem. (2024). N-(p-Amylcinnamoyl) anthranilic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/N-_p-Amylcinnamoyl_anthranilic-acid
- Salcher, S., Spoden, G. A., Hagenbuchner, J., Führer, S., Kaserer, T., Tollinger, M., Huber-Cantonati, P., Gruber, T., Schuster, D., Gust, R., Zwierzina, H., Müller, T., Kiechl‐Kohlendorfer, U., Ausserlechner, M. J., & Obexer, P. (2019). A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene, 39(5), 1080–1097. https://doi.org/10.1038/s41388-019-1044-7
- Le, V. T., Knight, S., Watrous, J. D., Najhawan, M., Dao, K., McCubrey, R. O., Bair, T. L., Horne, B. D., May, H. T., Muhlestein, J. B., Nelson, J. R., Carlquist, J. F., Knowlton, K. U., Jain, M., & Anderson, J. L. (2023). Higher docosahexaenoic acid levels lower the protective impact of eicosapentaenoic acid on long-term major cardiovascular events. Frontiers in cardiovascular medicine, 10, 1229130. https://doi.org/10.3389/fcvm.2023.1229130
- Alcocer, L., Bryce, A., Brasil, D. P., Terán, J. L., Cortés, J. E., Quesada, D., & Rodríguez, P. (2023). The pivotal role of Angiotensin-Converting enzyme inhibitors and Angiotensin II receptor blockers in hypertension management and cardiovascular and renal Protection: A Critical appraisal and comparison of international guidelines. American Journal of Cardiovascular Drugs, 23(6), 663–682. https://doi.org/10.1007/s40256-023-00605-5
- Kornej, J., Börschel, C. S., Benjamin, E. J., & Schnabel, R. B. (2020). Epidemiology of Atrial fibrillation in the 21st century. Circulation Research, 127(1), 4–20. https://doi.org/10.1161/circresaha.120.316340
- O’Brien, T. J., Fenton, K., Sidahmed, A., Barbour, A., & Harralson, A. F. (2021). Race and Drug Toxicity: A Study of Three Cardiovascular Drugs with Strong Pharmacogenetic Recommendations. Journal of Personalized Medicine, 11(11), 1226. https://doi.org/10.3390/jpm11111226
- Joukar S. (2021). A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and humans: extrapolation of experimental insights to clinic. Laboratory animal research, 37(1), 25. https://doi.org/10.1186/s42826-021-00102-3
- Chrysafides, S. M. (2023, April 10). Physiology, resting potential. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538338/
- Wei, X. (2023, April 17). Physiology, cardiac repolarization dispersion and reserve. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537194/
- Pirahanchi, Y. (2023, March 13). Physiology, Sodium potassium pump. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537088/
- Goyal, A. (2023, July 4). Reentry arrhythmia. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537089/
- An, Z., Yang, G., Liu, X., Zhang, Z., & Liu, G. (2018). New progress in understanding the cellular mechanisms of anti-arrhythmic drugs. Central European Journal of Biology, 13(1), 335–339. https://doi.org/10.1515/biol-2018-0041
- Osadchii, O. E. (2017). Effects of Na+ channel blockers on the restitution of refractory period, conduction time, and excitation wavelength in perfused guinea-pig heart. PLOS ONE, 12(2), e0172683. https://doi.org/10.1371/journal.pone.0172683
- Wołowiec, Ł., Grześk, G., Osiak, J., Wijata, A., Mędlewska, M., Gaborek, P., Banach, J., Wołowiec, A., & Głowacka, M. (2023). Beta-blockers in cardiac arrhythmias–Clinical pharmacologist’s point of view. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.1043714
- Naji, A. (2023, May 8). Muscarinic antagonists. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK557541/
- Marcu, D., Adam, C. A., Dorobantu, D. M., Șalaru, D. L., Sascău, R. A., Balasanian, M., Macovei, L., Arsenescu-Georgescu, C., & Stătescu, C. (2022). Beta-Blocker-Related Atrioventricular Conduction Disorders—A single tertiary referral Center experience. Medicina-lithuania, 58(2), 320. https://doi.org/10.3390/medicina58020320
