Course
Rhode Island APRN Bundle
Course Highlights
- In this Rhode Island APRN Bundle we will learn about the most common types of substance abuse, and why it is important for nurses to recognize each type and treat accordingly.
- You’ll also learn to understand the difference between Alzheimer’s and dementia.
- You’ll leave this course with a broader understanding of FDA-approved and off-label indications for non-opioid medications and other various therapies for chronic pain management.
About
Contact Hours Awarded: 10
Including 7 Pharmacology Hours
Course By:
Various Authors
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Rhode Island Substance Abuse
Introduction
Substance abuse is described as “a pattern of using a substance (drug) that causes significant problems or distress” (1). As of 2020, 37.309 million Americans were currently using illegal drugs (2). Medical professionals are on the front lines of recognizing, treating and providing support to individuals who suffer from substance abuse. This Rhode Island Substance Abuse course will walk you through the different types of substances abused, the prevalence of that abuse, the symptoms one experiences while using that substance, overdose symptoms, and how to counteract an overdose. You will also learn about substance abuse in adolescents, and prevention methods currently being used to combat substance abuse in adolescents.

Self-Quiz
Ask Yourself...
- What do you think are the most abused substances?
- What knowledge do you hope to gain by completing this Rhode Island Substance Abuse course?
Alcohol
Alcohol abuse is the second most common type of substance abuse, with the first being tobacco use (3). While many individuals in the United States can drink alcohol and it is not considered abuse, there are some individuals whose drinking causes harm or distress. In the case of alcohol use disorder, harm or distress is described as alcohol leading to health problems, or trouble while at home, work, school or with law enforcement (3).
There are several signs and symptoms of alcohol use disorder that help one to determine if their loved one needs help. As health care providers, it is important to understand the signs and symptoms to properly help and treat those who are experiencing alcohol use disorder. Symptoms can range from mild to severe, depending on the number of symptoms experienced (5) and include:
- Unable to limit the amount of alcohol consumed
- Wanting to decrease the amount consumed, but being unsuccessful
- Spending a large amount of time obtaining alcohol, drinking alcohol, or recovering from alcohol use
- Having a strong craving or urge to drink alcohol
- Not completing major obligations at work, school or home due to alcohol use
- Continuing to drink alcohol even though you know it is causing problems physically, at work, home, or in relationships
- No participating in social activities or work-related functions to consume alcohol
- Developing a tolerance to alcohol so more is needed to elicit the same effect
- Experiencing symptoms of withdrawal, such as, nauseas, sweating and shaking when you are not drinking
While the above signs and symptoms are typically ones that do not have a medical component attached, alcohol use disorder impacts nearly every organ and system in the body. This widespread impact can have a detrimental effect on an individual suffering from alcohol use disorder (4) such as:
- Neurologic
– Ischemic stroke
– Hemorrhagic stroke - Cardiac
– Cardiomyopathy
– Arrhythmias
– Ischemic heart disease
– Hypertension - Lung
– Acute respiratory distress syndrome
– Pneumonia - Liver
– Steatosis
– Steatohepatitis
– Fibrosis
– Cirrhosis
– Alcohol associated hepatitis
– Liver cancer - Pancreas
– Acute and chronic pancreatitis - Gastrointestinal
– Gut leakiness
– Microbial dysbiosis
– Colorectal cancer
Clear patterns have emerged between alcohol use disorder and increased risk for certain types of cancers (4):
- Head and neck cancer
– Oral cavity
– Pharynx
– Larynx - Esophageal Cancer
- Liver Cancer
- Breast
- Colorectal Cancer
Knowing the effects of chronic alcohol use on the body is important in understanding the treatment methods that will be needed. Treatment options range from spiritual to medical, with many individuals utilizing more than one option (6).
- Detox and withdrawal
– This treatment option is typically done in an inpatient setting. Treatment begins with detoxification, which leads to withdrawal symptoms. These symptoms can be medically managed, and occasionally require sedating medications. Detox and withdrawal generally take 2 to 7 days. - Psychological counseling
– This treatment option will help the individual better understand their problem with alcohol and provide support on the psychological aspects of alcohol use disorder. This type of treatment can be done individually or in a group setting. - Oral medications
– Disulfiram is a medication that helps to curb one’s want for alcohol. While the drug doesn’t remove the urge to drink, it will produce a physical reaction to consuming alcohol in the form of flushing, nausea, vomiting and headaches.
– Naltrexone is used to block the good feelings alcohol causes, which can aid in recovery.
– Acamprosate is used to help curb cravings of alcohol and is generally used in combination with Naltrexone. - Injected medication
– Vivitrol is the injected version of Naltrexone and is injected once a month. Injected medications may be easier, or more consistently used than oral medications. - Medical treatment
– As we’ve learned, alcohol use disorder comes with a large amount of health concerns. These concerns typically require medical treatment in the form of medication, surgery, outpatient care, etc. - Spiritual practice
– It has been shown that individuals involved in some type of spiritual practice find it easier to maintain recovery.

Self-Quiz
Ask Yourself...
- What are the five most common types of cancers associated with alcohol use disorder?
- Have you personally taken care of someone with alcohol use disorder? Did they exhibit any symptoms or illnesses listed above?
Marijuana
Marijuana, also known as cannabis, weed or pot, refers to the dried flowers, leaves, stems and seeds of the cannabis plant. In one cannabis plant there are over 100 compounds ranging from tetrahydrocannabinol (THC) to cannabidiol (CBD) (7). While THC and CBD have the same molecular structure, the difference in how the atoms are arranged accounts for the different effects on the body. THC is the main psychoactive compound in cannabis, which produces the high sensation, while CBD, although psychoactive, does not produce the high sensation (8).
Marijuana is the most used federally illegal drug in the United Stated. In 2019, the CDC reported that 48.2 million, or ~18% of Americans have used marijuana at least once. There are several ways to use marijuana, including: smoking in joints, blunts, or bongs, vaping via electronic vaporizing devices, mixing or infusing into foods or drinks, or inhaling oil concentrates (7).
There are many health risks associated with using marijuana, in any form. It is estimated that 3 in 10 people who use marijuana have marijuana use disorder (7).
The risks include:
Brain Health
Since marijuana is a psychoactive drug, the main effect is on brain function. Marijuana specifically affects the parts of the brain responsible for memory, learning, attention, decision making, coordination, emotions and reaction time.
Heart Health
Marijuana is known to make the heart beat faster, which can make blood pressure higher immediately after use. This can lead to an increased risk of stroke, heart disease or vascular disease.
Lung Health
Inhaled marijuana can cause damage to lung tissues and small blood vessels, as well as scarring to the lung. More research is being done to understand the effects of secondhand marijuana smoke.
Mental Health
While the relationship is not fully understood, marijuana has been linked to social anxiety, depression and schizophrenia.
Unintentional Poisoning
There is a greater risk for unintentional poisoning with edibles (marijuana baked or put into food or drinks) than with inhaled marijuana. This risk is because children can easily mistake food with marijuana in it. In some instances, emergency medical care has been required.
As marijuana becomes legal, and readily available across many states, teenagers are gaining better access to it. The CDC reports that 37% of high school students in the United States have reported using marijuana. This use can come with impacts to their developing brains, resulting in (7):
- Difficulty thinking and problem-solving
- Problems with memory and learning
- Reduced coordination
- Difficulty maintaining attention
- Problems with school and social life

Self-Quiz
Ask Yourself...
- What is the difference between THC and CBD?
- What were the 5 health risks listed?
- Were you aware of the health risks associated with marijuana used? Do the health risks surprise you?
Prescription Medicines
Prescription drug abuse is classified as the abuse of a prescription medication that is taken in a way not intended by the prescriber. This abuse can be by the person who the drug was initially prescribed for, or from someone taking another person’s prescription medication. The National Center for Drug Abuse Statistics show that 6% of Americans over the age of 12 abuse prescriptions in a year, and 12% of prescription drug abusers are addicted. This is perpetuated by 4 out of 5 pharmacy filled prescriptions being opioids (10).
Prescription medication is sectioned off into three categories: opioids, anti-anxiety medications/sedatives/hypnotics, and stimulants. Signs and symptoms of prescription drug abuse vary depending on the type of drug used (9). Opioids are a type of medication that is used to treat pain. These medications usually contain oxycodone or hydrocodone. Opioids are the leading cause of drug overdose death, with 74.8% of drug overdose death being from Opioids (11).
The signs of symptoms of opioid drug abuse include (9):
- Constipation
- Nausea
- Feeling high
- Slowed breathing rate
- Drowsiness
- Confusion
- Poor coordination
- Increased dose needed for pain relief
- Worsening or increased sensitivity to pain with higher doses
Anti-anxiety medication, sedatives and hypnotics are used to treat anxiety and sleep disorders. Some medications used for these disorders are alprazolam (Xanax), diazepam (Valium), and zolpidem (Ambien).
The signs and symptoms of drug abuse by these types of medications are (9):
- Drowsiness
- Confusion
- Unsteady walking
- Slurred speech
- Poor concentration
- Dizziness
- Problems with memory
- Slowed breathing
Stimulants are a type of medication used to treat attention-deficit/hyperactivity disorder (ADHD) and certain sleep disorders. Some medications used to treat these disorders include methylphenidate (Ritalin, Concerta, and others), dextroamphetamine-amphetamine (Adderall XR, Mydayis) and dextroamphetamine (Dexedrine).
Signs and symptoms of drug abuse by these types of medications are (9):
- Increased alertness
- Feeling high
- Irregular heartbeat
- High blood pressure
- High body temperature
- Reduced appetite
- Insomnia
- Agitation
- Anxiety
- Paranoia
Medical complications differ depending on the type of medication abused. Opioids can decrease respiratory rate with the potential for breathing to stop altogether. They can also cause a coma, and lead to death. Anti-anxiety/sedatives/hypnotics can cause memory problems, low blood pressure and slowed breathing. Like opioids, they can also lead to coma or death. Abrupt withdrawal of these medications can lead to an overactive nervous system and seizures. Stimulants can increase the bodies temperature, produce heart problems, high blood pressure, seizures or tremors, hallucinations, aggressiveness, and paranoia (9).
Opioids can be reversed with a medication called Naloxone. This medication works by binding to the opioid receptors, then reversing and blocking the effects of other opioids. It is used to restore an individual’s breathing and can be given through injection or nasal spray. If Naloxone is given outside of a medical facility, emergency personnel should be contacted immediately (18).

Self-Quiz
Ask Yourself...
- Were you surprised to learn that most prescriptions filled in pharmacies are opioids?
- Think about the number of children who are on ADHD medication. Do you think they or their guardians should receive in-depth training and education on the potential dangers of that medication?
- What symptoms were similar? What symptoms were different?
Methamphetamine
Methamphetamine is a highly addictive, man-made, central nervous system stimulant. This drug increases heart rate, body temperature, respiration, and blood pressure. It also enhances one’s energy, attention, focus, pleasure, and excitement (12). It has commonly been referred to as meth, ice, speed, and crystal. Research has shown that 2.5 million Americans aged 12 or older reported using methamphetamine within the past year. 53% of those individuals met diagnostic criteria for methamphetamine use disorder, but less than 1 in 3 received substance use treatment within the past year (13).
There are four ways methamphetamine can be used: smoking, swallowing (pill), snorting, or injecting the powder that has been dissolved in water or alcohol. While methamphetamine produces a high quickly, it also fades quickly. This produces what is called and “binge and crash” pattern of use. This type of use is where an individual will take the drug every few hours for several days at a time, resulting in lack of food and sleep (14).
There is a substantial amount of long-term health effects from methamphetamine use. Those who inject methamphetamine are at a higher risk for contracting infectious diseases like HIV and hepatitis C.
Other long-term problems include (14):
- Extreme weight loss
- Severe dental problems
- Intense itching which can lead to skin sores and infection from scratching
- Anxiety
- Changes in brain structure and function
– Changes have been noted to the brain’s dopamine system which has resulted in problems with coordination and verbal learning.
– Severe changes have also been noted to the areas of the brain that deal with emotion and memory - Confusion and memory loss
- Sleeping problems
- Violent behavior
- Paranoia
- Hallucinations
Due to the effect methamphetamine has on the body, an overdose often leads to a stroke, heart attack, or organ problems. Because of this, it is imperative health care providers restore blood flow to the affected part of the brain for a stroke, restore blood flow to the heart in the event of a heart attack, or treat the organ issues that present (14). Treatment for methamphetamine use disorder focuses on cognitive-behavioral therapy and motivational incentives, such as vouchers or small cash rewards that encourage individuals to remain drug-free. There is currently no FDA approved medication to treat a methamphetamine addiction (14).

Self-Quiz
Ask Yourself...
-
What are the 10 long-term effects methamphetamine can have on the body as noted in this Rhode Island Substance Abuse course?
-
Have you seen any of these long-term effects in your nursing practice?
-
Does it surprise you that there is no medication to treat a methamphetamine overdose?
Cocaine
Cocaine is a highly addictive stimulant drug that is derived from the leaves of the coco plant that is native to South America. Dealers of cocaine may add in other drugs to the cocaine, such as amphetamine or synthetic opioids, like fentanyl. Adding in synthetic opioids can be especially dangerous and lead to overdose and even death (15). Over 5 million Americans reported current cocaine use in 2020, with nearly 1 in 5 overdose deaths reported (13).
There are several ways in which cocaine can be used: in powder form it can be snorted or rubbed into an individual’s gums, the powder can be dissolved and injected into the bloodstream, or if the cocaine is in crystal form, it can be heated and smoked. Injecting cocaine produces a faster and more intense high but is short-lasting. Cocaine affects the brain by increasing the amount of dopamine produced. This increase of dopamine floods the brain’s reward circuit, which reinforces drug-taking behavior. Repeated cocaine use can lead to the brain’s reward circuit becoming less sensitive, which leads to individuals taking stronger and more frequent doses to achieve the same high as before (15).
The effects of cocaine are felt almost immediately and can disappear within a few minutes to an hour. There are several health effects from using cocaine (15):
- Extreme happiness and energy
- Mental alertness
- Hypersensitivity to sight, sound, and touch
- Irritability
- Paranoia
- Constricted blood vessels
- Dilated pupils
- Nausea
- Increase in body temperature and blood pressure
- Increased or irregular heartbeat
- Tremors/muscle twitches
- Restlessness
There are several long-term effects of cocaine use. These effects can range from common, to being dependent on the method of use (15)
- Malnourished due to a decreased appetite
- Movement disorders
- Irritability
- Restlessness
- Auditory hallucinations
- Snorting cocaine
– Loss of smell
– Nosebleeds
– Frequent runny nose
– Problems with swallowing - Smoking cocaine
– Cough
– Asthma
– Respiratory distress
– Higher risk for infections like pneumonia - Consuming cocaine by mouth
– Severe bowel decay due to reduced blood flow - Injecting cocaine
– Increased risk of contracting HIV, hepatitis B and C, and other blood-borne diseases
– Skin or soft tissue infections
– Scarring or collapsed veins
A cocaine overdose is similar to that of a methamphetamine overdose, with the inclusion of seizures. Like methamphetamine, it is critical that health care providers restore blood flow to the heart and brain in the event of a heart attack or stroke. If an individual presents with a seizure due to a cocaine overdose, the first action to be taken is to stop the seizure. Cocaine mirrors that of methamphetamine use in terms of increased dopamine in the brain. This leads to an addictive nature, as well as needing more drug overtime to produce the same high (15).
Unfortunately, there is no FDA medication approved to treat cocaine use disorder.
There are several behavioral therapy options available (15):
- Cognitive-behavioral therapy
- Contingency management or motivational incentives
- Therapeutic communities
– These are residences in which people can recover from substance use disorders with other individuals who understand their addiction, all while being drug-free - Community based recovery groups

Self-Quiz
Ask Yourself...
- How many Americans stated they had used cocaine in 2020? Did that number surprise you? Did you think it would be higher or lower?
- While a cocaine overdose may be similar to that of a methamphetamine overdose, what additional overdose symptom can happen with cocaine use?
- There are four methods in which cocaine can be used, what long-term side effects stem from those four methods?
Heroin
Heroin is a type of drug made from morphine, which is derived from the seed pod of opium poppy plants (16). According to the CDC, over 19% of all opioid overdose deaths in 2020 involved the use of heroin (17). Heroin can be found as a white or brown powder, or a black tar like substance. Like cocaine and methamphetamine, heroin can be injected into the bloodstream, snorted, or heated and smoked. Additionally, some individuals mix heroin with cocaine, or alcohol. This created an even higher risk for an overdose, and potentially death (16).
The effects of heroin on the body are like those of prescribed opioids. When heroin reaches the brain, it is turned into morphine, which binds to opioid receptors. This causes the user to feel what is described as a rush, or a pleasurable sensation. How intense the rush is, is determined by how much drug has been taken and how quickly it attaches itself to the opioid receptor (16).
Along with the rush, there are several short-term effects that people may experience when using heroin (16):
- Dry mouth
- Warm, flushing of the skin
- Heaving feeling in their arms and legs
- Nausea
- Vomiting
- Severe itching
- Clouded mental functioning
- Being in a back-and-forth state of consciousness and semi-consciousness
Individuals with heroin use disorder may experience some of the following long term health effects (16):
- Insomnia
- Collapsed or damaged veins from injecting the drug
- Damaged tissues on the inside of the nose due to snorting the drug
- Infection in the lining of the heart and the valves
- Abscesses
- Constipation and stomach cramping
- Liver and kidney disease
- Lung complications, like pneumonia
- Mental disorders like depression and antisocial personality disorder
- Sexual dysfunction in men
- Irregular menstrual cycle in women
- Increased risk for blood borne diseases such as HIV and hepatitis C
Heroin overdoses, along with opioid overdoses, have been increasing in the United States. A heroin overdose depresses one’s heart rate as well as breathing, leading to hypoxia. However, Naloxone is a medication that can reverse opioid overdoses, if given the correct way. Naloxone can be injected or snorted and has recently been approved for over the counter dispense in several states (18).
Those who suffer from heroin use disorder have a wider variety of treatments at their disposal. Behavioral therapies include cognitive-behavioral therapy as well asl contingency management. It has been shown that these behavioral therapies work best when used in-conjunction with medications.
There are three different types of medications available to those with heroin use disorder (16):
Methadone
This is an opioid receptor full agonist, which means it attaches itself to and actives an opioid receptor to help ease withdrawal symptoms of heroin cravings
Buprenorphine
This is an opioid receptor partial agonist, which means it attaches itself to and partially activates opioid receptors to help ease withdrawal symptoms and heroin cravings
Naltrexone
This is an opioid receptor antagonist, which means it prevents heroin from binding to opioid receptors, blocking the effects

Self-Quiz
Ask Yourself...
- What plant is heroin derived from? Were you aware of this before this course Rhode Island Substance Abuse course?
- Have you been educated on the proper way to administer Narcan to an individual suffering from a heroin or opioid overdose? Do you feel like this is something all health care professionals should be educated on?
- What are the three medications approved for treatment of heroin use disorder?
Hallucinogens
Hallucinogenic drugs are described as a group of drugs that alter a person’s awareness of their surroundings, thoughts, and feelings (19). In 2019 it was estimated that 5.5 million people in the United States used hallucinogens within that past year (20). Hallucinogenic drugs are split into two categories: classic hallucinogens and dissociative drugs. Like the name suggests, both types of hallucinogens can cause the user to experience hallucinations, but dissociative drugs can also cause the user to feel out of control or disconnected from their body (19).
Common classic hallucinogens include (19):
D-lysergic acid diethylamide (LSD)
Considered one of the most powerful mind-altering chemicals. This drug is a clear or white odorless material, made from lysergic acid. Lysergic acid is found on fungus that will grow on rye and other grains.
4-phosphoryloxy-N, N-dimethyltryptamine (Psilocybin)
This hallucinogenic is also referred to “magic mushrooms” or “shrooms” since it is found on certain types of mushrooms in South America, Mexico, and the United States.
Mescaline (Peyote)
Peyote comes from a small, spineless cactus, but may also be synthetic. While it is illegal in the United States, it can be used in religious ceremonies in the Native American Church.
N, N-dimethyltryptamine (DMT)
A chemical found in some Amazonian plants. It can be made into a tea called Ayahuasca or smoked if synthetically made.
251-NBOMe
This is a synthetic hallucinogen that is like LSD and MDMA but is more potent. It was originally developed for use in brain research. It has also been referred to as “N Bomb” or “251”.
Common dissociative drugs include (19):
Phencyclidine (PCP)
This drug was initially developed for surgery in the 1950’s, but due to its serious side effects it is no longer used. It can be found in several forms, such as: tablets, liquid, and white crystal powder.
Ketamine
This drug is used as an anesthetic for both humans and animals and is typically stolen or sold illegally from veterinary offices. Ketamine comes in powder, pills, or liquid form.
DXM (Dextromethorphan)
This drug is used as a cough suppressant and muscus-clearing ingredient in over-the-counter cold and cough medicines. It can be found in syrup, tablet or gel capsule form.
Saliva divinorum (Salvia)
This is a plant that is common to southern Mexico and Central and South America. It’s leaves or typically chewed, or the juice that is extracted from them is drank. Saliva can also be inhaled.
The short- and long-term side effects of hallucinogens are different depending on the type and category of hallucinogenic used. Short-term side effects for classic hallucinogens are (19):
- Hallucinations
- Increased heart rate
- Nausea
- Intensified feelings and sensory experiences
- Changes in sense of time
- Increased blood pressure, breathing and body temperature
- Loss of appetite
- Dry mouth
- Sleep problems
- Uncoordinated movements
- Excessive sweating
- Panic
- Paranoia
- Psychosis
There are two specific long-term side effects of classic hallucinogens. These side effects are typically seen in individuals with a history of mental illness but can happen to anyone (19).
Persistent Psychosis
- This refers to a series of continuing mental problems that include:
- Visual disturbances
- Disorganized thinking
- Paranoia
- Mood changes
Hallucinogen Persisting Perception Disorder (HPPD)
This is a recurrence of certain drug experiencs like hallucinations or visual disturbances. These typically happen without warning and can occur any time after drug use.
Antidepressants and antipsychotic medicals have been used to improve an individual’s mood, as well as treat psychosis. Behavioral therapies have been used to help individuals cope with fear or confusion associated with visual disturbances.
Short-term side effects for dissociative drugs have been known to appear within a few minutes of taking the drug and can last hours or days. If the dosage is low, dissociative drugs can cause the following effects (19):
- Numbness
- Disorientation and loss of coordination
- Hallucinations
- Increase in the user’s blood pressure, heart rate and body temperature
If higher doses of dissociative drugs are taken the following side effects may occur (19):
- Memory loss
- Panic and anxiety attacks
- Seizures
- Psychotic symptoms
- Amnesia
- Inability to move
- Mood swings
- Trouble breathing
The long-term side effects of dissociative drugs are still being researched. However, repeated and prolonged use of PCP has been known to result in addiction. The following long-term effects may continue for a year or more after the drug use stops (19):
- Speech problems
- Memory Loss
- Weight Loss
- Anxiety
- Depression and suicidal thoughts
Most classic hallucinogen use will not result in an overdose but tend to have extremely unpleasant experiences when taken in higher doses. There have been some serious medical emergencies and fatalities that have been reported by 251-NBOMe. Overdose becomes more likely with dissociative drugs. High doses of PCP have been known to cause seizures, coma, and death (19).
Due to the nature of hallucinogens, there is a high risk of bodily harm due to the alteration of the user’s perception and mood (19):
- Users could attempt things they wouldn’t normally do when not under the influence, such as jumping out of a window or off a building.
- Users could experience a profound sadness or feeling of hopelessness leading to suicidal feelings and/or suicidal actions.
- Psilocybin users could accidently consume a poisonous mushroom that look like psilocybin, which can result in severe illness or death.

Self-Quiz
Ask Yourself...
-
What are the two categories of hallucinogens?
-
How many hallucinogens are derived from plants? What plants are they?
Substance Abuse in Adolescents
Substance abuse and opioid overdose deaths are beginning to affect school systems. In 2017, 2.2 million adolescents between the ages of 12-17 stated they were currently using illicit substances (21).
Brain growth and development, particularly during one’s adolescent years, has been highly studied and reviewed. One area of the brain that is still developing during adolescents is the prefrontal cortex. This area of the brain is responsible for allowing one to assess situations, make decisions, and keep emotions and desires under control (21). Because this area of the brain is still developing, it places adolescents at an increased risk of trying drugs and continuing them (21).
Substance use during one’s adolescent years has the potential to create several long-term negative effects. It is estimated that 90% of individuals with addictions began using substances during their adolescent years (22). There are several factors that can lead to substance use. These risk factors include family history of addiction, mental health concerns, behavioral or impulse control problems, exposure to trauma, and environmental factors (22).
Multiple studies have shown that the science of prevention may affect the probability of later problems (23). The main goal in adolescent substance abuse prevention is to reduce risk factors and overall enhance/reinforce protective factors (23). Depending on the addiction, medication may be used in combination with a form of behavioral therapy or counseling.
There are several types of behavioral therapies:
Cognitive-Behavioral Therapy
Helps individuals recognize, avoid and cope with situations in which they may use drugs.
Contingency Management
Uses positive reinforcement for attending counseling sessions, remaining drug-free, or taking prescribed medications.
Motivational Enhancement Therapy
Focuses on strategies that make the most of an individual’s readiness to change their current behavior and enter treatment.
Family Therapy
Focuses on utilizing the family to address influences on drug patterns and improve overall family function.
Twelve-Step Facilitation
Delivered in 12-week sessions. There are no medical treatments, but allow the individual to social and complementary support.
Follows 12 steps of acceptance, surrender, and active involvement in recovery

Self-Quiz
Ask Yourself...
- How many adolescents stated they had tried illicit substances in 2017?
- What is the estimated percentage of individuals with addictions who began using substances in their adolescent years?
- There are five different forms of behavior therapy listed in this Rhode Island Substance Abuse course, what are they?
Conclusion
Substance abuse in the United States is on the rise, with many hospitals and health care centers seeing an increase in patients. Understanding the different types of substances used, their short- and long-term symptoms, overdose symptoms, and medication options will help prepare you to care for these individuals. It is equally important to understand the behavioral therapy options for those with substance use disorders, and advocate for them while they are in your care.
Alzheimers Nursing Care
Introduction
Alzheimer’s disease is a destructive, progressive, and irreversible brain disorder that slowly destroys memory and thinking. Alzheimer’s is the most common cause of dementia in older adults (1). For most people who have Alzheimer’s disease, symptoms first appear in their mid 60’s (1).
Studies suggest more than 5.5 million Americans, most 65 or older, may have dementia caused by Alzheimer’s (1). It is currently listed as the sixth leading cause of death in the United States. It is essential to understand the signs and symptoms of Alzheimer’s dementia and how to manage the care of a patient, family member, or friend suffering from the disease.
Dementia is the loss of cognitive functioning, such as thinking, remembering, reasoning, and behavioral abilities, such as a decreased ability to perform activities of daily living (1). The severity of dementia ranges from mild to severe. Dmentia’s mildest stage often begins with forgetfulness, while its most severe stage consists of complete dependence on others for general activities of daily living (1).
History of Alzheimer’s
Alzheimer’s disease is named after Dr. Alois Alzheimer. In the early 1900s, Dr. Alzheimer noticed changes in the brain tissue of a patient who had died of an unknown mental illness. The patient’s symptoms included memory loss, language problems, and unpredictable behavior.
After her death, her brain was examined and was noted to have abnormal clumps known as amyloid plaques and tangled bundled fibers, known as neurofibrillary or tau tangles (1). These plaques and tangles within the brain are considered some of the main features of Alzheimer’s disease. Another feature includes connections of neurons in the brain. Neurons are a type of nerve cell responsible for sending messages between different parts of the brain and from the brain to other parts of the body (1).
Scientists are continuing to study the complex brain changes involved with the disease of Alzheimer’s. The changes in the brain could begin ten years or more before cognitive problems start to surface.
During this stage of the disease, people affected seem to be symptom-free; however, toxin changes occur within the brain (1). Initial damage in the brain occurs within the hippocampus and entorhinal cortex, which are the parts of the brain that are necessary for memory formation. As the disease progresses, additional aspects of the brain become affected, and overall brain tissue shrinks significantly (1).

Signs and Symptoms & Diagnosis of Alzheimer’s Disease
Memory problems are typically among the first signs of cognitive impairment related to Alzheimer’s disease. Some people with memory problems have Mild Cognitive Impairment (MCI) (2). In this condition, people have more memory problems than usual for their age; however, their symptoms do not interfere with their daily lives.
Older people with MCI are at increased risk of developing Alzheimer’s disease. The first symptoms of Alzheimer’s may vary from person to person. Many people display a decline in non-memory-related aspects of cognition, such as word-finding, visual issues, impaired judgment, or reasoning (2).
Healthcare providers use several methods and tools to determine the diagnosis of Alzheimer’s Dementia. Diagnosis and evaluation involve memory, problem-solving, attention, counting, and language tests. Healthcare providers may perform brain scans, including CVT. MRI or PET is used to rule out other causes of symptoms.
Various tests may be repeated to give doctors information about how memory and cognitive functions change over time. They can help diagnose different causes of memory problems, such as stroke, tumors, Parkinson’s disease, and vascular dementia. Alzheimer’s disease can be diagnosed only after death by linking clinical measures with an examination of brain tissue in an autopsy (3).

Self-Quiz
Ask Yourself...
- Have you experienced a patient in your practice with dementia or Alzheimer’s disease? What did their symptoms look like?
- What standard diagnostic tools do healthcare providers use to diagnose this disease?
- What is the definitive diagnosis of Alzheimer’s disease?
Stages of Disease
Mild Alzheimer’s
People experience significant memory loss and other cognitive problems as the disease progresses. Most people are diagnosed in this stage (1).
- Wandering/getting lost
- Trouble handling money or paying bills
- Repeating questions
- Taking longer to complete basic daily tasks
- Personality/behavioral changes (1)
Moderate Alzheimer’s
In this stage, damage occurs in the area of the brain that controls language, reasoning, sensory processing, and conscious thought (1).
- Memory and confusion worsen.
- Problems recognizing family and friends
- Unable to learn new things
- Trouble with multi-step tasks such as getting dressed
- Trouble coping with situations
- Hallucinations/delusions/paranoia (1)
Severe Alzheimer’s
- Plaques and tangles spread throughout the brain, and brain tissue shrinks significantly.
- Cannot communicate
- Entirely dependent on others for care
- Bedridden – most often as the body shuts down

Self-Quiz
Ask Yourself...
- What are some of the signs and symptoms that differentiate each stage of Alzheimer’s disease?
- A person is in what stage of Alzheimer’s disease when they struggle to recognize family members and friends?
Prevention
Many aging patients worry about developing Alzheimer’s disease and dementia. Especially if they have had a family member who suffered from the disease, patients may worry about genetic risk. Although there have been many ongoing studies on the prevention of the disease, nothing has been proven to prevent or delay dementia caused by Alzheimer’s disease (2).
More research suggests that women are more likely to develop dementia and Alzheimer’s compared to men. Further research is needed to determine the role between genetics, sex, and Alzheimer’s risk (4).
A review led by experts from the National Academies of Sciences, Engineering, and Medicine found encouraging yet inconclusive evidence for three types of interventions related to ways to prevent or delay Alzheimer’s Dementia or age-related cognitive decline (2):
- Increased physical activity
- Blood pressure control
- Cognitive training

Treatment of the Disease
Alzheimer’s disease is complex and is continuously being studied. Current treatment approaches focus on helping people maintain their mental function, manage behavioral symptoms, and lower the severity of symptoms. The FDA has approved several prescription drugs to treat those diagnosed with Alzheimer’s (3).
Treating symptoms of Alzheimer’s can provide patients with comfort, dignity, and independence for a more significant amount of time while simultaneously assisting their caregivers. The approved medications are most beneficial in the early or middle stages of the disease (3).
Cholinesterase inhibitors are prescribed for mild to moderate Alzheimer’s disease; they may help to reduce symptoms. Medications include Rzadyne®, Exelon®, and Aricept® (3). Scientists do not fully understand how cholinesterase inhibitors work to treat the disease; however, research indicates that they prevent acetylcholine breakdown. Acetylcholine is a brain chemical believed to help memory and thinking (3).
For those suffering from moderate to severe Alzheimer’s disease, a medication known as Namenda®, which is an N-methyl D-aspartate (NMDA) antagonist, can be prescribed. This drug helps to decrease symptoms, allowing some people to maintain certain essential daily functions slightly longer than they would without medication (3).
For example, this medication could help a person in the later stage of the disease maintain their ability to use the bathroom independently for several more months, benefiting the patient and the caregiver (3). This drug works by regulating glutamate, an essential chemical in the brain. When it is produced in large amounts, glutamate may lead to brain cell death. Because NMDA antagonists work differently from cholinesterase inhibitors, these rugs can be prescribed in combination (3).

Self-Quiz
Ask Yourself...
- Is there a cure for this disease?
- What are some of the treatment forms that have been used for the management of Alzheimer’s disease?
- Can medications be used in conjunction with one another to treat the disease?
Medications to be Used with Caution in those Diagnosed with Alzheimer’s
Some medications, such as sleep aids, anxiety medications, anticonvulsants, and antipsychotics, should only be taken by a patient diagnosed with Alzheimer’s after the prescriber has explained the risks and side effects of the medications (3).
Sleep aids: They help people get to sleep and stay asleep. People with Alzheimer’s should not take these drugs regularly because they could make the person more confused and at a higher risk for falls.
Anti-anxiety: These treat agitation and can cause sleepiness, dizziness, falls, and confusion (3).
Anticonvulsants: These are used to treat severe aggression and have possible side effects of mood changes, confusion, drowsiness, and loss of balance.
Antipsychotics: they are used to treat paranoia, hallucinations, agitation, and aggression. Side effects can include the risk of death in older people with dementia. They would only be given when the provider agrees the symptoms are severe enough to justify the risk (3).
Caregiving
Coping with Agitation and Aggression
People with Alzheimer’s disease may become agitated or aggressive as the disease progresses. Agitation causes restlessness and causes someone to be unable to settle down. It may also cause pacing, sleeplessness, or aggression (5). As a caregiver, it is essential to remember that agitation and aggression are usually happening for reasons such as pain, depression, stress, lack of sleep, constipation, soiled underwear, a sudden change in routine, loneliness, and the interaction of medications (5). Look for the signs of aggression and agitation. It is helpful to prevent problems before they happen.
Ways to cope with agitation and aggression (5):
- Reassure the person. Speak calmly. Listen to concerns and frustrations.
- Allow the person to keep as much control as possible.
- Build in quiet times along with activities.
- Keep a routine.
- Try gently touching, soothing music, reading, or walks.
- Reduce noise and clutter.
- Distract with snacks, objects, or activities.
Common Medical Problems
In addition to the symptoms of Alzheimer’s disease, a person with Alzheimer’s may have other medical conditions over time. These additional health conditions can cause confusion and behavior changes. The person may be unable to communicate with you about their circumstances. As a caregiver, it is essential to watch for various signs of illness and know when to seek medical attention for the person being cared for (6).
Fever
Fever could indicate potential infection, dehydration, heatstroke, or constipation (6).
Flu and Pneumonia
These are easily transmissible. Patients 65 years or older should get the flu and Pneumonia shot each year. Flu and Pneumonia may cause fever, chills, aches, vomiting, coughing, or trouble breathing (6).
Falls
As the disease progresses, the person may have trouble with balance and ambulation. They may also have changes in depth perception. To reduce the chance of falls, clean up clutter, remove throw rugs, use armchairs, and use good lighting inside (6).
Dehydration
It is important to remember to ensure the person gets enough fluid. Signs of dehydration include dry mouth, dizziness, hallucinations, and rapid heart rate (6).
Wandering
Many people with Alzheimer’s disease wander away from their homes or caregivers. As the caregiver, it is essential to know how to limit wandering and prevent the person from becoming lost (7).
Steps to follow before a person wanders (7)
- Ensure the person carries an ID or wears a medical bracelet.
- Consider enrolling the person in the Medic Alert® + Alzheimer’s Association Safe Return Program®.
- Alert neighbors and local police that the person tends to wander and ask them to alert you immediately if they are seen alone.
- Place labels on garments to aid in identification.
Tips to Prevent Wandering (7)
- Keep doors locked. Consider a key or deadbolt.
- Use loosely fitting doorknob covers or safety devices.
- Place STOP, DO NOT ENTER< or CLOSED signs on doors.
- Divert the attention of the person away from using the door.
- Install a door chime that will alert when the door is opened.
- Keep shoes, keys, suitcases, coats, and hats out of sight.
- Make sure not to leave a person who has a history of wandering unattended.

Self-Quiz
Ask Yourself...
- What are the basic implementations you can make as a caregiver to make handling confusion and aggression easier in a patient with Alzheimer’s?
- What are some of the types of medical problems that people with Alzheimer’s may face, and how can they be monitored for prevention?
Conclusion
Alzheimer’s is a sad, debilitating, progressive disease that robs patients of their lives and dignity. As research continues on the causes, treatment, and prevention of the disease, healthcare workers and caregivers need to know the signs and symptoms of a patient with Alzheimer’s disease and potential coping mechanisms and management strategies of the disease. More information on the disease is available through several various resources, including:
Family Caregiver Alliance
800-445-8106
NIA Alzheimer’s and Related Dementias Education and Referral Center
800-438-4380
Controlled Substances
Introduction
Pain is complex and subjective. The experience of pain can significantly impact an individual’s quality of life. According to the National Institute of Health (NIH) (40), pain is the most common complaint in a primary care office, with 20% of all patients reporting pain. Chronic pain is the leading cause of disability, and effective pain management is crucial to health and well-being, particularly when it improves functional ability. Effective pain treatment starts with a comprehensive, empathic assessment and a desire to listen and understand. Nurse Practitioners are well-positioned to fill a vital role in providing comprehensive and empathic patient care, including pain management (23).
While the incidence of chronic pain has remained a significant problem, how clinicians manage pain has significantly changed in the last decade, primarily due to the opioid epidemic. This education aims to discuss pain and the assessment of pain, federal guidelines for prescribing, the opioid epidemic, addiction and diversion, and recommendations for managing pain.
Definition of Pain
Understanding the definition of pain, differentiating between various types of pain, and recognizing the descriptors patients use to communicate their pain experiences are essential for Nurse practitioners involved in pain management. By understanding the medical definition of pain and how individuals may communicate it, nurse practitioners can differentiate varying types of pain to target assessment.
According to the International Association for the Study of Pain (27), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or terms of described such in damage.” The IASP, in July 2020, expanded its definition of pain to include context further.
Their expansion is summarized below:
- Pain is a personal experience influenced by biological, psychological, and social factors.
- Pain cannot be inferred solely from activity in sensory neurons.
- Individuals learn the concept of pain through their life experiences.
- A person’s report of an experience in pain should be respected.
- Pain usually serves an adaptive role but may adversely affect function and social and psychological well-being.
- The inability to communicate does not negate the possibility of the experience of pain.


Self Quiz
Ask yourself...
- Analyze how changes to the definition of pain may affect your practice.
- Discuss how you manage appointment times, knowing that 20% of your scheduled patients may seek pain treatment.
- How does the approach to pain management change in the presence of a person with a disability?
Types of Pain
Pain originates from different mechanisms, causes, and areas of the body. As a nurse practitioner, understanding the type of pain a patient is experiencing is essential for several reasons (23).
- Determining an accurate diagnosis. This kind of pain can provide valuable clues to the underlying cause or condition.
- Creating a treatment plan. Different types of pain respond better to specific treatments or interventions.
- Developing patient education. A nurse practitioner can provide targeted education to patients about their condition, why they may experience the pain as they do, its causes, and treatment options. Improving the patient's knowledge and control over their condition improves outcomes.
Acute Pain
Acute pain is typically short-lived and is a protective response to an injury or illness. Patients are usually able to identify the cause. This type of pain resolves as the underlying condition improves or heals (12).
Chronic Pain
Chronic pain is diagnosed when it continues beyond the expected healing time. Pain is defined as chronic when it persists for longer than three months. It may result from an underlying disease or injury or develop without a clear cause. Chronic pain often significantly impacts a person's physical and emotional well-being, requiring long-term management strategies. The prolonged experience of chronic pain usually indicates a central nervous system component of pain that may require additional treatment. Patients with centralized pain often experience allodynia or hyperalgesia (12).
Allodynia is pain evoked by a stimulus that usually does not cause pain, such as a light touch. Hyperalgesia is the effect of a heightened pain response to a stimulus that usually evokes pain (12).
Nociceptive Pain
Nociceptive pain arises from activating peripheral nociceptors, specialized nerve endings that respond to noxious stimuli. This type of pain is typically associated with tissue damage or inflammation and is further classified into somatic and visceral pain subtypes.
Somatic pain is most common and occurs in muscles, skin, or bones; patients may describe it as sharp, aching, stiffness, or throbbing.
Visceral pain occurs in the internal organs, such as indigestion or bowel spasms. It is more vague than somatic pain; patients may describe it as deep, gnawing, twisting, or dull (12).
Neuropathic pain
Neuropathic pain is a lesion or disease of the somatosensory nervous system. Examples include trigeminal neuralgia, painful polyneuropathy, postherpetic neuralgia, and central poststroke pain (10).
Neuropathic pain may be ongoing, intermittent, or spontaneous pain. Patients often describe neuropathic pain as burning, prickling, or squeezing quality. Neuropathic pain is a common chronic pain. Patients commonly describe allodynia and hyperalgesia as part of their chronic pain experience (10).
Affective pain
Affective descriptors reflect the emotional aspects of pain and include terms like distressing, unbearable, depressing, or frightening. These descriptors provide insights into the emotional impact of pain on an individual's well-being (12).

Self Quiz
Ask yourself...
- How can nurse practitioners effectively elicit patient descriptors to accurately assess the type of pain the patient is experiencing?
- Expand on how pain descriptors can guide interventions even if the cause is not yet determined.
- What strategies ensure patients feel comfortable describing their pain, particularly regarding subjective elements such as quality and location?
Case Study
Mary Adams is a licensed practical nurse who has just relocated to town. Mary will be the utilization review nurse at a local long-term care facility. Mary was diagnosed with Postherpetic Neuralgia last year, and she is happy that her new job will have her mostly doing desk work and not providing direct patient care as she had been before the relocation. Mary was having difficulty at work at her previous employer due to pain. She called into work several times, and before leaving, Mary's supervisor had counseled her because of her absences.
Mary wants to establish primary care immediately because she needs ongoing pain treatment. She is hopeful that, with her new job and pain under control, she will be able to continue a successful career in nursing. When Mary called the primary care office, she specifically requested a nurse practitioner as her primary care provider because she believes that nurse practitioners tend to spend more time with their patients.
Assessment
The assessment effectively determines the type of treatment needed, the options for treatment, and whether the patient may be at risk for opioid dependence. Since we know that chronic pain can lead to disability and pain has a high potential to negatively affect the patient's ability to work or otherwise, be productive, perform self-care, and potentially impact family or caregivers, it is imperative to approach the assessment with curiosity and empathy. This approach will ensure a thorough review of pain and research on pain management options. Compassion and support alone can improve patient outcomes related to pain management (23).
Record Review
Regardless of familiarity with the patient, reviewing the patient's treatment records is essential, as the ability to recall details is unreliable. Reviewing the records can help identify subtle changes in pain description and site, the patient's story around pain, failed modalities, side effects, and the need for education, all impacting further treatment (23).
Research beforehand the patient's current prescription and whether or not the patient has achieved the maximum dosage of the medication. Analysis of the patient's past prescription could reveal a documented failed therapy even though the patient did not receive the maximum dose (23).
A review of documented allergens may indicate an allergy to pain medication. Discuss with the patient the specific response to the drug to determine if it is a true allergy, such as hives or anaphylaxis, or if the response may have been a side effect, such as nausea and vomiting.
Research whether the patient tried any non-medication modalities for pain, such as physical therapy (PT), occupational therapy (OT), or Cognitive Behavioral Therapy (CBT). Note any non-medication modalities documented as failed therapies. The presence of any failed therapies should prompt further discussion with the patient, family, or caregiver about the experience. The incompletion of therapy should not be considered failed therapy. Explore further if the patient abandoned appointments.
Case Study
You review the schedule for the week, and there are three new patient appointments. One is Mary Adams. The interdisciplinary team requested and received Mary's treatment records from her previous primary care provider. You make 15 minutes available to review Mary's records and the questionnaire Mary filled out for her upcoming appointment. You see that Mary has been diagnosed with Postherpetic Neuralgia and note her current treatment regimen, which she stated was ineffective. You write down questions you will want to ask Mary. You do not see evidence of non-medication modalities or allergies to pain medication.