- Westergaard, L. M., Alhakak, A., Rørth, R., Fosbøl, E. L., Kristensen, S. L., Svendsen, J. H., Graff, C., Jb, N., Gislason, G., Køber, L., Torp‐Pedersen, C., Lee, C. J., & Weeke, P. (2023). Ventricular rate in atrial fibrillation and the risk of heart failure and death. Europace, 25(5). https://doi.org/10.1093/europace/euad088
- Grubb A, Mentz RJ. Pharmacological management of atrial fibrillation in patients with heart failure with reduced ejection fraction: review of current knowledge and future directions. Expert Rev Cardiovasc Ther. 2020 Feb;18(2):85-101
- David, M. N. V. (2023, January 19). Digoxin. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK556025/
- Alqahtani, M. S., Kazi, M., Alsenaidy, M. A., & Ahmad, M. Z. (2021). Advances in oral drug delivery. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.618411
- Cheng, L., & Wong, H. (2020). Food effects on oral drug absorption: Application of Physiologically Based Pharmacokinetic Modeling as a predictive tool. Pharmaceutics, 12(7), 672. https://doi.org/10.3390/pharmaceutics12070672
- Herman, T. F. (2023, November 3). First-Pass effect. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK551679/
- Mar, P. L., Horbal, P., Chung, M. K., Dukes, J. W., Ezekowitz, M. D., Lakkireddy, D., Lip, G. Y. H., Miletello, M., Noseworthy, P. A., Reiffel, J. A., Tisdale, J. E., Olshansky, B., & Gopinathannair, R. (2022). Drug interactions affecting antiarrhythmic drug use. Circulation: Arrhythmia and Electrophysiology, 15(5). https://doi.org/10.1161/circep.121.007955
- Florek, J. B. (2023, November 12). Amiodarone. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482154/
- Dean, L. (2017, April 4). Propafenone therapy and CYP2D6 genotype. Medical Genetics Summaries – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK425391/
- Rollason, V., Lloret‐Linares, C., Lorenzini, K. I., Daali, Y., Gex‐Fabry, M., Piguet, V., Besson, M., Samer, C. F., & Desmeules, J. A. (2020). Evaluation of phenotypic and genotypic variations of drug metabolizing enzymes and transporters in chronic pain patients facing adverse drug reactions or Non-Response to analgesics: a retrospective study. Journal of Personalized Medicine, 10(4), 198. https://doi.org/10.3390/jpm10040198
- Chu, R. (2020, October 10). Intravenous lidocaine infusion for the management of early postoperative pain: A Comprehensive review of controlled trials. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7901134/
- Weibel, S., Jelting, Y., Pace, N. L., Helf, A., Eberhart, L., Hahnenkamp, K., Hollmann, M. W., Poepping, D., Schnabel, A., & Kranke, P. (2018). Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. The Cochrane Library, 2018(6). https://doi.org/10.1002/14651858.cd009642.pub3
- Larson, J., Rich, L., Deshmukh, A., Judge, E. C., & Liang, J. J. (2022). Pharmacologic Management for Ventricular arrhythmias: Overview of Anti-Arrhythmic Drugs. Journal of Clinical Medicine, 11(11), 3233. https://doi.org/10.3390/jcm11113233
- Tun, M. M., Pandey, S., Adhikari, S., Mainali, A., Thapa, A., Bisural, R., Bista, P. B., Htet, S. Y., Chhetri, B., & Panigrahi, K. (2023). Amiodarone-Induced liver attenuation on CT scan: alarming signal for toxicity and prompt discontinuation. Cureus. https://doi.org/10.7759/cureus.39844
- Sasi, N., MD. (2024). Medscape Registration. https://emedicine.medscape.com/article/813046-overview?form=fpf
- Ohyama, K., Nakajima, M., Suzuki, M., Shimada, N., Yamazaki, H., & Yokoi, T. (2000). Inhibitory effects of amiodarone and its N‐deethylated metabolite on human cytochrome P450 activities: Prediction of in vivo drug interactions. British Journal of Clinical Pharmacology, 49(3), 244–253. https://doi.org/10.1046/j.1365-2125.2000.00134.x
- Beavers, C. J., Rodgers, J. E., Bagnola, A., Beckie, T. M., Campia, U., Di Palo, K. E., Okwuosa, T. M., Przespolewski, E., & Dent, S. (2022). Cardio-Oncology Drug Interactions: A scientific statement from the American Heart Association. Circulation, 145(15). https://doi.org/10.1161/cir.0000000000001056
- Kamaraju, S., Mohan, M., Zaharova, S., Wallace, B., McGraw, J., Lokken, J., Tierney, J. F., Weil, E., Fatunde, O., & Brown, S. (2021). Interactions between cardiology and oncology drugs in precision cardio-oncology. Clinical Science, 135(11), 1333–1351. https://doi.org/10.1042/cs20200309
- Chakraborty, R. K. (2023, July 28). Calcium channel blocker toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537147/
- Konieczny, K. M., & Dorian, P. (2019). Clinically important Drug–Drug interactions between antiarrhythmic drugs and anticoagulants. The Journal of Innovations in Cardiac Rhythm Management, 0(3), 3552–3559. https://doi.org/10.19102/icrm.2019.100304
- Ferri, N., Colombo, E., Tenconi, M., Baldessin, L., & Corsini, A. (2022). Drug-Drug interactions of direct oral anticoagulants (DOACs): From pharmacological to clinical practice. Pharmaceutics, 14(6), 1120. https://doi.org/10.3390/pharmaceutics14061120
- Hügl, B., Horlitz, M., Fischer, K., & Kreutz, R. (2023). Clinical significance of the rivaroxaban–dronedarone interaction: insights from physiologically based pharmacokinetic modelling. European Heart Journal Open, 3(1). https://doi.org/10.1093/ehjopen/oead004
- Lucà, F., Oliva, F., Abrignani, M. G., Di Fusco, S. A., Parrini, I., Canale, M. L., Giubilato, S., Cornara, S., Nesti, M., Rao, C. M., Pozzi, A., Binaghi, G., Maloberti, A., Ceravolo, R., Bisceglia, I., Rossini, R., Temporelli, P. L., Amico, A., Calvanese, R., . . . Gulizia, M. M. (2023). Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. Journal of Clinical Medicine, 12(18), 5955. https://doi.org/10.3390/jcm12185955
- Wiggins, B. S., Dixon, D. L., Neyens, R., Page, R. L., & Gluckman, T. J. (2020). Select Drug-Drug interactions with direct oral anticoagulants. Journal of the American College of Cardiology, 75(11), 1341–1350. https://doi.org/10.1016/j.jacc.2019.12.068
- Tovia-Brodie, O. (2020). Pharmacological therapy in Brugada syndrome. Radcliffe Cardiology. https://www.aerjournal.com/articles/pharmacological-therapy-brugada-syndrome
- Sanchez‐Nadales, A., Anampa-Guzmán, A., & Khan, A. (2019). Disopyramide for hypertrophic cardiomyopathy. Cureus. https://doi.org/10.7759/cureus.4526
- Blotner, M., Betageri, O., Miles, W. M., & Kong, X. (2022). WorkUp for Suspected Brugada Syndrome: Two case reports for the general practitioner. Cureus. https://doi.org/10.7759/cureus.21921
- Chhabra, L. (2023, August 7). Wolff-Parkinson-White Syndrome. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK554437/
- Bohnen, M. S., Peng, G., Robey, S., Terrenoire, C., Iyer, V., Sampson, K. J., & Kass, R. S. (2017). Molecular Pathophysiology of Congenital Long QT Syndrome. Physiological Reviews, 97(1), 89–134. https://doi.org/10.1152/physrev.00008.2016
- Narayanan, K. (2020). Strategies for rhythm control in Atrial fibrillation. Indian Journal of Clinical Cardiology, 1(2), 94–107. https://doi.org/10.1177/2632463620936018
- Arunachalam, K. (2023, August 8). Flecainide. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK542291/
- Sreenivas, S. (2021, March 3). Beta-Blocker Medications for AFIB. WebMD. https://www.webmd.com/heart-disease/atrial-fibrillation/beta-blocker-medications-afib
- Viskin, S., Chorin, E., Viskin, D., Hochstadt, A., Schwartz, A. L., & Rosso, R. (2021). Polymorphic Ventricular Tachycardia: Terminology, mechanism, diagnosis, and emergency therapy. Circulation, 144(10), 823–839. https://doi.org/10.1161/circulationaha.121.055783