Self Quiz
Ask yourself...
- What potential risks or complications can arise from neglecting to conduct a thorough chart review before initiating a pain management assessment?
- In your experience, what evidence supports reviewing known patient records?
- What is an alternative to reviewing past treatment if records are not available?
Pain Assessment
To physically assess pain, several acronyms help explore all the aspects of the patient's experience. Acronyms commonly used to assess pain are SOCRATES, OLDCARTS, and COLDERAS. These pain assessment acronyms are also helpful in determining treatment since they include a character and duration of pain assessment (23).
| O-Onset | S-Site | C-Character |
| L-Location | O-Onset | O-Onset |
| D-Duration | C-Character | L-Location |
| C-Character | R-Radiate | D-Duration |
| A-Alleviating | A-Associated symptoms | E-Exacerbating symptoms |
| R-Radiating, relieving | T-Time/Duration | R-Relieving, radiating |
| T-Temporal patterns (frequency) | E-Exacerbating | A-Associated symptoms |
| S-Symptoms | S-Severity | S-Severity of illness |
Inquire where the patient is feeling pain. The patient may have multiple areas and types of pain. Each type and location must be explored and assessed. Unless the pain is from a localized injury, a body diagram map, as seen below, is helpful to document, inform, and communicate locations and types of pain. In cases of Fibromyalgia, rheumatoid arthritis, or other centralized or widespread pain, it is vital to inquire about radiating pain. The patient with chronic pain could be experiencing acute pain or a new pain site, such as osteoarthritis, that may need further evaluation and treatment (23).
Inquire with the patient how long their pain has been present and any associated or known causative factors. Pain experienced longer than three months defines chronic versus acute pain. Chronic pain means that the pain is centralized or a function of the Central Nervous system, which should guide treatment decisions.
To help guide treatment, ask the patient to describe their pain. The description helps identify what type of pain the patient is experiencing: Allodynia and hyperalgesia indicate centralized pain; sharp, shooting pain could indicate neuropathic pain. Have the patient rate their pain. There are various tools, as shown below, for pain rating depending on the patient's ability to communicate. Not using the pain rating number alone is imperative. Ask the patient to compare the severity of pain to a previous experience. For example, a 1/10 may be experienced as a bumped knee or bruise, whereas a 10/10 is experienced on the level of a kidney stone or childbirth (23).
Besides the 0-10 rating scale and depending on the patient's needs, several pain rating scales are appropriate. They are listed below.
The 0-5 and Faces scales may be used for all adult patients and are especially effective for patients experiencing confusion.
The Defense and Veterans Pain Rating Scale (DVPRS) is a five-item tool that assesses the impact of pain on sleep, mood, stress, and activity levels (20).
For patients unable to self-report pain, such as those intubated in the ICU or late-stage neurological diseases, the FLACC scale is practical. The FLACC scale was initially created to assess pain in infants. Note: The patient need not cry to be rated 10/10.
| Behavior | 0 | 1 | 2 |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent or constant quivering chin, clenched jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn |
| Activity | Lying quietly, in a normal position, or relaxed | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry wake or asleep | Moans or whimpers: occasional complaints | Crying steadily, screams, sobs, frequent complaints |
| Consolability | Content, relaxed | Distractable, reassured by touching, hugging, or being talked to | Difficult to console or comfort |
(21).
Assess contributors to pain such as insomnia, stress, exercise, diet, and any comorbid conditions. Limited access to care, socioeconomic status, and local culture also contribute to the patient's experience of pain (23). Most patients have limited opportunity to discuss these issues, and though challenging to bring up, it is compassionate and supportive care. A referral to social work or another agency may be helpful if you cannot explore it fully.
Assess for substance abuse disorders, especially among male, younger, less educated, or unemployed adults. Substance abuse disorders increase the likelihood of misuse disorder and include alcohol, tobacco, cannabis, cocaine, and heroin (29).
Inquire as to what changes in function the pain has caused. One question to ask is, "Were it not for pain, what would you be doing?" As seen below, a Pain, Enjoyment, and General Activity (PEG) three-question scale, which focuses on function and quality of life, may help determine the severity of pain and the effect of treatment over time.
| What number best describes your pain on average in the past week? 0-10 |
| What number best describes how, in the past week, pain has interfered with your enjoyment of life? 0-10 |
| What number determines how, in the past week, pain has interfered with your general activity? 0-10 |
(21).
Assess family history, mental health disorders, chronic pain, or substance abuse disorders. Each familial aspect puts patients at higher risk for developing chronic pain (23).
Evaluate for mental health disorders the patient may be experiencing, particularly anxiety and depression. The Patient Health Questionnaire (PHQ4) is a four-question tool for assessing depression and anxiety.
In some cases, functional MRI or imaging studies effectively determine the cause of pain and the treatment. If further assessment is needed to diagnose and treat pain, consult Neurology, Orthopedics, Palliative care, and pain specialists (23).
Case Study
You used OLDCARTS to evaluate Mary's pain and completed a body diagram. Mary is experiencing allodynia in her back and shoulders, described as burning and tingling. It is exacerbated when she lifts, such as moving patients at the long-term care facility and, more recently, boxes from her move to the new house. Mary has also been experiencing anxiety due to fear of losing her job, the move, and her new role. She has moved closer to her family to help care for her children since she often experiences fatigue. Mary has experienced a tumultuous divorce in the last five years and feels she is still undergoing some trauma.
You saw in the chart that Mary had tried Gabapentin 300 mg BID for her pain and inquired what happened. Mary explained that her pain improved from 8/10 to 7/10 and had no side effects. Her previous care provider discontinued the medication and documented it as a failed therapy. You reviewed the minimum and maximum dosages of Gabapentin and know Mary can take up to 1800mg/day.
During the assessment, Mary also described stiffness and aching in her left knee. She gets a sharp pain when she walks more than 500 steps, and her knee is throbbing by the end of the day. Mary rated the pain a 10/10, but when she compared 10/10 to childbirth, Mary said her pain was closer to 6/10. Her moderate knee pain has reduced Mary's ability to exercise. She used to like to take walks. Mary stated she has had knee pain for six months and has been taking Ibuprofen 3 – 4 times daily.
Since Mary's pain is moderate, you evaluate your options of drugs for moderate to severe pain.


Self Quiz
Ask yourself...
- How do you assess and evaluate a patient's pain level?
- What are the different types of pain and their management strategies?
- How do you determine the appropriate dosage of pain medications for a patient?
- How do you assess the effectiveness of pain medications in your patients?
- How do you adjust medication dosages for elderly patients with pain or addiction?
- How do you address the unique challenges in pain management for pediatric patients?
- What is the role of non-pharmacological interventions in pain management?
- How do you incorporate non-pharmacological interventions into your treatment plans?
Opioid Classifications and Drug Schedules
A comprehensive understanding of drug schedules and opioid classifications is essential for nurse practitioners to ensure patient safety, prevent drug misuse, and adhere to legal and regulatory requirements. Nurse practitioners with a comprehensive understanding of drug schedules and opioid classifications can effectively communicate with colleagues, ensuring accurate medication reconciliation and facilitating interdisciplinary care. Nurse practitioners’ knowledge in facilitating discussions with pharmacists regarding opioid dosing, potential interactions, and patient education is essential (49).
Drug scheduling became mandated under the Controlled Substance Act. The Drug Enforcement Agency (DEA) Schedule of Controlled Drugs and the criteria and common drugs are listed below.
|
Schedule |
Criteria | Examples |
|
I |
No medical use; high addiction potential |
Heroin, marijuana, PCP |
|
II |
Medical use; high addiction potential |
Morphine, oxycodone, Methadone, Fentanyl, amphetamines |
|
III |
Medical use; high addiction potential |
Hydrocodone, codeine, anabolic steroids |
|
IV |
Medical use, low abuse potential |
Benzodiazepines, meprobamate, butorphanol, pentazocine, propoxyphene |
| V | Medical use; low abuse potential |
Buprex, Phenergan with codeine |
(Pain Physician, 2008)
Listed below are drugs classified by their schedule and mechanism of action. "Agonist" indicates a drug that binds to the opioid receptor, causing pain relief and also euphoria. An agonist-antagonist indicates the drug binds to some opioid receptors but blocks others. Mixed antagonist-agonist drugs control pain but have a lower potential for abuse and dependence than agonists (7).
| Schedule I | Schedule II | Schedule III | Schedule IV | Schedule V | |
| Opioid agonists |
BenzomorphineDihydromor-phone, Ketobemidine, Levomoramide, Morphine-methylsulfate, Nicocodeine, Nicomorphine, Racemoramide |
Codeine, Fentanyl, Sublimaze, Hydrocodone, Hydromorphone, Dilaudid, Meperidine, Demerol, Methadone, Morphine, Oxycodone, Endocet, Oxycontin, Percocet, Oxymorphone, Numorphan |
Buprenorphine Buprenex, Subutex, Codeine compounds, Tylenol #3, Hydrocodone compounds, Lortab, Lorcet, Tussionex, Vicodin |
Propoxyphene, Darvon, Darvocet | Opium, Donnagel, Kapectolin |
| Mixed Agonist -Antagonist | BuprenorphineNaloxone, Suboxone |
Pentazocine, Naloxone, Talwin-Nx |
|||
| Stimulants | N-methylampheta-mine 3, 4-methylenedioxy amphetamine, MDMA, Ecstacy | Amphetamine, Adderal, Cocaine, Dextroamphetamine, Dexedrine, Methamphetamine, Desoxyn, Methylphenidate, Concerta, Metadate, Ritalin, Phenmetrazine, Fastin, Preludin | Benapheta-mine, Didrex, Pemolin, Cylert, Phendimetra-zine, Plegine | Diethylpropion, Tenuate, Fenfluramine, Phentermine Fastin | 1-dioxy-ephedrine-Vicks Inhaler |
| Hallucinogen-gens, other | Lysergic Acid Diamine LSD, marijuana, Mescaline, Peyote, Phencyclidine PCP, Psilocybin, Tetrahydro-cannabinol | Dronabinol, Marinol | |||
| Sedative Hypnotics |
Methylqualine, Quaalude, Gamma-hydroxy butyrate, GHB
|
Amobarbitol, Amytal, Glutethamide, Doriden, Pentobarbital, Nembutal, Secobarbital, Seconal |
Butibarbital. Butisol, Butilbital, Florecet, Florinal, Methylprylon, Noludar |
Alprazolam, Xanax, Chlordiazepoxide, Librium, Chloral betaine, Chloral hydrate, Noctec, Chlorazepam, Clonazepam, Klonopin, Clorazopate, Tranxene, Diazepam, Valium, Estazolam, Prosom, Ethchlorvynol, Placidyl, Ethinamate, Flurazepam, Dalmane, Halazepam, Paxipam, Lorazepam, Ativan, Mazindol, Sanorex, Mephobarbital, Mebaral, Meprobamate, Equanil, Methohexital, Brevital Sodium, Methyl-phenobarbital, Midazolam, Versed, Oxazepam, Serax, Paraldehyde, Paral, Phenobarbital, Luminal, Prazepam, Centrax, Temazepam, Restoril, Triazolam, Halcion, Sonata, Zolpidem, Ambien |
Diphenoxylate preparations, Lomotil |
(41).

Self Quiz
Ask yourself...
- What are the potential risks and benefits of using opioids for pain management?
- How can nurse practitioners effectively monitor patients on long-term opioid therapy?
- What are the potential risks and benefits of using long-acting opioids for chronic pain?
- How do you monitor patients on long-acting opioids for safety and efficacy?
Commonly Prescribed Opioids, Indications for Use, and Typical Side Effects
Opioid medications are widely used for managing moderate to severe pain. Referencing NIDA (2023), this section aims to give healthcare professionals an overview of the indications and typical side effects of commonly prescribed Schedule II opioid medications, including hydrocodone, oxycodone, morphine, Fentanyl, and hydromorphone.
Opioids are derived and manufactured in several ways. Naturally occurring opioids come directly from the opium poppy plant. Synthetic opioids are manufactured by chemically synthesizing compounds that mimic the effects of a natural opioid. Semi-synthetic is a mix of naturally occurring and man-made (35).
Understanding the variations in how an opioid is derived and manufactured is crucial in deciding the type of opioid prescribed, as potency and analgesic effects differ. Synthetic opioids are often more potent than naturally occurring opioids. Synthetic opioids have a longer half-life and slower elimination, affecting the duration of action and timing for dose adjustments. They are also associated with a higher risk of abuse and addiction (38).
Hydrocodone
Mechanism of Action and Metabolism
Hydrocodone is a Schedule II medication. It is an opioid agonist and works as an analgesic by activating mu and kappa opioid receptors located in the central nervous system and the enteric plexus of the bowel. Agonist stimulation of the opioid receptors inhibits nociceptive neurotransmitters' release and reduces neuronal excitability (17).
- Produces analgesia.
- Suppresses the cough reflex at the medulla.
- Causes respiratory depression at higher doses.
Hydrocodone is indicated for treating severe pain after nonopioid therapy has failed. It is also indicated as an antitussive for nonproductive cough in adults over 18.
Available Forms
Hydrocodone immediate release (IR) reaches maximum serum concentrations in one hour with a half-life of 4 hours. Extended-release (ER) Hydrocodone reaches peak concentration at 14-16 hours and a half-life of 7 to 9 hours. Hydrocodone is metabolized to an inactive metabolite in the liver by cytochrome P450 enzymes CYP2D6 and CYP3A4. Hydrocodone is converted to hydromorphone and is excreted renally. Plasma concentrations of hydromorphone are correlated with analgesic effects rather than hydrocodone.
Hydrocodone is formulated for oral administration into tablets, capsules, and oral solutions. Capsules and tablets should never be crushed, chewed, or dissolved. These actions convert the extended-release dose into immediate release, resulting in uncontrolled and rapid release of opioids and possible overdose.
Dosing and Monitoring
Hydrocodone IR is combined with acetaminophen or ibuprofen. The dosage range is 2.5mg to 10mg every 4 to 6 hours. If formulated with acetaminophen, the dosage is limited to 4gm/day.
Hydrocodone ER is available as tablets and capsules. Depending on the product, the dose of hydrocodone ER formulations in opioid-naïve patients is 10 to 20 mg every 12 to 24 hours.
Nurse practitioners should ensure patients discontinue all other opioids when starting the extended-release formula.
Side Effects and Contraindications
Because mu and kappa opioid receptors are in the central nervous system and enteric plexus of the bowel, the most common side effects of hydrocodone are constipation and nausea (>10%).
Other adverse effects of hydrocodone include:
- Respiratory: severe respiratory depression, shortness of breath
- Cardiovascular: hypotension, bradycardia, peripheral edema
- Neurologic: Headache, chills, anxiety, sedation, insomnia, dizziness, drowsiness, fatigue
- Dermatologic: Pruritus, diaphoresis, rash
- Gastrointestinal: Vomiting, dyspepsia, gastroenteritis, abdominal pain
- Genitourinary: Urinary tract infection, urinary retention
- Otic: Tinnitus, sensorineural hearing loss
- Endocrine: Secondary adrenal insufficiency (17)
Hydrocodone, being an agonist, must not be taken with other central nervous system depressants as sedation and respiratory depression can result. In formulations combined with acetaminophen, hydrocodone can increase the international normalized ratio (INR) and cause bleeding. Medications that induce or inhibit cytochrome enzymes can lead to wide variations in absorption.
The most common drug interactions are listed below:
- Alcohol
- Benzodiazepines
- Barbiturates
- other opioids
- rifampin
- phenytoin
- carbamazepine
- cimetidine,
- fluoxetine
- ritonavir
- erythromycin
- diltiazem
- ketoconazole
- verapamil
- Phenytoin
- John’s Wort
- Glucocorticoids
Considerations
Use with caution in the following:
- Patients with Hepatic Impairment: Initiate 50% of the usual dose
- Patients with Renal Impairment: Initiate 50% of the usual dose
- Pregnancy: While not contraindicated, the FDA issued a black-boxed warning since opioids cross the placenta, and prolonged use during pregnancy may cause neonatal opioid withdrawal syndrome (NOWS).
- Breastfeeding: Infants are susceptible to low dosages of opioids. Non-opioid analgesics are preferred.
Pharmacogenomic: Genetic variants in hydrocodone metabolism include ultra-rapid, extensive, and poor metabolizer phenotypes. After administration of hydrocodone, hydromorphone levels in rapid metabolizers are significantly higher than in poor metabolizers.
Oxycodone
Mechanism of Action and Metabolism
Oxycodone has been in use since 1917 and is derived from Thebaine. It is a semi-synthetic opioid analgesic that works by binding to mu-opioid receptors in the central nervous system. It primarily acts as an agonist, producing analgesic effects by inhibiting the transmission of pain signals (Altman, Clark, Huddart, & Klein, 2018).
Oxycodone is primarily metabolized in the liver by CYP3A4/5. It is metabolized in the liver to noroxycodone and oxymorphone. The metabolite oxymorphone also has an analgesic effect and does not inhibit CYP3A4/5. Because of this metabolite, oxycodone is more potent than morphine, with fewer side effects and less drug interactions. Approximately 72% of oxycodone is excreted in urine (Altman, Clark, Huddart, & Klein, 2018).
Available Forms
Oxycodone can be administered orally, rectally, intravenously, and as an epidural. For this sake, we will focus on immediate-release and extended-release oral formulations.
- Immediate-release (IR) tablets
- IR capsules
- IR oral solutions
- Extended-release (ER) tablets
Dosing and Monitoring
The dosing of oxycodone should be individualized based on the patient's pain severity, previous opioid exposure, and response. Initial dosages for opioid naïve patients range from 5-15 mg for immediate-release formulations, while extended-release formulations are usually initiated at 10-20 mg. Dosage adjustments may be necessary based on the patient's response, but caution should be exercised. IR and ER formulations reach a steady state at 24 hours and titrating before 24 hours may lead to overdose.
Regular monitoring is essential to assess the patient's response to treatment, including pain relief, side effects, and signs of opioid misuse or addiction. Monitoring should include periodic reassessment of pain intensity, functional status, and adverse effects (Altman, Clark, Huddart, & Klein, 2018).
Side Effects and Contraindications
Common side effects of oxycodone include:
- constipation
- nausea
- sedation
- dizziness
- respiratory depression
- respiratory arrest
- hypotension
- fatal overdose
Oxycodone is contraindicated in patients with known hypersensitivity to opioids, severe respiratory depression, paralytic ileus, or acute or severe bronchial asthma. It should be used cautiously in patients with a history of substance abuse, respiratory conditions, liver or kidney impairment, and those taking other medications that may interact with opioids, such as alcohol (4).
It is also contraindicated with the following medications and classes:
- Antifungal agents
- Antibiotics
- Rifampin
- Carbamazepine
- Fluoxetine
- Paroxetine
Considerations
- Nurse practitioners should consider the variations in the mechanism of action for the following:
- Metabolism differs between males and females: females have been shown to have less concentration of oxymorphone and more CYP3A4/5 metabolites.
- Infants have reduced clearance of oxycodone, increasing side effects.
- Pediatrics have 20-40% increased clearance over adults.
- Reduced clearance with age increases the half-life of oxycodone.
- Pregnant women have a greater clearance and reduced half-life.
- Impairment of the liver reduces clearance.
- Cancer patients with cachexia have increased exposure to oxycodone and its metabolite.
- Maternal and neonate concentrations are similar, indicating placenta crossing (4)
Morphine