- Muser, D., Tritto, M., Mariani, M. V., Di Monaco, A., Compagnucci, P., Accogli, M., De Ponti, R., & Guarracini, F. (2021). Diagnosis and treatment of idiopathic Premature ventricular contractions: A stepwise approach based on the site of origin. Diagnostics, 11(10), 1840. https://doi.org/10.3390/diagnostics11101840
- Khachatryan, A., Merino, J. L., De Abajo, F. J., Botto, G. L., Kirchhof, P., Breithardt, G., Stambler, B. S., Abenhaim, L., & Grimaldi-Bensouda, L. (2021). International cohort study on the effectiveness of dronedarone and other antiarrhythmic drugs for atrial fibrillation in real-world practice (EFFECT-AF). Europace, 24(6), 899–909. https://doi.org/10.1093/europace/euab262
- Clinical use of dofetilide – UpToDate. (2024). UpToDate. https://www.uptodate.com/contents/clinical-use-of-dofetilide
- Mubarik, A. (2022, June 10). Sotalol. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK534832/
- Szymanski, M. W. (2023, April 3). Ibutilide. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK526021/
- Phillips, C. T., Wang, J., Celi, L. A., Zhang, Z., & Feng, M. (2019). Association of hypokalemia with an increased risk for medically treated arrhythmias. PLOS ONE, 14(6), e0217432. https://doi.org/10.1371/journal.pone.0217432
- PROPAFENONE HYDROCHLORIDE TABLETS. (2024). https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a7c8f090-c48c-44f7-9973-2cf4b491e35c&type=display
- Intro_to_antiarrhythmics [TUSOM | Pharmwiki]. (2024). https://tmedweb.tulane.edu/pharmwiki/doku.php/intro_to_antiarrhythmics
- Singh, S. (2023, August 28). Adenosine. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK519049/
- Alobaida, M., & Alrumayh, A. (2021). Rate control strategies for atrial fibrillation. Annals of Medicine, 53(1), 682–692. https://doi.org/10.1080/07853890.2021.1930137
- Fatima, N., Mandava, K., Khatoon, F., Badar, J., Begum, S. F., Narasimhan, C., Daljeet, K., & Mohammed, W. (2022). CLInical Profile and Side Effects of chronic use of oral Amiodarone in cardiology outpatient department (CLIPSE-A Study)- A prospective observational study. Annals of Medicine and Surgery, 80. https://doi.org/10.1016/j.amsu.2022.104167
- Dunn, J. (2023, February 13). Physiology, adenosine triphosphate. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK553175/
- Lavonas, E. J., Akpunonu, P., Arens, A. M., Babu, K. M., Cao, D., Hoffman, R. S., Hoyte, C., Mazer‐Amirshahi, M., Stolbach, A., St‐Onge, M., Thompson, T. M., Wang, G. S., Hoover, A. V., & Drennan, I. R. (2023). 2023 American Heart Association Focused Update on the Management of Patients with Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation, 148(16). https://doi.org/10.1161/cir.0000000000001161
- Rehman, R. (2023, May 1). Digitalis toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK459165/
- Pincus M. (2016). Management of digoxin toxicity. Australian prescriber, 39(1), 18–20. https://doi.org/10.18773/austprescr.2016.006
- Crosby, J., Bhopalwala, H., Kharawala, A., Dewaswala, N., Ganti, S. S., & Bhopalwala, A. (2021). Refractory Torsades de Pointes Due to Dofetilide Overdose. Journal of Investigative Medicine High Impact Case Reports, 9, 232470962110564. https://doi.org/10.1177/23247096211056492
- Khalid, M. M. (2023, July 28). Beta-Blocker toxicity. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK448097/
- Bologa, C., Lionte, C., Popescu, A., Sorodoc, V., & Sorodoc, L. (2021). First Case of Acute Poisoning with Amiodarone and Flecainide in Attempted Suicide Successfully Managed with Lipid Emulsion Therapy in the Emergency Department: Case Report and Literature Review. Healthcare (Basel, Switzerland), 9(6), 671. https://doi.org/10.3390/healthcare9060671