Mechanism of Action and Metabolism
Morphine is a naturally occurring opioid alkaloid extracted from the opium poppy. It was isolated in 1805 and is the opioid against which all others are compared. Morphine binds to mu-opioid receptors in the brain and spinal cord, inhibiting the transmission of pain signals and producing analgesia. It is a first-line choice of opioid for moderate to severe acute, postoperative, and cancer-related pain (8).
Morphine undergoes first-pass metabolism in the liver and gut. It is well absorbed and distributed throughout the body. Its main metabolites are morphine-3-glucuronide and morphine-6-glucuronide. Its mean plasma elimination half-life after intravenous administration is about 2 hours. Approximately 90% of morphine is excreted in the urine within 24 hours (8).
Available Forms
Morphine is available in various forms, including.
- immediate-release tablets
- extended release tablets
- oral IR solutions
- injectable solutions
- transdermal patches
Dosing and Monitoring
Morphine is hydrophilic and, as such, has a slow onset time. The advantage of this is that it is unlikely to cause acute respiratory depression even when injected. However, because of the slow onset time, there is more likelihood of morphine overdose due to the ability to “stack” doses in patients experiencing severe pain (Bistas, Lopez-Ojeda, & Ramos-Matos, 2023).
The dosing of morphine depends on the patient's pain severity, previous opioid exposure, and other factors. It is usually initiated at a low dose and titrated upwards as needed. Monitoring pain relief, adverse effects, and signs of opioid toxicity is crucial. Reevaluate benefits and harms with patients within 1 to 4 weeks of starting opioid therapy or of dose escalation. General recommendations for initiating morphine (Bistas, Lopez-Ojeda, & Ramos-Matos, 2023).
Prescribe IR opioids instead of ER opioids.
Prescribe the lowest effective dosage, below 50 Morphine Milligram Equivalents (MME) /day.
Side Effects and Contraindications
Because morphine binds to opioid receptors in the brain and spinal cord, is metabolized in the liver and gut, and has a slow onset, the following side effects are common:
- Constipation
- Nausea
- Vomiting
- Sedation
- Dizziness
- Respiratory depression
- Pruritis
- Sweating
- Dysphoria/Euphoria
- Dry mouth
- Anorexia
- Spasms of urinary and biliary tract
Contraindications of morphine are:
- Known hypersensitivity or allergy to morphine.
- Bronchial asthma or upper airway obstruction
- Respiratory depression in the absence of resuscitative equipment
- Paralytic ileus
- Risk of choking in patients with dysphagia, including infants, children, and the elderly (8)
Concurrent use with other sedating medications: Amitriptyline, diazepam, haloperidol, chlorpromazine
Morphine interacts with the following medications:
- Ciprofloxacin
- Metoclopramide
- Ritonavir
Considerations for Nurse Practitioners
Assess for medical conditions that may pose serious and life-threatening risks with opioid use, such as the following:
- Sleep-disordered breathing, such as sleep apnea.
- Pregnancy
- Renal or hepatic insufficiency
- Age >= 65
- Certain mental health conditions
- Substance use disorder
- Previous nonfatal overdose
Fentanyl
Mechanism of Action and Metabolism
Fentanyl is a synthetic opioid more potent than morphine and was approved in 1968. Fentanyl is an agonist that works by binding to the mu-opioid receptors in the central nervous system. This binding inhibits the transmission of pain signals, resulting in analgesia. Fentanyl is often used for severe pain management, particularly in the perioperative and palliative care settings, or for severe pain in patients with Hepatic failure (8).
It is a mu-selective opioid agonist. However, it can activate other opioid receptors in the body, such as the delta and kappa receptors, producing analgesia. It also activates the Dopamine center of the brain, stimulating relaxation and exhilaration, which is responsible for its high potential for addiction (8).
Indications for fentanyl are as follows:
- Preoperative analgesia
- Anesthesia adjunct
- Regional anesthesia adjunct
- General anesthesia
- Postoperative pain control
- Moderate to severe acute pain (off-label)
Available Forms
- Fentanyl is available in various forms, including:
- transdermal patches
- injectable solutions
- lozenges
- nasal sprays
- oral tablets (8)
Dosing and Monitoring
Fentanyl is metabolized via the CYP3A4 enzyme in the liver. It has a half-life of 3 to 7 hours, and 75% of Fentanyl is excreted in the urine and 9% in feces.
The dosing of fentanyl depends on the route of administration and the patient's needs. For example, transdermal patches are typically applied every 72 hours, while injectable solutions are titrated to achieve the desired analgesic effect. Monitoring should include assessing pain levels, respiratory rate, blood pressure, and sedation scores (8).
Fentanyl is most dosed as follows:
- Post-operative pain control
- 50 to 100 mcg IV/IM every 1 to 2 hours as needed; alternately 0.5 to 1.5 mcg/kg/hour IV as needed. Consider lower dosing in patients 65 and older.
PCA (patient-controlled analgesia): 10 to 20 mcg IV every 6 to 20 minutes as needed; start at the lowest effective dose for the shortest effective duration - refer to institutional protocols (8).
Moderate to severe acute pain (off-label) 1 to 2 mcg/kg/dose intranasally each hour as needed; the maximum dose is 100 mcg. Use the lowest effective dose for the shortest effective duration (8).
Side Effects and Contraindications
Common side effects of fentanyl include:
- respiratory depression
- sedation
- constipation
- nausea
- vomiting
- euphoria
- confusion
- respiratory depression/arrest
- visual disturbances
- dyskinesia
- hallucinations
- delirium
- narcotic ileus
- muscle rigidity
- addiction
- loss of consciousness
- hypotension
- coma
- death (8).
The use of fentanyl is contraindicated in patients in the following situations:
- After operative interventions in the biliary tract, these may slow hepatic elimination of the drug.
- With respiratory depression or obstructive airway diseases (i.e., asthma, COPD, obstructive sleep apnea, obesity hyperventilation, also known as Pickwickian syndrome)
- With liver failure
- With known intolerance to fentanyl or other morphine-like drugs, including codeine or any components in the formulation.
- With known hypersensitivity (i.e., anaphylaxis) or any common drug delivery excipients (i.e., sodium chloride, sodium hydroxide) (8).
Considerations for Nurse Practitioners
Nurse practitioners prescribing fentanyl should thoroughly assess the patient's pain, medical history, and potential risk factors for opioid misuse. They should also educate patients about the proper use, storage, and disposal of fentanyl. It should be used cautiously in patients with respiratory disorders, liver or kidney impairment, or a history of substance abuse. Fentanyl is contraindicated in patients with known hypersensitivity to opioids and those without exposure to opioids.
Alcohol and other drugs, legal or illegal, can exacerbate fentanyl's side effects, creating multi-layered clinical scenarios that can be complex to manage. These substances, taken together, generate undesirable conditions that complicate the patient's prognosis (8).
Hydromorphone
Mechanism of Action and Metabolism
Hydromorphone is a semi-synthetic opioid derived from morphine. It binds to the mu-opioid receptors in the central nervous system. It primarily exerts its analgesic effects by inhibiting the release of neurotransmitters involved in pain transmission, thereby reducing pain perception. Hydromorphone also exerts its effects centrally at the medulla level, leading to respiratory depression and cough suppression (1).
Hydromorphone is indicated for:
- moderate to severe acute pain
- severe chronic pain
- refractory cough suppression (off-label) (1)
Available Forms
Hydromorphone is available in various forms, depending on the patient’s needs and severity of pain.
- immediate-release tablet
- extended release tablets
- oral liquid
- injectable solution
- rectal suppositories
Dosing and Monitoring
The immediate-release oral formulations of hydromorphone have an onset of action within 15 to 30 minutes. Peak levels are typically between 30 and 60 minutes with a half-life of 2 to 3 hours. Hydromorphone is primarily excreted through the urine.
The dosing of hydromorphone should be individualized based on the patient's pain intensity, initiated at the lowest effective dose, and adjusted gradually as needed. Close monitoring of pain relief, adverse effects, and signs of opioid toxicity is essential. Patients should be assessed regularly to ensure they receive adequate pain control without experiencing excessive sedation or respiratory depression.
The following are standard dosages that should only be administered when other opioid and non-opioid options fail.
- Immediate-release oral solutions dosage: 1 mg/1 mLoral tablets are available in 2 mg, 4 mg, and 8 mg.
- Extended-release oral tablets are available in dosages of 8 mg, 12 mg, 16 mg, and 32 mg.
- Injection solutions are available in concentrations of 1 mg/mL, 2 mg/mL, 4 mg/mL, and 10 mg/mL.
- Intravenous solutions are available in strengths of 2 mg/1 mL, 2500 mg/250 mL, ten mg/1 mL, and 500 mg/50 mL.
- Suppositories are formulated at a strength of 3 mg (1).
Side Effects and Contraindications
Hydromorphone has potential adverse effects on several organ systems, including the integumentary, gastrointestinal, neurologic, cardiovascular, endocrine, and respiratory.
Common side effects of hydromorphone include:
- Constipation
- Nausea
- Vomiting
- Dizziness
- Sedation
- respiratory depression
- pruritus
- headache
- Somnolence
- Severe adverse effects of hydromorphone include:
- Hypotension
- Syncope
- adrenal insufficiency
- coma
- raised intracranial pressure.
- seizure
- suicidal thoughts
- apnea
- respiratory depression or arrest
- drug dependence or withdrawal
- neonatal drug withdrawal syndrome
- Hydromorphone is contraindicated in patients with:
- known allergies to the drug, sulfites, or other components of the formulation.
- known hypersensitivity to opioids.
- severe respiratory depression
- paralytic ileus
- acute or severe bronchial asthma (1).
Caution should be exercised in patients with:
- respiratory insufficiency
- head injuries
- increased intracranial pressure.
- liver or kidney impairment.
Considerations for Nurse Practitioners
As nurse practitioners, it is crucial to assess the patient's pain intensity and overall health status before initiating Hydromorphone. Start with the lowest effective dose and titrate carefully for optimal pain control. Regular monitoring for adverse effects, signs of opioid toxicity, and therapeutic response is essential. Educate patients about the potential side effects, proper dosing, and the importance of not exceeding prescribed doses. Additionally, nurse practitioners should be familiar with local regulations and guidelines regarding opioid prescribing and follow appropriate documentation and monitoring practices.
Additional Considerations
In terminal cancer patients, clinicians should not restrain opioid therapy even if signs of respiratory depression become apparent.
Hydromorphone requires careful administration in cases of concurrent psychiatric illness.
Specific Patient Considerations:
- Hepatic impairment and Renal Impairment: Initiate hydromorphone treatment at one-fourth to one-half of the standard starting dosage, depending on the degree of impairment.
- Pregnancy considerations: Hydromorphone can traverse the placental barrier and induce NOWS.
- Breastfeeding considerations: Nonopioid analgesic agents are preferable for breastfeeding women.
- Older patients: hydromorphone is categorized as a potentially inappropriate medication for older adults (1).
Tramadol

Mechanism of Action and Metabolism
Tramadol is a Schedule IV opioid medication with a higher potential for dependency and misuse than non-opioid medications. It binds to opioid receptors in the central nervous system, inhibiting the reuptake of norepinephrine and serotonin. It also has weak mu-opioid receptor agonist activity.
The liver metabolizes tramadol mediated by the cytochrome P450 pathways (particularly CYP2D6) and is mainly excreted through the kidneys.
Tramadol is used for moderate to severe pain.
Available Forms of Tramadol include:
- Immediate-release-typically used for acute pain management.
- Extended-release-used for chronic pain.
Dosing and Monitoring
Tramadol has an oral bioavailability of 68% after a single dose and 90–100% after multiple doses and reaches peak concentrations within 2 hours. Approximately 75% of an oral dose is absorbed, and the half-life of tramadol is 9 hours (18).
Tramadol dosing should be individualized based on the patient's pain severity and response.
The initial dose for adults is usually 50-100 mg orally every 4-6 hours for pain relief. The maximum daily dose is 400 mg for immediate-release formulations and 300 mg for extended-release formulations (18).
It is essential to monitor the patient's pain intensity, response to treatment, and any adverse effects. Regular reassessment and adjustment of the dosage may be necessary.
Side Effects and Contraindications
Tramadol is responsible for severe intoxications leading to consciousness disorder (30%), seizures (15%), agitation (10%), and respiratory depression (5%). The reactions to Tramadol suggest that the decision to prescribe should be carefully considered.
Common Side Effects of Tramadol Include:
- Nausea
- Vomiting
- Dizziness
- Constipation
- Sedation
- Headache
- CNS depression
- Seizure
- Agitation
- Tachycardia
- Hypertension
- reduced appetite
- pruritus and rash
- gastric irritation
Serious side effects include:
- respiratory depression
- serotonin syndrome
- seizures
Contraindications
Tramadol is contraindicated in patients with:
- history of hypersensitivity to opioids
- acute intoxication with alcohol
- opioids, or other psychoactive substances
- Patients who have recently received monoamine oxidase inhibitors (MAOIs)
Additionally, the following can be observed in tramadol intoxication:
- miosis
- respiratory depression
- decreased level of consciousness
- hypertension
- tremor
- irritability
- increased deep tendon reflexes
Poisoning leads to:
- multiple organ failure
- coma
- cardiopulmonary arrest
- death
Considerations for Nurse Practitioners
Tramadol has been increasingly misused with intentional overdoses or intoxications. Suicide attempts were the most common cause of intoxication (52–80%), followed by abuse (18–31%), and unintentional intoxication (1–11%). Chronic tramadol or opioid abuse was reported in 20% of tramadol poisoning cases. Fatal tramadol intoxications are uncommon except when ingested concurrent with depressants, most commonly benzodiazepines and alcohol (18).
Tramadol poisoning can affect multiple organ systems:
- gastrointestinal
- central nervous system: seizure, CNS depression, low-grade coma, anxiety, and over time anoxic brain damage
- Cardiovascular system: palpitation, mild hypertension to life-threatening complications such as cardiopulmonary arrest
- respiratory system
- renal system: renal failure with higher doses of tramadol intoxication
- musculoskeletal system: rhabdomyolysis
- endocrine system: hypoglycemia, serotonin syndrome (18)
Cannabis
Mechanism of Action and Metabolism
Cannabis is classified as a Schedule I status. It contains various cannabinoids, with delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) being the most studied. THC primarily acts on cannabinoid receptors in the brain, producing psychoactive effects, while CBD has more diverse effects on the nervous system. These cannabinoids interact with the endocannabinoid system, modulating neurotransmitter release and influencing various physiological processes (32).
Similar to opioids, cannabinoids are synthesized and released in the body by synapses that act on the cannabinoid receptors present in presynaptic endings (32). They perform the following actions related to analgesia:
- Decrease the release of neurotransmitters.
- Activate descending inhibitory pain pathways.
- Reduce postsynaptic sensitivity and alleviate neural inflammation.
- Modulate CB1 receptors within central nociception processing areas and the spinal cord, resulting in analgesic effects.
- Attenuate inflammation by activating CB2 receptors (32).
- Emerging research shows cannabis is indicated for:
- Migraines
- chronic pain
- back pain
- arthritic pain
- pain associated with cancer and surgery.
- neuropathic pain
- diabetic neuropathic pain when administered early in the disease progression.
- sickle cell disease
- cancer
- inflammatory bowel disease (32)
Available Forms
Cannabis refers to products sourced from the Cannabis sativa plant. There are differences between cannabis, cannabinoids, and cannabidiol (CBD). Cannabinoids are extracted from the cannabis plants. Cannabinoid-based treatments, such as dronabinol and CBD, are typically approved medical interventions for specific indications. THC (9-tetrahydrocannabinol) is the psychoactive component of the cannabis plant. CBD is a non-psychoactive component (32).
Cannabis can be consumed in different forms, each with a different onset and duration. Patients may have individual preferences, including:
- smoking/vaporizing dried flowers.
- consuming edibles
- tinctures or oils
- applying topicals (32)
Dosing and Monitoring
Inhaling marijuana via the lungs by smoking or vaping causes maximum plasma concentration within minutes. Psychiatric effects begin within seconds to a few minutes after inhalation and peak after 15 to 30 minutes. The effect diminishes throughout 2 to 3 hours (32).
Oral ingestion of marijuana causes psychiatric effects that typically occur between 30 and 90 minutes and reach maximum effect after 2 to 3 hours. Ingested marijuana effects last about 4 to 12 hours (32).
Dosing cannabis is challenging due to variations in potency and individual responses. Start with low doses and titrate slowly to achieve the desired effect while minimizing side effects. Regular monitoring is crucial, including assessing symptom relief, adverse effects, and potential drug interactions. Encourage patients to keep a diary to track their cannabis use and its effects (32).
Side Effects and Contraindications
Cannabis can exacerbate mental health conditions such as anxiety and psychosis. Common side effects of cannabis include (32):
- Dizziness
- dry mouth
- increased heart rate
- impaired memory
- psychoactive effects
Contraindications include:
- Pregnancy
- Breastfeeding
- heart disease
- respiratory conditions
- history of substance abuse
- mental health disorders

Self Quiz
Ask yourself...
- How do you address patients' misconceptions about pain medications?
- What are the mechanisms of action for commonly prescribed pain medications?
- How do these mechanisms of action contribute to pain relief?
- What are the potential side effects and risks associated with commonly prescribed pain medications?
- How do you educate patients about the risks and benefits of pain medications?
- How do you manage patients who require high-dose opioids for pain management?
- Is medical cannabis legal in your State? If yes, are you familiar with the prescribing guidelines?
- Do you have any personal biases against the use of medical cannabis? Why or why not?
Case Study
Mary is agreeable to trying an increased dose of Gabapentin. Mary would also like to see a counselor to discuss her past and get help with her anxiety. You made an appointment for Mary to see a Licensed Clinical Social Worker in your clinic.
You read the side effects and warnings for Gabapentin, and it is unsafe to use Gabapentin and Tramadol together since they are both depressants. You order a non-steroidal drug for Mary's somatic knee pain and make a consult for imaging studies on her left knee. You also make a referral to Orthopedics.
You educated Mary about the side effects of Gabapentin and scheduled a follow-up appointment. The day after Mary began her treatment with the increased Gabapentin, you called Mary to follow up on its effect. Mary still has pain, but she is not having any untoward side effects. Gabapentin may not work immediately so you will schedule a follow-up call in 3 days.


Self Quiz
Ask yourself...
- In this case study, Mary has insurance. How might your practice be different were Mary not insured?
- In your experience, what are the possible reasons for Mary's knee pain not being a part of her previous treatment record?
- Consider how your assessment of Mary's needs differs from the above-mentioned case study.
- Explain the rationale for decisions made by the nurse practitioner in the case study mentioned above and if your decisions would differ.
Opioid Use, the Opioid Epidemic, and Statistics
The use and misuse of opioids has become a pressing public health concern, leading to a global epidemic. The history of opioid use, the opioid epidemic, and associated statistics provide essential context for healthcare professionals in addressing this public health crisis. More importantly, it is estimated that 1 in 4 patients receiving prescription opioids in primary care settings will misuse them. In addition, 50% of opioid prescriptions are written by primary care providers, including nurse practitioners (22). Understanding the factors contributing to the epidemic and the magnitude of its impact is crucial for effective prevention, intervention, and treatment strategies.
History of Opioid Use
Opioids have a long history of medicinal use, dating back to ancient civilizations. They have been a drug of choice for pain relief for thousands of years. The introduction of synthetic opioids in the 19th century, such as morphine and later heroin, revolutionized pain management. However, their potential for addiction and misuse soon became apparent (16).
The Opioid Epidemic
The opioid epidemic refers to the surge in opioid misuse, addiction, and overdose deaths. The epidemic gained momentum in the late 1990s with increased prescribing of opioids for chronic pain (43).
No doubt, increased prescribing put opioids in the hands of consumers, but increased prescribing resulted from a multifactorial influence. One of the main influences was aggressive marketing by pharmaceutical companies, which has been well publicized. However, due to the long history of underprescribing pain medications for fear of misuse and addiction, the medical community was primed to expand its opioid prescribing practices (31).
A historical event that increased comfort with prescribing opioids, in the writer's opinion, was the introduction of the Medicare Hospice Benefit in 1986. Medical directors must be contracted or employed by hospices, and these medical directors had or soon gained pain management expertise. To further promote hospice and effective pain management, the hospice medical directors, with newly acquired skills, provided education throughout medical communities about pain management and specifically to decrease the fear of using opioids. Pharmacies and attending physicians grew accustomed to giving opioids for home use. Hospice care is for terminally ill patients, defined as a life expectancy of 6 months or less. Still, the reality is that hospice discharges 12 to 40% of patients for ineligibility and other reasons.
A more prominent factor in increasing opioid prescribing was the 1996 American Pain Society's introduction of pain as "the 5th Vital sign." Soon after, The Joint Commission promoted pain as "the 5th Vital Sign" and began compliance surveys in healthcare organizations requiring pain assessment details to be as prominent as blood pressure and heart rate. The Joint Commission cited a quote from 1968 by a nurse from the University of California Los Angeles, Margo McCaffrey, who defined pain as "…Whatever the experiencing person says it is, existing whenever s/he says it does." The Joint Commission accreditation programs pursued pain management as part of the accreditation process throughout its healthcare accreditation programs, including hospice accreditation by 1989 per TJC Timeline (48).
The National Institute of Health published an article about the Joint Commission's role in the opioid epidemic, particularly regarding the definition of pain, "This definition emphasizes that pain is a subjective experience with no objective measures. It also stresses that the patient, not the clinician, is the authority on the pain and that their self-report is the most reliable indicator of pain. This set the tone for clinicians: Patients are always to be trusted to report pain accurately” (45).
Statistics on the Opioid Epidemic
In the United States alone, over 500,000 people died from opioid overdoses between 1999 and 2017. The number of opioid-related overdose deaths continues to increase, with synthetic opioids, mainly illicitly manufactured Fentanyl, playing a significant role in recent years (46). Fentanyl-laced drugs, such as marijuana, are increasingly sold knowing and unknowingly to introduce medications with a high addiction rate, thus creating new consumers. This practice can potentially increase deaths due to the imprecise nature of manufacturing (16).
Opioid-related hospitalizations have also risen substantially. In 2014, there were approximately 1.27 million hospitalizations related to opioids in the United States. These hospitalizations not only place a burden on healthcare systems but also reflect the severe consequences of opioid misuse (3).


Self Quiz
Ask yourself...
- Have you experienced changes to your practice because of the opioid epidemic? If so, what are the changes?
- What is your opinion on the validity of Margo McCaffrey's definition of pain?
- What factors influence your willingness or unwillingness to prescribe opioids?
Federal Regulations on Opioid Prescribing
The history of substance use disorder prevention that promotes opioid recovery and treatment for patients and communities can be traced back to the early 20th century. However, the current approach to addressing opioid addiction and promoting healing has evolved significantly in recent times (36).
In the early 1900s, health professionals treated opioid addiction with punitive measures, including incarceration and moralistic approaches. The focus was on punishing individuals rather than providing effective treatment. This approach persisted for several decades until the mid-20th century when the medical community started recognizing addiction as a medical condition rather than a moral failing (36).
The Controlled Substances Act (CSA), introduced in 1970, was a response to increasing drug abuse and illicit drug trafficking in the United States. The CSA is a federal law regulating the manufacture, possession, distribution, and use of certain substances, including drugs and medications, that can potentially cause abuse and dependence. Its primary purpose is to combat drug abuse, reduce drug-related crimes, and protect public health and safety. The Drug Enforcement Agency (DEA) plays a crucial role in enforcing the CSA by monitoring and controlling controlled substance production, distribution, and use (31).
In the 1990s, the significant increase in opioid prescribing, leading to a surge in opioid addiction and overdose deaths, prompted a shift in focus toward prevention. Efforts were made to educate healthcare providers about the risks of overprescribing opioids and to implement prescription drug monitoring programs to track and prevent abuse (36).
The Comprehensive Addiction and Recovery Act (CARA) was signed into law in 2016 to expand access to treatment and recovery services for opioid addiction. This legislation allocated funding for prevention, treatment, recovery, and support services while promoting evidence-based practices and programs (36).
The Centers for Disease Control and Prevention (CDC) published guidelines in 2016 for prescribing opioids for chronic pain, which was updated in 2022. These guidelines emphasize the importance of non-opioid alternatives, using the lowest effective dose for the shortest duration, and assessing the benefits and risks of continued opioid therapy (13).
Furthermore, the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT) was signed into law in 2018, providing additional resources to address the opioid crisis. This legislation expanded access to medication-assisted treatment (MAT), increased the availability of naloxone, a medication used to reverse opioid overdose, and enhanced support for recovery housing (36).
In recent years, there has been a growing recognition of the importance of a comprehensive approach to opioid addiction, including harm reduction strategies, increased access to naloxone, and the integration of mental health services. Communities and organizations have been working together to address the underlying issues contributing to addiction, such as poverty, trauma, and social determinants of health (50).
Overall, the history of substance use disorder prevention that promotes opioid recovery and treatment has evolved from a punitive approach to a more compassionate and evidence-based model. Efforts are now focused on prevention, early intervention, and expanding access to comprehensive treatment and support services for individuals and communities affected by opioid addiction (36).
The most current federal regulations on opioid prescribing for healthcare providers are the amendments to the CSA in 2018, which added new rules to limit the quantity and duration of opioid prescriptions for acute pain to seven days. In 2022, the CDC updated recommendations to the Clinical Practice Guidelines for Prescribing Opioids for Pain.
The 2022 CDC guidelines are summarized below (13):
- Non-opioid therapies should be considered the first-line treatment for chronic pain.
- Establish clear treatment goals with patients, including realistic pain management and functional improvement expectations.
- Conduct a thorough risk assessment for potential harms before initiating opioid therapy.
- When opioids are used, start with the lowest effective dose and consider immediate-release opioids instead of extended-release or long-acting opioids.
- Prescribe the lowest effective dose for the shortest duration possible, typically three days or less and rarely exceeding seven days.
- Reassess benefits and risks within one day after prescribing opioids, including checking the prescription drug monitoring database.
- Avoid prescribing opioids and benzodiazepines concurrently whenever possible due to the increased risk of overdose and death.
- Offer naloxone to patients at increased risk of opioid overdose, including those with a history of overdose, substance use disorder, or concurrent benzodiazepine use.
- When opioids are no longer needed, taper the dose gradually to minimize withdrawal symptoms.
- Arrange an evidence-based treatment for patients with opioid use disorder, including medication-assisted treatment (Naltrexone, Buprenorphine, or Methadone).

Self Quiz
Ask yourself...
- What are the guidelines general for prescribing opioids for acute pain?
- How do these guidelines differ for chronic pain management?
- Discuss how federal regulations impact the practice of nurse practitioners in terms of opioid prescribing.
- Describe the potential benefits and challenges nurse practitioners face when adhering to federal regulations on opioid prescribing.
- How can nurse practitioners navigate and stay updated with evolving federal regulations surrounding opioid prescribing to ensure safe and effective care?
- How do you ensure appropriate documentation when prescribing controlled substances?
Safe Prescribing and Prescription Monitoring Program
Prescription Drug Monitoring Programs (PDMP) are state-run electronic databases that track.
the prescribing and dispensing of controlled substances. PDMPs are designed to improve patients.
care and safety by giving clinicians access to patients' prescription histories, allowing them to make informed decisions when prescribing controlled substances. PDMPs help identify patients at risk of substance misuse or prescription drug overdose. They also enable clinicians to identify potential drug interactions and prevent opioid diversion (14).
PDMPs collect and store data from pharmacies and prescribers in a centralized database. Clinicians can access this database to review a patient's prescription history, including the types of medications prescribed, the prescribers involved, and the dispensing pharmacies (14).
In many states, PDMP use is mandated by law, and nurse practitioners may be required to register and use the system. It is essential to understand state-specific laws and regulations regarding PDMP use.
PDMPs have some limitations, such as incomplete data or delays in reporting. The CDC emphasizes that clinicians should use PDMP data for their clinical assessment and other relevant information to make informed decisions about prescribing controlled substances. Still, PDMP cannot be used as the sole basis for denying or providing treatment (14).
Case Study
After five days on Gabapentin, Mary was doing well, and her neuropathic pain had decreased to 3/10. However, Mary suffered a fall after her knee "gave out" and injured her knee and back. She was in severe pain, and her family drove her to the ER. The ER doctors saw Mary, and orthopedics were consulted. Mary has surgery scheduled for a knee replacement a week from now.
Mary was prescribed Vicodin because she was in excruciating pain, but her prescription only allowed enough medication for two days. Mary has made an appointment with you to renew her prescription.
You evaluate Mary because you know that concomitant use of Gabapentin and opioids puts Mary at risk for respiratory depression and possible side effects, including accidental overdose.
Mary stated she has been more alert the past 24 hours and is afraid her functional status will continue to decline if she does not have more Vicodin because the pain in her back and knee makes it difficult to stand. You assess Mary. Mary stated she occasionally drinks alcohol but has not had a drink since she moved. She has no familial history of substance abuse or mental health disorders.
Mary's mother stayed at her house to help her for the first 24 hours after Mary's return from the ER, but Mary is providing her care now.
You check the PDMP database and see that Mary was prescribed eight pills she has taken over the last 48 hours.
Since the Vicodin has been effective without untoward side effects, and Mary's function is improving, you decide to refill the prescription of Vicodin. You will taper the dose to three Vicodin daily for two days and two for one day. Mary will be near her appointment for a knee replacement as well.
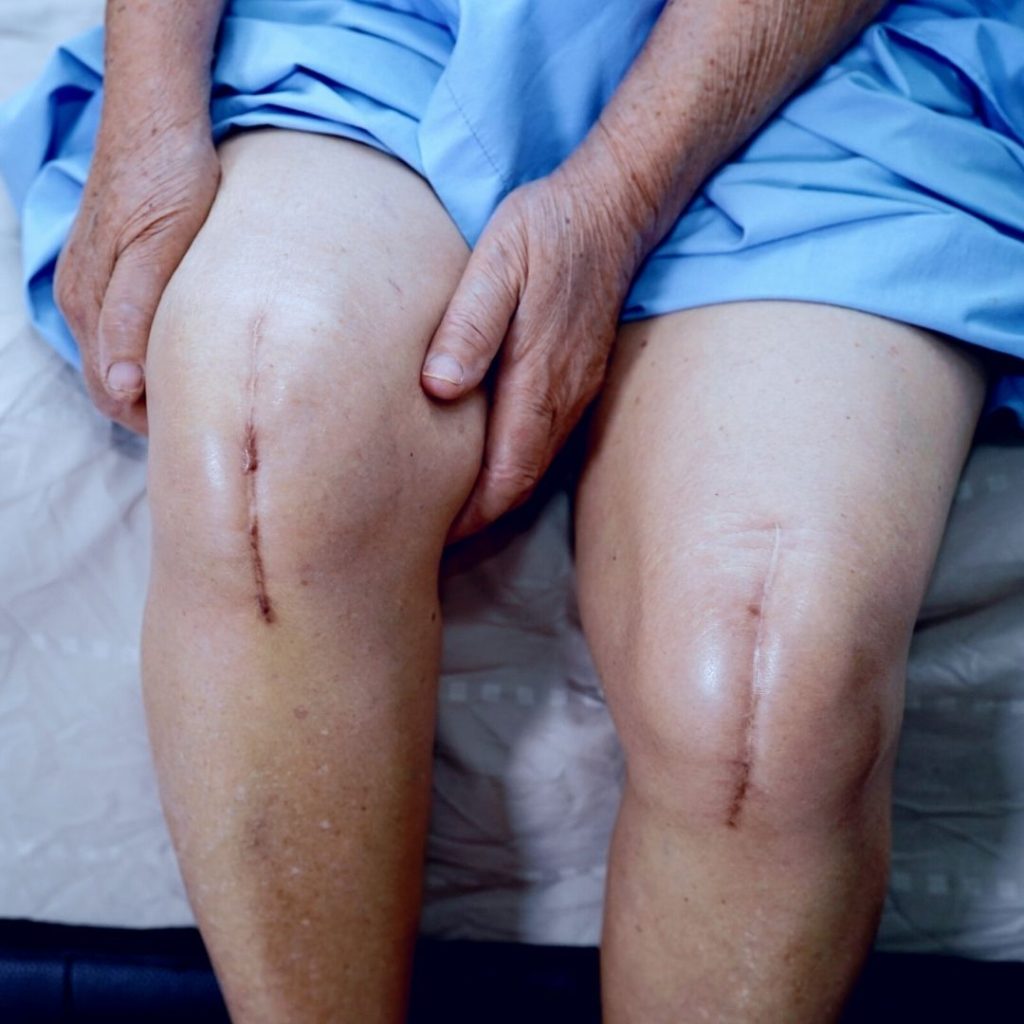

Self Quiz
Ask yourself...
- What are the potential benefits and drawbacks of using PDMPs in your practice?
- How can PDMPs help you identify potential drug abuse or diversion cases among your patients? Can you provide examples from your own experience?
- In what ways do PDMPs impact your decision-making process when prescribing controlled substances?
- What are the key considerations when prescribing controlled substances?
- How do you ensure responsible prescribing practices for controlled substances?
Preventing Opioid Use Disorder
As previously discussed, opioid addiction is a growing concern worldwide, affecting individuals from all walks of life. According to the CDC, "Anyone who takes prescription opioids can become addicted to them" (14).
As frontline healthcare professionals, nurse practitioners must recognize the signs of opioid addiction to provide timely intervention and support. This section will outline the key indicators of opioid addiction.
Physical Symptoms
Physical symptoms are often the first noticeable signs of opioid addiction. These symptoms may include constricted pupils, drowsiness, slurred speech, impaired coordination, and increased sensitivity to pain. Additionally, individuals struggling with opioid addiction may exhibit frequent flu-like symptoms, such as a runny nose, sweating, itching, or gastrointestinal issues.
Behavioral Changes
Opioid addiction can significantly impact an individual's behavior. These may include increased secrecy, frequent requests for early prescription refills, doctor shopping (seeking prescriptions from multiple healthcare providers), neglecting personal hygiene, and experiencing financial difficulties due to excessive spending on opioids (37).

Social Isolation
Opioid addiction often leads to social withdrawal and isolation. Individuals struggling with opioid addiction may distance themselves from family, friends, and social activities they once enjoyed. They may exhibit erratic mood swings, become defensive or hostile when confronted about their drug use, and display a general lack of interest in previously important activities (30).
Psychological Changes
The psychological impact of opioid addiction is significant. Individuals with opioid addiction may exhibit increased anxiety, depression, irritability, and restlessness. They may also experience cognitive impairments, memory lapses, and difficulties in decision-making. Healthcare professionals should be attentive to these changes, as they can indicate opioid addiction (51).
Tolerance and Withdrawal Symptoms
The development of tolerance and withdrawal symptoms are critical signs of opioid addiction. Individuals may require increased dosages of opioids to achieve the desired effect, indicating a growing tolerance. Furthermore, withdrawal symptoms such as muscle aches, nausea, vomiting, insomnia, and intense cravings for opioids may occur when the drug is discontinued or reduced abruptly (51).

Self Quiz
Ask yourself...
- Discuss how nurse practitioners can contribute to preventing opioid use disorder.
- Explain how nurse practitioners effectively communicate the risks and signs of opioid misuse without stigmatizing or alienating patients.
- What are the signs of opioid addiction or misuse in patients?
- How do you approach patients who may be at risk for opioid addiction?
- How do you ensure appropriate documentation when prescribing controlled substances?
Opioid Overdose
The management of opioid overdose, withdrawal, and addiction requires a comprehensive approach that combines pharmacological interventions with psychosocial support. Naloxone remains a vital tool for reversing opioid overdose, while medications such as Methadone, buprenorphine, and naltrexone play crucial roles in withdrawal and addiction treatment (National Institute of Health, 2023). Nurse practitioners must stay vigilant and informed about the evolving landscape of medications. This section aims to provide a comprehensive review of medications and treatment strategies for opioid overdose, withdrawal, and addiction and is excerpted from the NIH (40).
Naloxone
Mechanism of Action and Metabolism
Naloxone is an opioid receptor antagonist. It works by binding to opioid receptors and displacing any opioids present, thereby reversing the effects of opioid overdose. It has a higher affinity for opioid receptors than most opioids, effectively blocking their action.
Naloxone is indicated for emergency intervention of opioid overdose. It effectively reverses respiratory depression and other life-threatening effects. Studies suggest the potential benefits of combining naloxone with other medications, such as buprenorphine (see below), to improve outcomes. Initiatives promoting community-based naloxone distribution programs have shown promising results in reducing opioid-related deaths.
Available Forms
Naloxone is available in various formulations:
- Intranasal
- Intramuscular
- Intravenous
- auto-injectors.
The most used form is the intranasal spray, which is easy to administer and requires no specialized training. Intranasal naloxone formulations have gained popularity due to their ease of use and increased availability. A recent study showed that the non-FDA-approved compound spray was far less effective than either FDA compound (15).
Dosing and Monitoring
The recommended initial dose of naloxone for opioid overdose is 2mg intranasally or 0.4mg to 2mg intramuscularly or intravenously. If the patient does not respond within 23- minutes, additional doses may be administered every 2-3 minutes. Continuous monitoring of the patient's respiratory status is essential, as repeat doses may be required due to the short half-life of naloxone.
Side Effects and Contraindications
Naloxone has been shown not to affect individuals without opioids in their system.
Common side effects of naloxone include
- Withdrawal symptoms: increased heart rate, sweating, and agitation
- nausea
- vomiting
- headache
Contraindications include known hypersensitivity to naloxone and situations where the use of naloxone may be unsafe or not feasible.
Considerations for Nurse Practitioners
Fentanyl and other opioids have a rapid onset, and the need to act quickly is paramount. As mentioned previously, the ease of use and higher plasma concentrations using the FDA-approved 4-mg FDANxSpray device compared with the locally compounded nasal sprays should be considered when ordering Naloxone (15).
Fentanyl and other potent synthetic opioids may require multiple administrations of naloxone to achieve reversal of an overdose (Chiang, Gyaw, & Krieter, 2019). As a nurse practitioner prescribing naloxone, it is crucial to assess the patient's risk factors for opioid overdose, such as a history of substance use disorder or chronic pain management. Education regarding the proper administration of naloxone should be provided to the patients and their caregivers. Additionally, it is essential to provide resources for follow-up care, including addiction treatment and ongoing support.
Methadone
Mechanism of Action and Metabolism
Methadone is a long-acting opioid agonist that effectively suppresses withdrawal symptoms and reduces cravings. It binds to the same opioid receptors in the brain as other opioids. It relieves withdrawal symptoms and reduces cravings by blocking the euphoric effects of opioids, thus helping individuals with opioid dependence to achieve stability (33).
Available Forms
Methadone is available in oral tablets and liquid formulations. The oral tablet is the most used form and is typically administered once daily (33).
Dosing and Monitoring
Methadone dosing is individualized based on the patient's response and needs. Initially, the dose often started low and gradually increased until the patient reached a stable dose. Dosing may need to be adjusted based on the patient's response, adherence, and any changes in their overall health. Regularly monitoring the patient's vital signs, urine drug screens, and assessment of their withdrawal symptoms and cravings is essential.
Side Effects and Contraindications
Common side effects of methadone include:
- Constipation
- dry mouth
- drowsiness
- sweating
- weight gain
- respiratory depression
Contraindications include:
- known hypersensitivity to methadone
- severe asthma
- respiratory depression
- certain heart conditions (33).
Considerations for Nurse Practitioners
As a nurse practitioner prescribing methadone, conducting a comprehensive assessment of the patient's medical history, current medications, and substance use history is crucial. Opioid treatment programs or specialized clinics are often involved in methadone treatment, so collaboration and coordination of care with these programs are essential. Regularly monitoring the patient's progress, adherence, and potential side effects or drug interactions is essential. Additionally, providing education on the risks and benefits of methadone and the importance of adherence to the prescribed regimen is crucial for successful treatment outcomes.
Buprenorphine
Mechanism of Action and Metabolism
Buprenorphine is a partial opioid agonist with a ceiling effect that minimizes the risk of overdose while reducing withdrawal symptoms. Buprenorphine is a partial opioid agonist that binds to the same receptors as other opioids but produces a weaker response. It has a high affinity for the mu-opioid receptors, which helps reduce cravings and withdrawal symptoms in individuals with opioid dependence.
Available Forms
Buprenorphine is available in different formulations, including sublingual tablets, buccal films, and extended-release injections. The sublingual tablets have different strengths, such as 2mg, 4mg, 8mg, and 12mg. Buprenorphine is taken as a daily tablet or weekly or monthly injection.
Dosing and Monitoring
The dosing of buprenorphine varies depending on the individual's opioid dependence severity and treatment phase. Initially, a low dose (e.g., 2-4mg) is given, and it may gradually increase to a maintenance dose of 8-24 mg daily. Regular monitoring is essential to assess the patient's response, adherence, and potential side effects.
Side Effects and Contraindications
Common side effects of buprenorphine include:
- Constipation
- Nausea
- Headache
- Insomnia
- Sweating
Serious side effects are rare but can include:
- Respiratory depression
- Allergic reactions
Buprenorphine is contraindicated in individuals with:
- Severe respiratory insufficiency
- Acute intoxication with opioids
- Known hypersensitivity
Considerations for Nurse Practitioners
Nurse practitioners can prescribe buprenorphine for opioid dependence treatment under the Drug Addiction Treatment Act (DATA). To become eligible, they must complete specific training requirements and obtain a waiver from the Substance Abuse and Mental Health Services Administration (SAMHSA). Nurse practitioners should assess patients thoroughly, including their opioid use history, comorbidities, and medication compatibility, while ensuring appropriate counseling and referral for comprehensive treatment (40).
Clonidine + Lofexidine
Mechanism of Action and Metabolism:
Both Clonidine and Lofexidine are alpha-2 adrenergic agonists. They work by stimulating alpha-2 receptors in the brain, which reduces sympathetic outflow and norepinephrine release. This results in decreased sympathetic activity, leading to various effects such as reduced blood pressure, decreased heart rate, and alleviated withdrawal symptoms (28).
Available Forms
Clonidine is available in oral tablets and patches. Lofexidine is available in oral tablets and is taken as needed (40).
Dosing and Monitoring
For opioid withdrawal, the Clonidine dose ranges from 0.1-0.3 mg every 4-6 hours. Lofexidine is usually initiated at 0.53 mg three times daily, and the dose can be increased to 2.88 mg daily. Monitoring blood pressure and heart rate is essential during treatment (40).
Side Effects and Contraindications:
Common side effects of both medications include:
- dry mouth
- sedation
- dizziness
- constipation
- orthostatic hypotension (40).
Both medications are contraindicated in patients with:
- Hypotension
- Bradycardia
- heart block
- history of hypersensitivity to the drugs (40).
Considerations for Nurse Practitioners:
An early study of lofexidine vs. clonidine for withdrawal symptoms showed that treatment with lofexidine resulted in lower withdrawal symptoms, fewer mood problems, less sedation, and hypotension. There were no significant differences in craving levels, morphine metabolites in urine, or dropout rates when both were compared.
Lofexidine can be a safe option for outpatient treatment as it does not lead to hypotension. However, nurse practitioners must closely monitor patients' blood pressure and heart rate during treatment and educate them about possible side effects. If patients experience any concerning symptoms, they should inform their nurse practitioner immediately.
Gradual dose reduction of Clonidine is crucial to prevent rebound hypertension. Before prescribing either medication, nurse practitioners should assess for any contraindications or potential drug interactions (19).
Emerging Therapies for Withdrawal
Extended-release naltrexone: Naltrexone is an opioid receptor antagonist that blocks the effects of opioids, reducing the risk of relapse. It is taken as a monthly injection.
Alpha-2 adrenergic agonists: Emerging evidence suggests the potential use of dexmedetomidine and guanfacine for managing opioid withdrawal symptoms.
Medication-Assisted Treatment (MAT):
Methadone was introduced in the 1960s and marked a significant turning point in opioid addiction treatment or MAT. Along with counseling and behavioral therapies, MAT became the cornerstone of opioid addiction recovery.
Examples of medications used:
- Methadone
- Buprenorphine:
- Naltrexone:
Adjunctive Pharmacotherapies:
Antidepressants: Selective serotonin reuptake inhibitors and tricyclic antidepressants may help manage co-occurring depression and anxiety.
Anticonvulsants:
Medications like Gabapentin and pregabalin show promise in reducing opioid cravings and improving treatment outcomes.