- Lei, M., Wu, L., Terrar, D. A., & Huang, C. L. (2018). Modernized Classification of Cardiac Antiarrhythmic Drugs. Circulation, 138(17), 1879–1896. https://doi.org/10.1161/CIRCULATIONAHA.118.035455
- Hakkola, J., Hukkanen, J., Turpeinen, M., & Pelkonen, O. (2020). Inhibition and induction of CYP enzymes in humans: an update. Archives of Toxicology, 94(11), 3671–3722. https://doi.org/10.1007/s00204-020-02936-7
- Aggarwal, N., Leslie, S.W., & Lotfollhzadeh, S. (Updated 2024, January 11). Recurrent Urinary Tract Infections. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557479
- Bono, M.J., Leslie, S.W., & Reygaert, W.C. (Updated 2023, November 13). Uncomplicated Urinary Tract Infections. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470195
- Butler, D., Ambite, I., Wan, M. L. Y., Tran, T. H., Wullt, B., & Svanborg, C. (2022). Immunomodulation therapy offers new molecular strategies to treat UTIs. Nature reviews. Urology, 19(7), 419–437. https://doi.org/10.1038/s41585-022-00602-4
- Evans, J., Hanoodi, M., & Wittler, M. (Updated 2023, August 16). Amoxicillin Clavulanate. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK538164/
- Fekete, T. (Last reviewed 2024, January). Asymptomatic bacteriuria in adults. UpToDate. Retrieved from https://www.uptodate.com/contents/asymptomatic-bacteriuria-in-adults
- Gupta, K. (Last reviewed 2024, January). Acute simple cystitis in adult and adolescent females. UpToDate. Retrieved from https://www.uptodate.com/contents/acute-simple-cystitis-in-adult-and-adolescent-females#H207461713
- Gupta, K. (Last reviewed 2024, January). Acute Complicated Urinary Tract Infection (including pyelonephritis) in Adults and Adolescents. UpToDate. Retrieved from https://www.uptodate.com/contents/acute-complicated-urinary-tract-infection-including-pyelonephritis-in-adults-and-adolescents
- Herman, T.F., & Hashmi, M.F. (Updated 2023, August 17). Cephalexin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK549780
- Kaye, K. S., Rice, L. B., Dane, A. L., Stus, V., Sagan, O., Fedosiuk, E., Das, A. F., Skarinsky, D., Eckburg, P. B., & Ellis-Grosse, E. J. (2019). Fosfomycin for Injection (ZTI-01) Versus Piperacillin-tazobactam for the Treatment of Complicated Urinary Tract Infection Including Acute Pyelonephritis: ZEUS, A Phase 2/3 Randomized Trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 69(12), 2045–2056. https://doi.org/10.1093/cid/ciz181
- Kemnic, T.R., & Coleman, M. (Updated 2022, November 28). Trimethoprim Sulfamethoxazole. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK513232/
- Lodise, T. P., Henriksen, A. S., Hadley, T., & Patel, N. (2021). US-Focused Conceptual Health Care Decision-Analytic Models Examining the Value of Pivmecillinam Relative to Current Standard-of-Care Agents Among Adult Patients With Uncomplicated Urinary Tract Infections due to Enterobacterales. Open forum infectious diseases, 8(10), ofab380. https://doi.org/10.1093/ofid/ofab380
- Loubet, P., Ranfaing, J., Dinh, A., Dunyach-Remy, C., Bernard, L., Bruyère, F., Lavigne, J. P., & Sotto, A. (2020). Alternative Therapeutic Options to Antibiotics for the Treatment of Urinary Tract Infections. Frontiers in microbiology, 11, 1509. https://doi.org/10.3389/fmicb.2020.01509
- MedlinePlus. (2024). Cefdinir. MedlinePlus. Retrieved from https://medlineplus.gov/druginfo/meds/a698001.html
- MedlinePlus. (2024). Fosfomycin. MedlinePlus. Retrieved from https://medlineplus.gov/druginfo/meds/a697008.html
- MedlinePlus. (2024). Piperacillin and Tazobactam Injection. MedlinePlus. Retrieved from https://medlineplus.gov/druginfo/meds/a694003.html
- Squadrito, F.J., & del Portal, D. (Updated 2023, May 29). Nitrofurantoin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470526
- Thai, T., Salisbury, B.H., & Zito, P.M. (Updated 2023, August 28). Ciprofloxacin. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK535454/