Self Quiz
Ask yourself...
- What are the mechanisms of action for commonly prescribed addiction medications?
- What are the potential risks and benefits of using benzodiazepines for pain management?
- How do you assess and manage patients with co-occurring pain and substance use disorders?
- What are the guidelines for prescribing addiction medications like buprenorphine or methadone?
- How do these medications work in the treatment of opioid use disorder?
- What are the potential side effects and risks associated with addiction medications?
- How do you support patients in their recovery from opioid use disorder?
- How do you address patients' concerns and fears about addiction medications?
- What are the federal guidelines around prescribing addiction medications for nurse practitioners?
- How do these guidelines influence your prescribing practices?
Other Substance Use Disorders
Patients in pain may struggle with Substance Use Disorders other than Opioid Use Disorder. Substance use disorders may often occur with mental health conditions such as anxiety, depression, and bipolar disorder. In addition, many individuals engage in polydrug use. Understanding the most common Substance Use Disorders aids in a comprehensive assessment of the patient and the development of appropriate treatment plans (28).
Alcohol Use Disorder (AUD):
The prevalence of AUD worldwide was estimated to be 9.8% in men and 5.5% in women in 2016 (28).
Cannabis Use Disorder (CUD):
the prevalence of CUD in the United States increased from 2.18% in 2001-2002 to 2.89% in 2012-2013. (28).
Cocaine Use Disorder:
According to the National Survey on Drug Use and Health (NSDUH), in 2019, approximately 1.9 million Americans aged 12 or older had cocaine use disorder in the past year (44).
Methamphetamine Use Disorder:
A study published in Drug and Alcohol Dependence reported that the prevalence of methamphetamine use disorder in the United States was estimated to be 0.2% in 2015-2016 (6).

Self Quiz
Ask yourself...
- What are the options available for managing opioid addiction and withdrawal?
- How can nurse practitioners support patients in their recovery from opioid addiction?
- What strategies can nurse practitioners employ to effectively engage and build trust with patients reluctant to disclose or seek help for substance abuse disorders?
- How can nurse practitioners collaborate with other healthcare professionals and community resources to provide comprehensive care and support for patients with substance abuse disorders?
- What techniques or tools can nurse practitioners employ to start these sensitive conversations with new patients?
- How do you assess and manage patients experiencing opioid withdrawal symptoms?
- What are the non-pharmacological interventions for managing opioid withdrawal?
- How do you educate patients about the risks and benefits of addiction medications?
- How do you monitor patients on addiction medications for adherence and progress?
- What are the drug potential interactions with commonly prescribed addiction medications?
Drug Diversion and Illegal opioids
Misuse of opioids is facilitated by diversion and is defined as "the transfer of drugs from lawful to unlawful use" (24). Most commonly, this occurs when family and friends share prescribed opioids with other family and friends. Opioids and other controlled drugs are also diverted from healthcare facilities. Statistics show that healthcare facility diversion has increased since 2015 (24)
Diversion affects patients, healthcare workers, healthcare facilities, and public health. Patients experience substandard care due to ineffective pain management and impaired healthcare workers. In addition, affected patients are at risk of infections from compromised syringes (24).
Healthcare employees who divert are at risk of overdose and death. If caught, they face criminal prosecution and malpractice suits. Healthcare facilities also bear the cost of diverted drugs via internal investigations, follow-up care for affected patients, regulatory fines for inadequate safeguards, and declining public trust (24).
Despite the enormous consequences of drug diversion, healthcare facilities have implemented few processes to detect and deter the diversion of controlled substances (24).

Self Quiz
Ask yourself...
- What protocols can nurse practitioners implement to prevent drug diversion within their healthcare setting?
Patient Teachings and Considerations
Opioids have significant side effects and carry a risk of addiction and overdose. Nurse practitioners can decrease the risks of misuse and addiction by educating patients on appropriate disposal, safe storage, and potential signs of addiction. Taking additional time to provide teaching nurse practitioners can promote patient safety, informed decision-making, and responsible opioid use.
Safe Storage and Disposal:
- Teach patients to store opioids securely, out of reach of children, pets, visitors, and non-caregiver family members, to prevent accidental ingestion or misuse (13). Only the caregiver, if applicable, or the patient should have access to pain medications.
- Instruct patients on proper disposal methods, such as using drug take-back programs or mixing opioids with undesirable substances (e.g., coffee grounds) before throwing them away (11) (13).
Medication Adherence:
- Emphasize the importance of taking opioids as prescribed, at the correct dose and frequency, to achieve optimal pain relief.
- Encourage patients to notify their healthcare provider if they experience inadequate pain control or side effects (35).
Potential Side Effects:
- Educate patients about common side effects of opioids, including constipation, nausea, sedation, and respiratory depression.
- Discuss strategies to manage side effects, such as maintaining adequate hydration, consuming a fiber-rich diet, and using over-the-counter laxatives as needed (11).
Risk of Dependence and Addiction:
- Explain the potential for opioid dependence and addiction, especially with long-term use or a history of substance abuse.
- Encourage patients to promptly report signs of opioid misuse, such as craving, loss of control, or continued use despite negative consequences (51).

Avoiding Alcohol and Other Central Nervous System Depressants:
- Instruct patients to avoid consuming alcohol or other medications that can enhance the sedative effects of opioids, increasing the risk of respiratory depression.
- Advise patients to contact the Nurse Practitioner before starting new medications, including over-the-counter drugs or herbal supplements (2).
Driving and Operating Machinery:
- Inform patients about the potential impairment caused by opioids, including reduced alertness, reaction time, and coordination.
- Advise patients to avoid driving or operating heavy machinery while taking opioids until they know how the medication affects them (14).

Self Quiz
Ask yourself...
- What strategies can nurse practitioners employ to effectively communicate the risks and benefits of opioid use while ensuring they clearly understand the potential side effects and the importance of adhering to the prescribed regimen?
- How can nurse practitioners promote patient engagement and shared decision-making regarding opioid pain management, considering the potential for dependence and addiction?
- How can nurse practitioners assess a patient's knowledge and understand the safe storage and disposal of opioids?
Case Study
You take some extra time with Mary to educate her on the taper dose of Vicodin, the potential for harm, and the risk of opioids, especially when used concomitantly with Gabapentin. You let Mary know it is unsafe to use alcohol, not only with Vicodin but also with Gabapentin. You let Mary know that Vicodin has a risk of dependency and misuse and, therefore, she will be monitored carefully. You also educate that Mary should store the Vicodin away from visibility by anyone but herself since she can self-administer her medication. You let Mary know that Vicodin can cause constipation and that she should increase her water intake and take a stool softener.
You ask Mary to call you if her pain is not adequately relieved or if her medications run out before the three days.
You let Mary know that if she does stop taking the Vicodin before she has completed all the medication, she should dispose of it by mixing the pills with liquid and coffee grounds to make them unpalatable to animals and others.
Mary complied with your education, completed her course of Vicodin, and was scheduled for surgery. Mary's social worker helped her communicate with her new employer and delayed her start date until after her recovery.
During her recovery, Mary received physical therapy and a short course of pain medication managed by her orthopedist.
Mary returned to the clinic for a follow-up visit after completing her therapy and before starting work. Mary's pain level in her knee is 3/10, and she already feels like she can walk further than pre-surgery. Gabapentin has continued to help Mary's neuropathic pain in her back, and she reports 2/10. Mary looks forward to beginning her new job and is optimistic about the future.
Conclusion
Pain management is the leading cause of primary care appointments and chronic pain is the leading cause of disability. Yet, prescribing opioids for primary care patients is also a factor in drug misuse and the opioid epidemic. Nurse practitioners are challenged to appropriately treat pain and effectively control diversion, addiction, and death from overdose.
It is imperative that nurse practitioners use evidence-based practices to assess, appropriately intervene, and educate about the benefits and potential harm caused by treatment with opioids. Nurse practitioners must stay up to date with the current federal regulations regarding PDMPs, clinical prescribing guidelines, and emerging treatments for pain and opioid abuse disorders.
Non-Opioid Chronic Pain Management
Introduction
Chronic pain is common and debilitating condition, affecting about one in five people globally (6). Musculoskeletal conditions such as back pain are typically the most common conditions leading to chronic pain, followed by headache, orofacial pain, and visceral pain (6). Fibromyalgia and neuropathic pain are also prevalent.
About one-third of people with chronic non-cancer-related pain are prescribed opioid analgesics; however, long-term research finds that the potential harms likely outweigh the benefits.
Chronic pain can be difficult to treat, and management is often suboptimal. The most common non-opioid drug treatment is paracetamol (acetaminophen) and non-steroidal anti-inflammatory drugs, but they need to be used with caution and for short periods because of the risk of serious adverse events with long-term use (6)
We will dive into the pharmacokinetics of various non-opioid pain management options and explore this option for optimal outcomes for patients with chronic pain.
Definition
Pain is a subjective term. The one experiencing the pain is responsible fordescribing and rating it. Pain can be associated with actual or potential tissue damage or abnormal functioning of nerves. It may be classified as acute, chronic, or cancer pain. Pain may also be categorized as adaptive or maladaptive.
- Adaptive (Physiologic) Pain
-
- Nociceptive Pain – Examples include touching something hot or sharp.
- Inflammatory pain – Examples include trauma or surgery.
- Maladaptive (Pathologic) Pain
-
- Pathophysiologic pain (e.g., postherpetic neuralgia, diabetic neuropathy, fibromyalgia, irritable bowel syndrome, chronic headaches) is often described as chronic pain.
- It results from damage or abnormal functioning of nerves in the central nervous system (CNS) or peripheral nervous system (PNS). Pain circuits sometimes rewire themselves anatomically and biochemically, resulting in chronic pain, hyperalgesia, or allodynia (condition in which the pain stimulus typically should not cause pain, like “touch” on sunburned skin).
The steps in processing pain are: (10)
- Transduction – Stimulation of nociceptors.
-
- Nociceptors, located in somatic and visceral structures, are activated by mechanical, thermal, and chemical stimuli.
- Noxious stimuli may cause release of cytokines and chemokines that sensitize and/or activate nociceptors.
- Conduction – Receptor activation leads to action potentials that continue along afferent fibers to the spinal cord.
-
- Stimulation of large-diameter, sparsely myelinated fibers stimulate sharp, wider spread pain.
- Stimulation of small-diameter, unmyelinated fibers produce aching, poorly localized pain.
- Transmission – Synapse occurs in the spinal cord’s dorsal horn, releasing excitatory neurotransmitters.
-
- The signal to the brain’s higher cortical structures.
- Perception – Experience of pain happens when signals reach higher cortical structures.
-
- Relaxation, meditation, and distraction can lessen pain.
- Anxiety and depression can exacerbate pain.
- Modulation – Attributing factors can possibly include glutamate, substance P, endogenous opioids, γ-aminobutyric acid (GABA), norepinephrine, and serotonin.
The margin between neurons and immune cells within the CNS may facilitate chronic pain (10).
Somatosensation encompasses sensations such as touch, pressure, temperature, itch, and pain. Somatosensory information is transmitted from primary afferent fibers in the periphery into the central nervous system via the dorsal horn of the spinal cord (9). There are many therapies that target the dorsal horn functions as an intermediary processing center for this information, comprising a complex network of excitatory and inhibitory interneurons.

Self-Quiz
Ask Yourself...
- How would you describe the factors associated with chronic pain?
- Do you have experience with patients that have various forms of chronic pain?
- Can you describe the involvement of neurotransmitters in the signaling of nerve impulses?
- How does conduction vary between sparsely myelinated and unmyelinated fibers within the nervous system?
Overview of Non-Opioid Therapies for Chronic Pain Management
|
Non-Opioid Therapies for Chronic Pain Management (** Pharmacokinetics discussed in this course) |
|
|
Non-Opioid Drug Class |
Description |
|
Acetaminophen |
|
|
Nonsteroidal Anti-Inflammatory Drugs |
|
|
Anticonvulsants** |
|
|
Antidepressants** |
|
|
Topicals: Medicated Creams, Foams, Gels, Lotions, Ointments, Patches |
|
|
Interventional Pain Management |
|
|
Chronic Pain Therapies |
Description |
|
Self-Care |
|
|
Complementary Therapies |
|
|
Rehabilitation Therapies |
|
|
Behavioral And Mental Health Therapies |
Psychiatrists, clinical social workers, and mental health counselors provide therapies that identify and treat mental disorders or substance abuse problems that may serve as barriers to pain management. |

Self-Quiz
Ask Yourself...
- Can you name the benefits of using non-opioid pain management therapies when compared with opioid medications?
- Are you familiar with complementary chronic pain therapy?
- What are some common roadblocks to therapeutic pain management for those with opioid addictions?
- Can you name other medical professions that can become involved in the care planning for patients experiencing chronic pain?
Pharmacokinetics of Anticonvulsants
Anticonvulsants
Gabapentin and pregabalin are the most common anticonvulsants, or anti-epileptics, used for the treatment of chronic pain. These medications inhibit the alpha-2-delta subunit of voltage-gated calcium channels, which are involved in releasing nociceptive neurotransmitters. A number of systematic reviews strongly recommended using gabapentin for neuropathic pain and was backed by high-quality evidence.
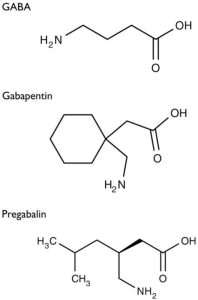
Figure 1. Structure of GABA, Gabapentin, and Pregabalin (1)
Gabapentin
Gabapentin [1-(aminomethyl)cyclohexane acetic acid] is an anti-epileptic agent and was originally developed as a gamma-aminobutyric acid (GABA)-mimetic compound to treat spasticity (1). Research found that it also has potent anticonvulsive effects. Initially approved only for use in partial seizures, it has evolved into an effective treatment agent for chronic pain syndromes, especially neuropathic pain.
Gamma-aminobutyric acid (GABA) was known to be a key inhibitory neurotransmitter, whose inhibition could cause seizures. Lipophilic groups were added to the carbon backbone to increase the bioavailability of GABA, as it does not penetrate the blood–brain barrier.
Gabapentin, available only as oral preparations, is absorbed in the small intestine by a combination of diffusion and facilitated transport. Its transport from the gut, following oral administration, is facilitated by its binding to a receptor (not yet identified) linked to a saturable l-amino acid transport mechanism (1).

Self-Quiz
Ask Yourself...
- Can you explain what Gabapentin was originally developed as?
- What is the cellular makeup of a GABA compound?
Gabapentin Drug Class
Gabapentin drug classes include: (16)
- Anticonvulsant, Miscellaneous
- GABA Analog
Gabapentin Uses
Gabapentin can be used in the management of postherpetic neuralgia (PHN) in adults, fibromyalgia, and various neuropathic pain (16).
Gabapentin Mechanism of Action
Gabapentinoids inhibit calcium-mediated neurotransmitter release through effects on α2δ-1 subunits, which inhibit forward movement of α2δ-1 that originates in the dorsal root ganglion within their endosomal compartments, causing processes that stimulate glutamate uptake by excitatory amino acid transporters (EAATs) (1). Na+-dependent excitatory amino acid transporters (EAATs) are the major transport mechanisms for extracellular glutamate removal in the CNS. EAATs-mediated clearance of amino acid glutamate released by neurons is vital to signaling and to prevent toxic accumulation of this amino acid in the extracellular space (11).
Glutamate is an amino acid and excitatory neurotransmitter that can stimulate all the CNS neurons— a capability that is unique to glutamate and explains why it is commonly known as the “master switch” (3). During this reuptake process, the cells can either reuse the glutamate or synthesize it back to glutamine, which is the form for storing for future use. Many different types of glutamate receptors exist, though they are classified into just two main categories: metabotropic and ionotropic. Ionotropic receptors are further divided into three main types of receptors: AMPA, harmac, and NMDA (3). Drugs can target these receptors to reduce glutamate release. Among the types of drugs that do so are anticonvulsants, mood stabilizers, and N -methylD-aspartate (NMDA) receptor antagonists (3).
Gamma-aminobutyric acid (GABA) is a powerful inhibitory neurotransmitter in the CNS. Glutamate and GABA work together to aid in the regulation of neurotransmitters and coordination with voltage-gated ion channels and G protein– coupled receptors (GPCRs) in the CNS (3).
GABA is synthesized from glutamate-by-glutamate decarboxylase (GAD) and is stored in synaptic vesicles. GABA-A receptors are the primary effector of the GABA-mediated inhibitory postsynaptic potential (IPSP). GABA-B receptors are responsible for the metabotropic effects of GABA and for the inhibition of voltage-gated calcium channels, the opening of potassium channels, and the release of glutamate and monoamines (3). Two main neurotransmitters regulate the sleep and wake function switch: histamine and GABA. The sleep encourager releases GABA, while the wake promoter, located within the tuberomammillary nucleus (TMN) of the hypothalamus, releases histamine (3). This is essentially why antihistamines can result in drowsiness.
Mechanisms not directly related to neurotransmitter release at dorsal horn include inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory actions, and influence on the affective component of pain.
Gabapentinoids do not bind to plasma proteins, and they are actively transported across the blood–brain barrier by LAT-1. The peak level in cerebrospinal fluid levels take significantly longer than the peak plasma levels, with a median time of 8 hours (1). Both are highly water-soluble and the volume of distribution of each is 0.8 and 0.5 L/kg for gabapentin and pregabalin, respectively.
Essential mechanisms of actions:
- α2δ-1 subunits are transported to the dorsal horn from their site of production in DRG (dorsal root ganglion) cell bodies. Elevated levels in the dorsal horn are associated with the development of neuropathic pain (1).
- Gabapentinoids inhibit the accumulation of α2δ-1 in the pre-synaptic terminals in the dorsal horn and reduce response to painful stimuli.
- α2δ-1 allows enhanced neurotransmitter release at decreased calcium influx.
- Analgesic effects are mediated by the facilitation of descending noradrenergic inhibition, decrease of descending serotonergic facilitation, and by cortical mechanisms affecting the limbic system (1).
- Stimulation of the uptake of glutamate by the excitatory amino acid transporters (EAAT).
- Suppression of the inflammatory response to injury.
- Modulation of the affective component of pain.

Self-Quiz
Ask Yourself...
- How would you describe the role of Na+-dependent excitatory amino acid transporters (EAATs) within the CNS?
- How do the gabapentinoids travel across the blood-brain barrier?
- Can you describe how analgesic effects can impact the limbic system?
- Can you name certain chronic conditions that may be associated with chronic inflammation?
Gabapentin Pharmacodynamics/Kinetics
The pharmacodynamics/kinetics of gabapentin is as follows: (17)
- Absorption: Variable
-
- Proximal small bowel by L-amino transport system
- Saturable process
- Dose-dependent
- Protein binding: <3%
- Metabolism: Not metabolized
- Immediate release:
-
- 900 mg/day: 60%
- 1,200 mg/day: 47%
- 2,400 mg/day: 34%
- 3,600 mg/day: 33%
- 4,800 mg/day: 27%
- Extended release: Variable; increased with higher fat content meal
- Half-life elimination:
-
- Adults, normal: 5 to 7 hours
- Increased half-life with decreased renal function
- Anuric adult patients: 132 hours
- Adults during hemodialysis: 3.8 hours
- Time to peak
-
- Adults: 2 to 4 hours
- Extended release: 8 hours
- Excretion: Proportional to renal function; urine (as unchanged drug)
- Clearance: Apparent oral clearance is directly proportional to CrCl
Gabapentin Adverse Effects
Adverse effects are common with gabapentinoids resulting in a discontinuation rate of at least 11%, but serious adverse events are uncommon. The substitution of gabapentin with pregabalin in gabapentin responders resulted in improved pain relief and fewer adverse events.
Central nervous system effects
Dizziness, somnolescence, and gait disturbances are the most common adverse effects. The effects often occur during the initiation of treatment and can diminish after several weeks of treatment. Visual blurring can also occur (1).
Other common side effects affecting the central nervous system (CNS) include impaired concentration, confusion, memory loss, altered mood, movement disorders, sleep disorder, speech impairment, and vertigo (1).
Most adverse reactions have a clear dose–response relationship with increased risk of complications with higher doses. However, ocular adverse effects such as amblyopia and blurred vision appeared at lower doses of pregabalin (1)
Respiratory depression
Respiratory depression has been reported when used in combination with opioids, resulting in an increased risk of accidental opioid-related mortality (1). Clinicians should be aware of this prior to prescribing multiple medications. A large primary care database review showed that 21.8% of patients with a new prescription for gabapentin and 24.1% of patients with a new prescription for pregabalin received additional prescriptions, primarily for opioids (1). Dose adjustments are recommended for patients with compromised respiratory function, respiratory or neurological diseases, renal impairment, and elderly people due to the higher risk of experiencing severe respiratory depression.
Weight gain
Weight gain is common with gabapentinoids and can affect up to one-fourth of all patients treated (This is a common reason for patients to stop taking it). However, the extent of weight gain appears to be moderate. The majority of patients treated with pregabalin for one year maintain weight within ±7% of their baseline or initial weight (1). Weight gain is related to dose and duration of use but not to body mass index, gender, age, and development of edema (1).
Gastrointestinal effects
Gastrointestinal adverse effects such as abdominal distension, abnormal appetite, constipation, dry mouth, and nausea are common side effects and are dose-related, all except constipation (1).
Misuse
There is potential for abuse of gabapentinoids, particularly in individuals with a history of opioid abuse (1). Both gabapentinoids have been reported to stimulate feelings of sociability, euphoria, calm and relaxation, which can lead to misuse. Abuse potential of pregabalin is higher as compared to gabapentin due to its pharmacokinetic properties (1). All anticonvulsants are associated with increased risk for suicidal thoughts and behavior (12).
Withdrawal
Withdrawal symptoms are common and appear between 12 hours and 7 days after cessation of use, with most cases occurring between 24 and 48 hours (1). Other symptoms include tachycardia, palpitations, anxiety, sweating, restlessness, hypertension, tremor, gastrointestinal symptoms, paranoia, auditory hallucinations, and suicidal ideation – all similar to the withdrawal effects of benzodiazepines and alcohol (1). Patients with psychiatric comorbidities and the elderly may be at an increased risk of withdrawal. Clinicians should do a slow tapering schedule for patients at higher risk for withdrawal. A slower tapering schedule such as a twice-weekly reduction of 10–25% of the dose has been suggested to minimize the risk of withdrawal effects (1).
Toxicity
The risk for toxicity is higher in patients with chronic kidney disease and those on dialysis (1). Toxicity may present with increased sedation, confusion, unsteady gait, myoclonus, ataxia, episodic leg spasm, asterixis and tremulousness (1).
Guidelines on recommendations for dose reductions based on creatinine clearance are available for prescribers. Patients on hemodialysis might require supplemental doses following dialysis due to it removing approximately 35% of gabapentin and 50–60% of pregabalin (1).
Gabapentin Dosing
Fibromyalgia
Initial: Oral: 100 to 300 mg once daily at bedtime; increase dose gradually based on response and tolerability every 1 to 2 weeks to a target dose of 1.2 to 2.4 g/day in divided doses (17)
Neuropathic pain
Note: For chronic use, an adequate trial with gabapentin may require two months or more. For critically ill patients with neuropathic pain, gabapentin may be a useful component of multimodal pain control (17).
Immediate release: Oral: Initial: 100 to 300 mg 1 to 3 times daily; increase dose based on response and tolerability to a target dose range of 300 mg to 1.2 g 3 times daily (17).
Extended release: Oral: Initial: 300 mg at bedtime; increase dose based on response and tolerability to a target dose of 900 mg to 3.6 g once daily (17).
Postherpetic neuralgia
Immediate release: Oral: 300 mg once on day one, 300 mg twice daily on day two, and 300 mg 3 times daily on day two, then increase as needed up to 1.8 to 3.6 g/day in divided doses (17).
Extended release: Oral: Initial: 300 mg once daily; increase by 300 mg each day up to 900 mg once daily. Further increase as needed up to 1.8 g once daily (17).


Self-Quiz
Ask Yourself...
- Can you discuss the side effects of gabapentin?
- How is gabapentin excreted?
- Can you name the contraindications for taking this medication, specifically associated with respiratory distress?
- Do you have experience with educating patients on a tapered dosing schedule?
Pregabalin
Pregabalin was originally approved by the U.S. Food and Drug Administration (FDA) as an anti-epileptic drug, also called an anticonvulsant. It works by reducing the conduction of impulses in the brain that cause seizures. Pregabalin also affects chemicals in the brain that send pain signals across the nervous system.
Pregabalin Drug Class
Pregabalin is classified as a Schedule V prescription drug (16). Schedule V drugs are defined as drugs with lower potential for abuse than Schedule IV by the U.S. Drug Enforcement Administration (DEA).
Pregabalin Uses
Pregabalin is used to treat pain caused by neuropathic pain associated with spinal cord injury, fibromyalgia, diabetic neuropathy, post-herpetic neuralgia, and management of postherpetic neuralgia (1, 12).
Pregabalin Mechanism of Action
Pregabalin binds to alpha-2-delta subunit of calcium channels within the CNS and lowers calcium influx at the nerve terminals, which inhibits excitatory neurotransmitter release (16). These excitatory neurotransmitters including glutamate, norepinephrine (noradrenaline), serotonin, dopamine, substance P, and calcitonin gene-related peptide (16).
Glutamate was discussed earlier in the course, but it is important to closely examine norepinephrine, as it relates to pain. Norepinephrine is another monoamine neurotransmitter in the catecholamine family, and it serves as both a neurotransmitter and a hormone (3). The sympathetic nervous system, which becomes activated during stressful or painful events, activates norepinephrine as the neurotransmitter primarily responsible for the “fight or flight” response (3). The sympathetic system is highly influenced by changes in the serum norepinephrine concentration and is associated with the regulation of heart rate and blood pressure.
By binding presynaptically to the alpha2-delta subunit of voltage-gated calcium channels in the CNS, it is essentially thought to calm the conduction of pain impulse. In addition, pregabalin prevents the alpha2-delta subunit from being transported from the dorsal root ganglia to the spinal dorsal horn, which may also contribute to the mechanism of action and use in chronic pain in those with spinal cord injuries (1).
Although structurally related to GABA, it does not bind to GABA or benzodiazepine receptors. Pregabalin may also affect descending noradrenergic and serotonergic pain transmission pathways from the brainstem to the spinal cord.
Alpha (α 2δ )-ligands modulate neurotransmitter release and increase membrane hyperpolarization and the seizure threshold.
Pregabalin Pharmacodynamics/Kinetics
The pharmacodynamics/kinetics of gabapentin is as follows: (16)
- Onset of action: Effects may be noted as early as the first week of therapy.
- Absorption
-
- Extended release: Approximately 30% lower when administered while fasting.
- Distribution: Vd: 0.5 L/kg
- Protein binding: 0%
- Bioavailability: ≥90%
- Half-life elimination: Adult: 6.3 hours
- Time to peak, plasma:
-
- Extended release: Median is 8 hours with food (Average 5 to 12 hours)
- Immediate release, Adults: Within 1.5 hours fasting; 3 hours with food
- Excretion: Urine (90% as unchanged drug; minor metabolites)
Pregabalin Adverse Effects
Central nervous system effects
Dizziness (3% to 45%), drowsiness (≤36), fatigue (4% to 11%) is included among adverse effects (16).
Ataxia, balance impairment, abnormal gait, euphoria, confusion, disturbance in attention abnormal thinking, neuropathy, myasthenia, insomnia, memory impairment, vertigo, speech disturbance, anxiety, paresthesia, intoxicated feeling, lethargy, and nervousness have also been reported (16).
Cardiovascular
Peripheral edema (4% to 16%), facial edema (1% to 3%), chest pain (2%), hypertension (2%), hypotension (2%) has been reported.
Endocrine & metabolic
Weight gain (2% to 14%) is noted.
Ophthalmic
Visual field loss (13%) and blurred vision (≤12%) have been noted.
Gastrointestinal
Constipation, increased appetite, nausea, flatulence, vomiting, abdominal distension, and abdominal pain are among the reported adverse effects.
Endocrine & metabolic: Fluid retention (2% to 3%), hypoglycemia (2% to 3%), decreased libido (≥1%)
Hematologic & oncologic
Thrombocytopenia (3%) has occurred.
Pregabalin Dosing
Fibromyalgia
Immediate release: Oral: Initial: 75 mg twice daily; may increase to 150 mg twice daily within one week based on response and tolerability; maximum dose: 450 mg/day (manufacturer’s labeling). Note: A lower initial doses of 25 to 50 mg at bedtime is suggested by many experts (8).
Neuropathic pain
Immediate release: Oral: Initial: 25 to 150 mg/day once daily or in two divided doses; may increase in increments of 25 to 150 mg/day at intervals ≥1 week based on response and tolerability up to a usual dose of 300 to 600 mg/day in two divided doses (16).
Postherpetic neuralgia
Immediate release: Oral: Initial: 150 mg/day in divided doses (75 mg twice daily or 50 mg three times daily); may increase to 300 mg/day within one week based on response and tolerability; after 2 to 4 weeks, may further increase up to the maximum dose of 600 mg/day (16).
Extended release: Oral: Initial: 165 mg once daily; may increase to 330 mg once daily within one week based on tolerability; after 2 to 4 weeks, may further increase up to the maximum dose of 660 mg/day (16).

Self-Quiz
Ask Yourself...
- Can you discuss the side effects of pregabalin?
- What is the half-life for this medication?
- Can you describe the mechanism of action of this medication?
- How can the classification or schedule of a drug impact prescribing?
Warnings for Gabapentin and Pregabalin
Warnings for gabapentin and pregabalin include the following risk factors, dosing and monitoring considerations, and patient education needs (1).
The FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Risk factors include the use of opioid pain medicines, and conditions that reduce lung function, such as chronic obstructive pulmonary disease (COPD) (14). The elderly are also at higher risk.
Health care providers should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation. Patients with underlying respiratory disease and elderly patients are also at increased risk and should be managed similarly.
Clinicians should assess the risk of misuse, dependence, and diversion.
Patients should be made aware of the importance of dosage titration, the titration process, and the requirement to take a stable regime for a few weeks before assessing for improvement in pain. Patients often misunderstand that gabapentinoids cannot be taken as needed and that taking an additional dose does not result in improved pain (1)
Patients must be warned about the potential alteration in concentration for certain tasks, such as using heavy equipment or driving.
Pharmacokinetics of Antidepressants
Antidepressants
Antidepressants, including tricyclic antidepressants (TCAs) and selective norepinephrine reuptake inhibitors (SNRIs), have shown to have analgesic effects by primarily blocking the reuptake of norepinephrine, thereby enhancing the pain-modulating pathway activity. TCAs also block peripheral sodium channels, which can also help reduce pain.
Serotonin and norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) are the most common classes of antidepressants used to manage chronic neuropathic pain.
All currently available antidepressants enhance monoamine neurotransmission by one of several mechanisms; the most common mechanism is inhibition of the activity of SERT, NET, or both. The serotonin transporter (SERT) is a glycoprotein with 12 transmembrane regions embedded within the axon terminal and cell body membranes of serotonergic neurons (2). As serotonin outside of the cells bind to receptors on the transporter, changes occur in the transporter and serotonin (2). Na+, and Cl− then are transported into the cell. Binding of intracellular K+ results in the release of serotonin inside the cell. The transporter is released into its original state.
At therapeutic doses, about 80% of the activity of the transporter is inhibited (2).
Ultimately, the increased availability of monoamines for binding in the synaptic cleft results in a cascade of events that enhance the transcription of some proteins and the inhibition of others (2). The inhibiting effects are most desirable for reduction of chronic pain.
We will use the pharmacokinetics of the tricyclic antidepressant (TCA), Amitriptyline, and selective norepinephrine reuptake inhibitor (SNRI), Duloxetine, to explore the research on use.
The SNRIs differ from the TCAs in that they lack the potent antihistamine, α-adrenergic blocking, and anticholinergic effects of the TCAs. As a result, the SNRIs tend to be favored over the TCAs in the treatment of pain syndromes because they are often tolerated better (2).
- Tricyclic antidepressant (TCA): Pharmacokinetics of Amitriptyline
- Serotonin norepinephrine reuptake inhibitors (SNRIs): Pharmacokinetics of Duloxetine
Tricyclic Antidepressants (TCA)
The first indication that tricyclic antidepressants (TCAs) may help with neuropathic pain came from a 1960 study that found that patients treated with imipramine experienced chronic pain relief. There have been many studies since that support the effectiveness of TCAs on chronic pain.
Amitriptyline
Example of tricyclic antidepressant (TCA) used in chronic pain management.
Amitriptyline Drug Class
Antidepressant, Tricyclic (Tertiary Amine)
Amitriptyline Uses
Used to treat major depressive disorder (unipolar), unipolar major depressive disorder, and management of chronic neuropathic pain.
Amitriptyline Mechanism of Action
The N-type calcium ion channel is an established target for the treatment of neuropathic pain. The channel consists of a unique α1 pore-forming subunit and auxiliary α2-δ and β subunits (6). The general structure of the α1 subunit is similar to that of other voltage-gated ion channels.
The mechanism of action involves increasing the synaptic concentration of serotonin and/or norepinephrine in the central nervous system by stopping their reuptake by the presynaptic neuronal membrane pump (Wil-A).
Amitriptyline is a tertiary amine with strong binding affinities for alpha-adrenergic, histamine (H1), and muscarinic (M1) receptors (13). Chronic treatment with amitriptyline desensitizes presynaptic receptors, producing long-lasting changes in monoaminergic neurotransmission (13).
Amitriptyline Pharmacodynamics/Kinetics
- Onset of action: Responses may vary per individual; however, 4 to 8 weeks of treatment are needed before determining if a patient is partially or non-responsive; desired therapeutic effect for pain reduction may take as long as 1 to 3 weeks.
- Absorption: Rapid, well absorbed.
- Distribution: Vd: 18 to 22 L/kg
- Protein binding: >90%
- Metabolism: Rapid; hepatic N to demethylation to nortriptyline (active), hydroxy derivatives and conjugated derivatives
- Bioavailability: 43% to 46%
- Time to peak, serum: 2 to 5 hours
(Wil-A)
- Amitriptyline has a half-life of 10 to 28 hours (13)
- Excretion: Amitriptyline and its metabolites are primarily excreted by the kidney (13)
- Special Populations: Elderly: May have increased plasma levels
- Amitriptyline can cross the placental barrier (13)
Amitriptyline Dosage Formulations
Amitriptyline dosage formulations come in oral tablets of 10 mg, 25 mg, 50 mg, 75 mg, 100 mg, and 150 mg.
Adult Dosing: For chronic pain, therapy can initiate a dose of 10 to 20 mg daily. The dose can be increased by 25 mg every 3 to 7 days, with a maximum of 150 to 300 mg/day. If the dose requires adjustment, it is preferable to change the bedtime dose. In cases of therapy cessation, the clinician should gradually taper to avoid withdrawal (13).
Plasma Levels: It is difficult to directly correlate plasma levels with desired, therapeutic effects. However, determining plasma levels might be useful in identifying toxicity with excessively high levels or in whom noncompliance is suspected.
Older adult patients have decreased hepatic metabolism and increased intestinal transport time, so plasma levels are usually higher for any given oral dose of amitriptyline for this population.
Amitriptyline Side Effects
Amitriptyline, due to its alpha-adrenergic receptor blockade, can cause orthostatic hypotension, dizziness, and sedation. Anticholinergic side effects include blurred vision, dry mouth, urinary retention, tachycardia, acute angle glaucoma, constipation, and confusion (13). Antihistamine side effects secondary to its histamine (H1) receptor binding property include sedation, increased appetite, weight gain, confusion, and delirium (13).
It can increase the risk of bone fracture and bone marrow suppression.
Amitriptyline Warnings
Anticholinergic Effects
May cause anticholinergic effects (constipation, xerostomia, blurred vision, urinary retention); use with caution in patients with decreased gastrointestinal motility, increased intraocular pressure (IOP), narrow-angle glaucoma, paralytic ileus, urinary retention, BPH, xerostomia, or visual problems (Wil-A).
CNS Depression
May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks that require mental alertness (e.g., operating machinery or driving).
Fractures
Bone fractures have been associated with antidepressant treatment (Wil-A)
Hematologic Effects
TCAs may rarely cause bone marrow suppression; monitor for any signs of infection and obtain CBC if symptoms are evident (e.g., fever, sore throat).
Orthostatic Hypotension
May cause orthostatic hypotension; use with caution in patients at risk of this (cerebrovascular disease, cardiovascular disease, hypovolemia, or concurrent medication use which may predispose to hypotension/bradycardia).
Therapy is relatively contraindicated in patients with symptomatic hypotension.
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and Hyponatremia
Associated with the development of syndrome of inappropriate antidiuretic hormone secretion (SIADH) and hyponatremia, predominately in the elderly (Wil-A).
Cardiovascular Risk
In a scientific statement from the American Heart Association, amitriptyline may exacerbate underlying myocardial dysfunction (WIL-A). Amitriptyline can also cause heart rate variability, slow intracardiac conduction, induce various arrhythmias, and cause QTc (corrected QT) prolongation (13).
Use with caution in patients with diabetes mellitus, hepatic impairment, or renal impairment (Wil-A).
A major contraindication is the coadministration with or within 14 days of Monoamine oxidase inhibitors (MAOIs); avoid coadministration with cisapride and avoid during the acute recovery phase following myocardial infarction (Wil-A).
Clinicians must monitor elderly patients carefully and obtain amitriptyline serum levels based on their clinical assessment. Clinicians should adjust amitriptyline dose according to the patient’s clinical response and not based on plasma levels (13).
Alternatives to Amitriptyline
Other TCAs that may be helpful in chronic pain management is Desipramine, Imipramine, and Nortriptyline.
Amitriptyline is more sedating and has increased anticholinergic properties than other TCAs (13).


Self-Quiz
Ask Yourself...
- Can you discuss the mechanism of action for Amitriptyline?
- What would you consider the most worrisome adverse effects?
- What are some common anticholinergic properties and effects?
- Why is the half-life and peak time important in the administration and prescribing of a medication?
Selective Norepinephrine Reuptake Inhibitors (SNRIs)
As mentioned earlier, serotonin and norepinephrine reuptake inhibitors (SNRIs) are one of the most common classes of antidepressants used to manage chronic neuropathic pain.
Duloxetine
Example of selective norepinephrine reuptake inhibitor (SNRI) used in chronic pain management.
Duloxetine Drug Class
Antidepressant, Serotonin/Norepinephrine Reuptake Inhibitor.
Duloxetine Uses
Duloxetine can be used to manage major depressive disorder (MDD), generalized anxiety disorder (GAD), fibromyalgia, diabetic peripheral neuropathy, and chronic musculoskeletal pain.
Duloxetine Mechanism of Action
Duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a weak inhibitor of dopamine reuptake.
Serotonin is another monoamine neurotransmitter derived from tryptophan; the majority of the serotonin found in the body is located in the enterochromaffin cells of the gastrointestinal tract, with the rest within in the CNS, where it regulates mood, sleep, and appetite (3).
Duloxetine inhibits serotonin and norepinephrine reuptake and enhances dopamine levels within the prefrontal cortex (5). The mechanism of action behind the increase in dopamine levels involves the inhibition of norepinephrine transporters; the transporters have an attraction for dopamine. Therefore, inhibition of norepinephrine transporters can lead to an increase in dopamine. This increase in dopamine specifically occurs in the prefrontal cortex, where dopamine transporters are scarce, and reuptake relies more heavily on norepinephrine transporters (5).
Duloxetine works to reduce neuropathic and chronic pain states by increasing the activity of noradrenergic and serotonergic neurons in the descending spinal pathway of the dorsal horn (5). These descending neurons decrease the activity of dorsal horn neurons, which essentially suppresses excessive input from reaching the brain. The hypothesis is that a deficiency in these inhibitory signals resulting in less signals perceived as pain being delivered.
Duloxetine has no significant activity for muscarinic cholinergic, H1-histaminergic, or alpha2-adrenergic receptors. Duloxetine does not possess MAO-inhibitory activity.
Duloxetine Pharmacodynamics/Kinetics
- Absorption: Well-absorbed.
- Protein binding: >90%; primarily to albumin and alpha1-acid glycoprotein.
- Metabolism: Hepatic, via CYP1A2 and CYP2D6; forms multiple metabolites (inactive).
- Half-life elimination (adults): ~12 hours (range: 8 to 22 hours); ~4 hours longer in elderly women.
- Time to peak: 5 to 6 hours; food delays by 1.7 to 4 hours (15).
Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are reliant on the dose (18). Plasma concentrations are typically stable after three days of dosing.
Elimination of duloxetine is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP1A2 (18). Orally administered duloxetine hydrochloride is well absorbed. There is a median two-hour lag until absorption begins with maximal plasma concentrations occurring six hours post dose (18).
Food does not affect the maximal concentration of duloxetine, but it delays the time to reach peak concentration from 6 to 10 hours and it marginally decreases the extent of absorption (AUC) by about 10% (18). There is a three-hour delay in absorption and a one-third increase in apparent clearance of duloxetine after an evening dose as compared to a morning dose (18).
Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and α1-acid glycoprotein (18). The plasma protein binding of duloxetine is not changed by renal or hepatic impairment. About 70% is excreted and found in the urine as metabolites of duloxetine; about 20% is excreted in the feces (18).
Duloxetine Dosing for Management of Chronic Pain
Low back pain: 30 mg can be given once daily for one week and increased up to 60 mg once daily as tolerated as an adjunct therapy. Maximum dose: 60 mg/day.
Neuropathy: 60 mg can be administered initially; however, lower starting doses may be appropriate depending on patient tolerance. Maximum dose: 60 mg/day.
Duloxetine Side Effects
Side and adverse effects of the cardiovascular, gastrointestinal, central nervous system, such as headaches and drowsiness, and fatigue, are common (5). Duloxetine has a very low anticholinergic impact.
Common adverse effects of duloxetine include: (5)
- Headache
- Drowsiness
- Fatigue
- Nausea
- Xerostomia
- Abdominal pain
- Weight loss
- Weakness
- Insomnia
- Dizziness
- Change in libido
- Diaphoresis
- Tremor
Serious adverse effects of duloxetine include:
- Suicidality
- Serotonin syndrome
- Hepatoxicity
- Mania
- Syncope
- Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
- Hyponatremia
Duloxetine Warnings
Warnings for duloxetine include the following information (5).
Duloxetine is contraindicated in patients with concurrent or recent (within 14 days) use of monoamine oxidase (MAO) inhibitors, uncontrolled angle-closure glaucoma, or hypersensitivity to duloxetine.
Duloxetine should also be avoided in patients with liver failure or severe renal dysfunction (5). Also avoid using duloxetine in patients receiving treatment with linezolid or intravenous methylene blue due to an increased risk of serotonin syndrome (5)
Precautions and Warning in Special Population:
- Duloxetine is FDA pregnancy category C, which means risk to fetal development cannot be ruled out.
- Caution is necessary when prescribing duloxetine in the geriatric population.
Monitor for suicidal ideation, especially when starting treatment, altering the dose, and after discontinuation of therapy.
Caution should be exercised when using anticoagulants or antiplatelet medications along with duloxetine therapy.
Laboratory workup should include monitoring serum creatinine, blood urea nitrogen (BUN), transaminase levels, blood glucose levels, and HgbA1c in diabetic patients.
Sodium levels require follow-up when prescribing duloxetine in the geriatric population.
Duloxetine Toxicity
Signs and symptoms of toxicity include serotonin syndrome, seizures, somnolence, syncope, tachycardia, diarrhea, and vomiting (5).
Signs of serotonin syndrome include agitation, disorientation, diaphoresis, hyperthermia, tachycardia, nausea, vomiting, myoclonus, dilated pupils, dry mucous membranes, and increased bowel sounds; clonus and hyperreflexia are particularly common in serotonin syndrome (5).
There is no antidote to duloxetine overdose; cyproheptadine and cooling measures may be considered for toxicity or overdose (5).