- Werth, B.J. (Last revised 2022, September). Cephalosporins. Merck Manual. Retrieved from https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/cephalosporins
- Werth, B.J. (Last revised 2022, September). Fluoroquinolones. Merck Manual. Retrieved from https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/fluoroquinolones
- Werth, B.J. (Last revised 2022, September). Fosfomycin. Merck Manual. Retrieved from https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/fosfomycin
- Werth, B.J. (Last revised 2022, September). Meropenem. Merck Manual. Retrieved from https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/carbapenems
- Werth, B.J. (Last revised 2022, September). Sulfonamides. Merck Manual. Retrieved from https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/sulfonamides
- How You Can Prevent Sexually Transmitted Diseases. (2023). Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/prevention/default.htm
- Mohseni, M., Sung, S., & Takov V. (2023). Chlamydia. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537286/
- Springer, C. and Salen, P. (2023). Gonorrhea. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK558903/
- Tudor, M. E., Al-Aboud, A. M., Leslie, S. W., & Gossman, W. (2023). Syphilis. In:StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK534780/
- Bacterial Vaginosis (2021). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Chancroid (2021). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/treatment-guidelines/chancroid.htm
- Mycoplasma Genitalium. (2021). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
- Genital Herpes. (2021). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/treatment-guidelines/herpes.htm
- Kombe Kombe, A. J., Li, B., Zahid, A., Mengist, H. M., Bounda, G. A., Zhou, Y., & Jin, T. (2021). Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Frontiers in public health, 8, 552028. https://doi.org/10.3389/fpubh.2020.552028
- Fletcher, C. V., Sax, P. E., & Mitty, J. (2023). Overview of antiretroviral agents used to treat HIV. UptoDate. Retrieved 22 Jan 2024 from https://www.uptodate.com/contents/overview-of-antiretroviral-agents-used-to-treat-hiv
- Trichomoniasis (2023). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/trichomonas/default.htm
- Rohatgi, K. W., Humble, S., McQueen, A., Hunleth, J. M., Chang, S. H., Herrick, C. J., & James, A. S. (2021). Medication Adherence and Characteristics of Patients Who Spend Less on Basic Needs to Afford Medications. Journal of the American Board of Family Medicine: JABFM, 34(3), 561–570. https://doi.org/10.3122/jabfm.2021.03.200361
- Patel, R.M. and Parmar, M. (2023). Doxycycline Hyclate. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK555888
- Graziani, A. L., Hooper, D. C., and Bogorodskaya, M. (2023). Azithromycin and clarithromycin. UptoDate. Retrieved 22 Jan 2024 from https://www.uptodate.com/contents/azithromycin-and-clarithromycin
- Podder, V. and Sadiq, N. M. (2022). Levofloxacin. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK545180/
- Gonorrhea (2023). The Centers for Disease Control and Prevention (CDC). Retrieved from https://www.cdc.gov/std/gonorrhea/treatment.htm
- Weir, C. B. and Le, J. K. (2023). Metronidazole. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539728/
- Thai, T., Salisbury, B. H., & Zito, P. M (2023). Ciprofloxacin. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK535454
- Taylor, M. and Gerriets, V. (2023). Acyclovir. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK542180
- Semaan, J. R. and Parmar, M. (2023). Famciclovir. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557563
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
Complete Survey
Give us your thoughts and feedback!