Self-Quiz
Ask Yourself...
- Can you discuss the mechanism of action of duloxetine?
- Can you name the signs of serotonin syndrome?
- How can inhibiting neuronal serotonin and norepinephrine reuptake improve symptoms of pain or depression?
- Is there an antidote to duloxetine overdose?
Nursing Considerations
It is meaningful to remember that pain is subjective and should be determined by each patient. Those with chronic pain spend a significant amount of time in out-of-hospital environments, in their homes and in their community settings.
There is no “one size fits all” care plan for those with chronic pain. Personalized care is a collaborative process that should be used in chronic condition management in which patients, caregivers, and healthcare providers can identify and discuss problems and develop plans and goals to empower them in their own care. Individualized care can improve aspects of physical health, mental health, and the ability to self-manage conditions.
Nursing should utilize appropriate pain assessment tools and identify changes in levels of pain. Pain intensity, pain relief, and medication side effects must be assessed regularly. The frequency of assessment depends on the type of pain, analgesic used, route of administration, and concomitant medications.
Current Research and Opportunities
Chronic pain affects more than 100 million people in the United States (4). The U.S. is currently in the midst of an opioid epidemic with increased opioid use over the last two decades. Research is extensively focused on treatment alternatives, including medications, physical therapy, exercise, injections, and neuromodulation.
Conclusion
Clinicians face difficult decisions when managing chronic pain in their patients. There is an increasing need for meaningful use of non-opioid options. Research supports the positive outcomes of therapies such as anticonvulsants, antidepressants, and various other alternatives. Knowledge of the unique pharmacokinetics of each therapy is essential for clinicians.
References + Disclaimer
- Substance Abuse / Chemical Dependency. Substance Abuse / Chemical Dependency | Johns Hopkins Medicine. (2019, November 19). Retrieved December 23, 2022, from https://www.hopkinsmedicine.org/health/conditions-and-diseases/substance-abuse-chemical-dependency
- Substance abuse and addiction statistics [2022]. NCDAS. (2022, June 18). Retrieved December 23, 2022, from https://drugabusestatistics.org/
- Alcohol abuse. Harvard Health. (2014, December 5). Retrieved December 23, 2022, from https://www.health.harvard.edu/addiction/alcohol-abuse
- U.S. Department of Health and Human Services. (n.d.). Alcohol’s effects on the body. National Institute on Alcohol Abuse and Alcoholism. Retrieved December 26, 2022, from https://www.niaaa.nih.gov/alcohols-effects-health/alcohols-effects-body
- Mayo Foundation for Medical Education and Research. (2022, May 18). Alcohol use disorder. Mayo Clinic. Retrieved December 26, 2022, from https://www.mayoclinic.org/diseases-conditions/alcohol-use-disorder/symptoms-causes/syc-20369243
- Mayo Foundation for Medical Education and Research. (2022, May 18). Alcohol use disorder. Mayo Clinic. Retrieved December 26, 2022, from https://www.mayoclinic.org/diseases-conditions/alcohol-use-disorder/diagnosis-treatment/drc-20369250
- Centers for Disease Control and Prevention. (2022, December 12). Marijuana and public health. Centers for Disease Control and Prevention. Retrieved December 26, 2022, from https://www.cdc.gov/marijuana/index.htm
- Holland, K. (2022, September 14). What’s the difference between CBD vs. THC? Healthline. Retrieved December 26, 2022, from https://www.healthline.com/health/cbd-vs-thc#psychoactive-components
- Mayo Foundation for Medical Education and Research. (2022, October 25). Prescription drug abuse. Mayo Clinic. Retrieved December 27, 2022, from https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/symptoms-causes/syc-20376813
- Prescription drug abuse statistics. NCDAS. (2022, May 2). Retrieved December 27, 2022, from https://drugabusestatistics.org/prescription-drug-abuse-statistics/
- Centers for Disease Control and Prevention. (2022, June 2). Death Rate Maps & Graphs. Centers for Disease Control and Prevention. Retrieved December 27, 2022, from https://www.cdc.gov/drugoverdose/deaths/index.html#:~:text=Opioids%E2%80%94mainly%20synthetic%20opioids%20(other,of%20all%20drug%20overdose%20deaths).
- Meth addiction: Facts, statistics & how meth changes you. American Addiction Centers. (2022, October 21). Retrieved December 28, 2022, from https://americanaddictioncenters.org/meth-treatment/facts
- Centers for Disease Control and Prevention. (2021, November 18). Other drugs. Centers for Disease Control and Prevention. Retrieved December 28, 2022, from https://www.cdc.gov/drugoverdose/deaths/other-drugs.html
- U.S. Department of Health and Human Services. (2022, December 19). Methamphetamine drug facts. National Institutes of Health. Retrieved December 28, 2022, from https://nida.nih.gov/publications/drugfacts/methamphetamine
- U.S. Department of Health and Human Services. (2022, December 19). Cocaine drug facts. National Institutes of Health. Retrieved December 28, 2022, from https://nida.nih.gov/publications/drugfacts/cocaine
- U.S. Department of Health and Human Services. (2022, December 19). Heroin drug facts. National Institutes of Health. Retrieved December 30, 2022, from https://nida.nih.gov/publications/drugfacts/heroin
- Centers for Disease Control and Prevention. (2022, May 23). Heroin. Centers for Disease Control and Prevention. Retrieved December 30, 2022, from https://www.cdc.gov/opioids/basics/heroin.html
- U.S. Department of Health and Human Services. (2022, December 19). Naloxone drug facts. National Institutes of Health. Retrieved December 30, 2022, from https://nida.nih.gov/publications/drugfacts/naloxone
- U.S. Department of Health and Human Services. (2022, December 20). Hallucinogens drug facts. National Institutes of Health. Retrieved December 30, 2022, from https://nida.nih.gov/publications/drugfacts/hallucinogens
- Shmulewitz, D., & Walsh, C. (2022, August 18). New Study estimates over 5.5 million U.S. adults use hallucinogens. Search the website. Retrieved December 30, 2022, from https://www.publichealth.columbia.edu/public-health-now/news/new-study-estimates-over-55-million-us-adults-use-hallucinogens
- Drugs, brains, and behavior: The science of addiction. (2020, June). Retrieved April 10, 2021, from https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/soa.pdf
- Risk factors for addiction. (2017, February 14). Retrieved April 10, 2021, from https://drugfree.org/print/article.php?id=60743
- Gray, K. M., & Squeglia, L. M. (2018). Research Review: What have we learned about adolescent substance use?. Journal of child psychology and psychiatry, and allied disciplines, 59(6), 618–627. https://doi.org/10.1111/jcpp.12783
- Alzheimer’s disease fact sheet. (n.d.). Retrieved February 10, 2021, from https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
- Preventing Alzheimer’s disease: What do we know? (n.d.). Retrieved February 10, 2021, from https://www.nia.nih.gov/health/preventing-alzheimers-disease-what-do-we-know
- How is Alzheimer’s disease treated? (n.d.). Retrieved February 10, 2021, from https://www.nia.nih.gov/health/how-alzheimers-disease-treated
- Castro-Aldrete, L., Moser, M. V., Putignano, G., Ferretti, M. T., Diemch, A. S., and Chadha, A. S. (2023). Sex and gender considerations in Alzheimer’s disease: The Women’s Brain Project contribution. Frontiers in Aging Neuroscience, 15, 1-11. https://doi.org/10.3389/fnagi.2023.1105620
- Carrarini, C., Russo, M., Dono, F., Barbone, F., Rispoli, M. G., Ferri, L., Di Pietro, M., Digiovanni, A., Ajdinaj, P., Speranza, R., Granzotto, A., Frazzini, V., Thomas, A., Pilotto, A., Padovani, A., Onofrj, M., Sensi, S. L., & Bonanni, L. (2021). Agitation and Dementia: Prevention and Treatment Strategies in Acute and Chronic Conditions. Frontiers in neurology, 12, 644317. https://doi.org/10.3389/fneur.2021.644317
- Alzhimer’s Disease: Common Medical Problems. (n.d.). Retrieved January 9, 2024, 10, 2021, from https://www.nia.nih.gov/health/alzheimers-caregiving/alzheimers-disease-common-medical-problems #7 Wandering and Alzhimer’s Diease. (n.d.). Retrieved January 9, 2024, from https://www.nia.nih.gov/health/alzheimers-changes-behavior-and-communication/wandering-and-alzheimers-disease
- Abi-Aad, K., & Derain, A. (2023, August 17). Hydromorphone. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK470393/
- Adler, J., Ballantyne, J., Chou, R., Davies, P., Fanciullo, G., & Fine, P. (2009). Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic NonCancer Pain. The Journal of Pain, 113-130.
- Ahmad, F., Rossen, L., Sutton, P., & & Talih, M. (2018). Provisional drug overdose. National Vital Statistics Reports, pp. 1-12.
- Altman, R., Clark, M., Huddart, R., & Klein, T. (2018, October 28). Pharm GKB Summary: Oxycodone Pathway, Pharmacokinetics. Retrieved from Pharmacogenet Genomics: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6602093/#:~:text=Approximately%2072%25%20of%20an%20oxycodone,oxycodone%20is%20unconjugated%20%5B70%5D
- Atrux-Tallau, N., naimi, Z., & Jaudinot, E. (2022, November 4). Pharmocokinetics of Morphine Sulfate Orodispersible tablets and Bioequivalence with Immediate-Release Oral Morphine Sulfate Formulations in Healthy Adult SubjectsUnder fastin Conditions. Retrieved from Clinical Drig Investigation: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9705466/
- Baldwin, G., Compton, W. M., Han, B., Houry, D., Jones, C. M., & Vivolo-Kantor, A. (2021, September 24). Methamphetamine use in the United States: epidemiological update and implocations for prevention, treatment and harm reduction. Annual New York Academy of Science. New York: National Institute of Health.
- Berg, K. A., & Clarke, W. P. (2018). Making Sense of Pharmacology: Inverse Agonism and Functional Selectivtiy. International Journal of Neuropsychopharmacology, 962-977.
- Bistas, K., Lopez-Ojeda, W., & Ramos-Matos, C. (2023, May 29). Fentanyl. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK459275/
- Blyth, F., Briggs, A., Hoy, D. G., March, L. M., & Schneider, C. H. (2019). The Global Burden of Musculoskeletal Pain-Where to From Here? American Journal of Public Health, 35-40.
- Bramanti, P., Cavalli, E., Mammana, S., Mazzon, E., & Nicoletti, F. (2019). The neuropathic pain: An Overview of the current treatment and future therapeutic approaches. International Journal of Immunopathology and Pharmacology, 33.
- Brennan, F., Carr, D., Cousins, M., & Pain, D. (2013). Pain Management: A Fundamental Human Right. Anesthesia and Analgesia, 224-227.
- Carver, A. C., & Foley, K. (2003). Types of Pain. In D. Kufke, & R. W. Pollock, Cancer Medicine. Hamilton: Holland Frei Cancer Medicine.
- Centers for Disease Control and Prevention. (2022). CDCs Clinical Practice Guidelines for Prescribing Opioids. Retrieved from Opioids: https://www.cdc.gov/opioids/healthcare-professionals/prescribing/guideline/index.html?s_cid=DOP_Clinician_Search_Paid_001&gclid=CjwKCAjwrranBhAEEiwAzbhNtVKOCMzLLnwhfj7B2D_aBCZM_SryNcEyXl0GRigmorFhKve2ekCVrhoCNYQQAvD_BwE
- Centers for Disease Control and Prevention. (2023, August 8). Opioids. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/opioids/basics/epidemic.html#print
- Chiang, N., Gyaw, S., & Krieter, P. A. (2019). Comparison of the Pharmacokinetic Properties of Naloxone Following the Use of FDA-Approved Intranasal and Intramuscular Devices Versus a Common Improvised Nasal Naloxone Device. Journal of Clinical Pharmacology, 1078-1084.
- Cicero, T., & Ellis, M. &. (2017). The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA, 673-681.
- Cofano, S., & Yellon, R. (2022, October 24). Stat Pearls: Hydrocodone. Retrieved from National Institutes of Health: https://www.ncbi.nlm.nih.gov/books/NBK537288/
- Dart, R., Hoyte, C., & Nakhee, S. (2021). A Review on Tramadol Toxicity: Mechanism of Action, Clinical Presentation, and Treatment. Forensic Toxicology, 293-310. Retrieved from Forensic Toxicology: https://link.springer.com/article/10.1007/s11419-020-00569-0
- Delsignor, R. e. (2001). Lofexidine versus clonidine in rapid opiate detoxification. Journal of Substance Abuse Treatment, 11-17.
- Department of Defense Veterans Association. (2023). Defense and Veterans Pain Rating Scale. Retrieved from VA.gov: https://www.va.gov/PAINMANAGEMENT/docs/DVPRS_2slides_and_references.pdf
- Department of Health and Aged Care. (2013, January 20). FLACC Pain Scale. Retrieved from health.gov: https://www1.health.gov.au/internet/publications/publishing.nsf/Content/triageqrg~triageqrg-pain~triageqrg-FLACC
- Donaldson, S. L. (2017). Provider-Based Interventions to Mitigate Risk for Opioid Pain Medication Abuse Among Adult Patients in a Primary Care Setting.
- Dydyk, A. M., & Grandhe, S. (2023, January 29). Pain Assessment. Retrieved from STAT PEARLS: https://www.ncbi.nlm.nih.gov/books/NBK556098/#:~:text=Pain%20is%20the%20most%20common,pain%20in%20the%20United%20States.
- Fan, M., Hamilton, M., Hyland, B., & Tscheng, D. (2019). Diversion of Controlled Drugs in Hospitals: A Scoping review of Contributors and Safeguards. Journal of Hospital Medicine.
- GeriatricPain.org. (2022, January). Fast Facts: Selected Pain Assessment Tools. Retrieved from Geriatricpain.org: Geriatricpain.org https://geriatricpain.org/selected-pain-assessment-tools-fc
- Haffajee, R., Jena, A., Weiner, S., & & Kesselheim, A. (2019). Mandatory use of prescription drug monitoring programs. Journal of the American Medical Association, 1667-1668.
- International Association for the Study of Pain . (2020, July 16). IASP Announces Revised Definition of Pain. Retrieved from International Association for the Study of Pain: https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/
- JAMA. (2023). Substance Use and Addiction Medicine. Retrieved from JAMA Network: https://jamanetwork.com/collections/5921/substance-use-and-addiction-medicine
- John, W. S., Schwartz, R. P., Subramanian, G., & Wu, L.-T. (2018). Prevalence, Patterns and correlates of multiple substance use disorders among adults. Drug and Alcohol Dependency, 79-87.
- Jones, C., Einstein, E., & Compton, W. (2018). Changes in synthetic opioid involvement in drug overdose deaths in the United States. JAMA, 1819-1821.
- Kalava, A., King, K. C., & Preuss, C. V. (2023, April 29). Perscription of Controlled Substances: Benefits and Risks. Retrieved from STAT PEARLS: https://www.ncbi.nlm.nih.gov/books/NBK537318/
- Khalid, N., Patel, P., & Singh, A. (2023, January). Cannabis Versus Opioids for Pain. Retrieved from Stat Pearls: https://www.ncbi.nlm.nih.gov/books/NBK573080/
- Kreutzwiser, D., & Tawfic, Q. (2020). Mathadone for Pain Management. A Pharmacotherapeutic Review, 827-839.
- Kroenke, K., Spiter, R., Williams, J., & Lowe, B. (2011, Nov). An ultra-brief screening scale for anxiety and depression: the PHQ-4. From Principles of Neuropathic Pain Assessment and Management, 613-21. Psychosomatics 2009.
- Manchikanti, L., Kaye, A., Knezevic, N., McAnally, H., Slavin, K., Trescot, A., & Hirsch, J. (2018). Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians guidelines. Pain Physician, S3-S92.
- Marquez, C. B. (2020). Changing the outlook toward the problem of opioid addiction: From “War on Drug” to “Public Health Emergency”. Seton Hall: Law Student Scholarship.
- Marsch, L., Borodovsky, J., & Dallery, J. (2019). Advances in the psychosocial treatment of addiction: The role of technology in the delivery of evidence-based psychosocial treatment. The Psyciatric Clinics of North America, 585-596.
- McLellan, T., & Volkow, N. (2016). Opioid Abuse in Chronic Pain-Misconceptions and Mitigation Strategies. New England Journal of Medicine, 1253-1263.
- National Institute of Drug Abuse (NIDA). (2023, August 30). Prescription Opioid Drug Facts. Retrieved from National Institute on Drug Abuse: https://nida.nih.gov/publications/drugfacts/prescription-opioids
- National Institute of Health. (2023, August 25). Medications for Opioid Overdose, Withdrawal, & Addiction. Retrieved from National Institute of Drug Addiction: https://nida.nih.gov/research-topics/opioids/medications-opioid-overdose-withdrawal-addiction-infographic
- Pain Physician. (2008). Opioid Special Issue. Pain Physician, S133-S153.
- Ransford, e. a. (2019, February 08). The Ransford Pain Drawing . Retrieved from compassionandsupport.org: https://compassionandsupport.org/wp-content/uploads/sites/2/2019/08/PainScaleRansford.pdf
- Rudd, R. A., Zibbell, J., & Gladden, M. (2016). Increases in drug and opioid overdose deaths-Untied States. Morbidity and Mortlity Weekly Report, 1378-1382.
- SAMHSA. (2020). Key Substance Use and Mental Health Indicators in the United States: Ressults from the 2019 National Survey on Drug Use and Health. SAMHSA.
- Scalpel, S. (2016, November). The Joint Commission Deserves Some Blame for the Opioid Crisis. Retrieved from Missouri Medicine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6139759/
- Scholl, L., Seth, P., Kariisa, M., Wilson, N., & Baldwin, G. (2019). Drug and opioid-involved overdose deaths-Unioted States, 2013-2017. Morbidity and Mortatlity Weekly Report 67, 1419-1427.
- Stauffer, L., & Gibson, S. (2017). The Nurse’s Role in Identifying and Educating Patients At Risk for Opioid Abuse. AAACN Viewpoint, 1.
- The Joint Commission. (2023, January). The Joint Commission History Timeline. Retrieved from JointCommission.org: https://www.jointcommission.org/-/media/tjc/documents/tjc-history-timeline-through-2022.pdf
- Toombs, K., Kral, L., Colleran, C., & & Varney, S. (2018). Nurse practitioner-pharmacist collaboration in pain management: Strategies for success. . Journal of the American Association of Nurse Practitioners, 141-148.
- U.S. Food and Drug Administration. (2023, May 23). FDA. Retrieved from Information about Medication Assisted Treatment (MAT): https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat
- Volkow, N., Jones, E., Einstein, E., & Wargo, E. (2019). Prevention and Treatment of Opioid mIsuse and Addiction: A Review. JAMA Psychiatry, 208-216.
- Chincholkar M. (2020). Gabapentinoids: pharmacokinetics, pharmacodynamics, and considerations for clinical practice. British journal of pain, 14(2), 104–114. https://doi.org/10.1177/2049463720912496
- DeBattista C (2024). Antidepressant agents. Vanderah T.W.(Ed.), Katzung’s Basic & Clinical Pharmacology, 16th Edition. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3382§ionid=281751777
- Demler, T. L., & Rhoads, J. (Eds.). (2018). Pharmacotherapeutics for advanced nursing practice. Jones & Bartlett Learning.
- Dey S, Vrooman BM. Alternatives to Opioids for Managing Pain. [Updated 2023 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574543/
- Dhaliwal JS, Spurling BC, Molla M. (2023). Duloxetine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Ferreira G E, Abdel-Shaheed C, Underwood M, Finnerup N B, Day R O, McLachlan A et al. Efficacy, safety, and tolerability of antidepressants for pain in adults: overview of systematic reviews BMJ 2023; 380 :e072415 doi:10.1136/bmj-2022-072415
- Fraser G.L., & Riker R.R. (2020). Critical care: pain, agitation, and delirium. DiPiro J.T., & Yee G.C., & Posey L, & Haines S.T., & Nolin T.D., & Ellingrod V(Eds.), Pharmacotherapy: A Pathophysiologic Approach, 11e. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2577§ionid=234136370
- Goldenberg, D.L. (2019). Treatment of fibromyalgia in adults not responsive to initial therapies. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com.
- Harding, E. K., Fung, S. W., & Bonin, R. P. (2020). Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches. Frontiers in neural circuits, 14, 31. https://doi.org/10.3389/fncir.2020.00031
- Macintyre, P. E., & Schug, S. A. (2021). Acute pain management : a practical guide (Fifth edition.). CRC Press.
- Malik, A. R., & Willnow, T. E. (2019). Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System. International journal of molecular sciences, 20(22), 5671. https://doi.org/10.3390/ijms20225671
- Schwinghammer T.L., & DiPiro J.T., & Ellingrod V.L., & DiPiro C.V.(Eds.), (2021). Pharmacotherapy Handbook, 11e. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3012§ionid=253436360
- Thour A, Marwaha R. Amitriptyline. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537225/
- U.S. Food and Drug Administration (FDA). (2020). FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). Retrieved from https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Amitriptyline. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426455#monoNumber=426455§ionID=243191387&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Pregabalin. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426994#monoNumber=426994§ionID=243281218&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Gabapentin. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426662#monoNumber=426662§ionID=243246336&tab=tab0
- Accessdata.fda.gov (FDA). (n.d.). Cymbalta. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021427s009s011s013lbl.pdf
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
