Course
Torsades de Pointes Identification
Course Highlights
- In this Torsades de pointes Identification course, we will learn about TdP on an electrocardiogram, including oscillatory changes in the QRS complexes.
- You’ll also learn how to manage TdP by addressing modifiable risk factors.
- You’ll leave this course with a broader understanding of long-term strategies to prevent TdP.
About
Contact Hours Awarded: 1
Course By:
R.E. Hengsterman MSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Torsades de Pointes (TdP) “twisting of the points or twisting of QRS complex on electrocardiogram” is a type of polymorphic ventricular tachycardia characterized on an electrocardiogram by oscillatory changes in the amplitude of the QRS complexes around the isoelectric line [1]. Torsades de Pointes relates to QTc prolongation, which involves the lengthening of the QT interval adjusted for heart rate [1]. QT prolongation measures delayed ventricular repolarization [2]. Excessive QT prolongation can predispose the myocardium to early after-depolarizations, which can trigger re-entrant tachycardias including TdP [2]. Torsades de Pointes (TdP) may either terminate without intervention or degenerate into ventricular fibrillation, leading to sudden death [1].
TdP is a rare but fatal arrhythmia if not diagnosed and treated with prompt interventions [3]. Patients may present with a range of symptoms, but the ECG is diagnostic. Conscious patients may experience palpitations and 50% of patients present as asymptomatic [1]. The most common symptoms reported are syncope, palpitations, and dizziness; however, in up to 10% of cases, cardiac death is the initial symptom [1]. Patients with Jervell and Lange-Nielsen syndrome may also have a history of deafness [1].
While physicians manage the arrhythmia, it is fundamental for all nurses to recognize it and consult with a cardiologist. Advise patients with congenital prolonged QT intervals to refrain from strenuous exercise [4][5]. Long QT syndrome (LQTS) can be congenital (cLQTS), acquired (aLQTS), or both [6]. TdP runs are often self-terminating but recurrent [1].
Several pathological conditions and drug toxicities can induce prolonged or abnormal repolarization (QT interval prolongation and/or twisted T or T/U wave morphology), resulting in TdP development, which can lead to ventricular fibrillation and sudden cardiac death [7]. QT intervals represent the duration of the total action potential during ventricular polarization and depolarization events [8].
Several non-cardiac drugs that modulate cardiac repolarization can prolong QT intervals and trigger TdP onset [34]. Both aLQTS and cLQTS may share similar genetic variants in their pathogenesis [9]. An individual’s genetic background is a critical factor, as even minor prolongation of the QT interval can result in life-threatening manifestations of aLQTS when appropriate pathogenic triggers are present [9].

Self-Quiz
Ask Yourself...
- How does the mechanism of QT prolongation contribute to the development of Torsades de Pointes, and what implications does this have for preventing and managing this arrhythmia in patients with congenital or acquired long QT syndrome?
- Considering that Torsades de Pointes can present with a range of symptoms or even be asymptomatic in some patients, what are the key diagnostic features on an electrocardiogram that healthcare providers should be vigilant about to ensure timely diagnosis and intervention?
Case Study
A 78-year-old male, recovering from a C. difficile infection, schedules a colonoscopy under sedation. His medical history is notable for hypertension and a history of paroxysmal supraventricular tachycardia (PSVT). The patient described self-resolving syncopal episodes which led to a suspected diagnosis of PSVT. His pertinent medications include diltiazem, amlodipine, and omeprazole. His social and family histories are non-contributory. He completed a bowel prep before the procedure as instructed and had minimal oral intake in the preceding days due to persistent nausea.

Self-Quiz
Ask Yourself...
- Given this patient’s medical history of hypertension and paroxysmal supraventricular tachycardia (PSVT), along with recent severe diarrhea and minimal oral intake, what are the potential risks to consider before proceeding with the colonoscopy, and how might these factors influence the management of his condition during the procedure?
Diagnostic Assessment
A pre-procedure EKG displayed inverted T waves, prominent U waves, and a prolonged QTc exceeding 600 milliseconds. The team evaluated the rhythm strip which indicated polymorphic ventricular tachycardia (Torsades de Pointes). Over 15 minutes, the providers administered 2 grams of IV magnesium. Laboratory evaluation showed a potassium level of 2.9 mmol/L (normal range 3.5–4.5), a sodium level of 132 mmol/L (normal range 135–145), glucose at 153 mg/dL (normal fasting level 60–100), normal magnesium at 2.1 mg/dL (normal range 1.6–2.4), low ionized calcium at 1.14 mmol/L (normal range 1.2–1.32), and a low phosphorus level of 0.98 mg/dL (normal range 0.97–1.45) [10].
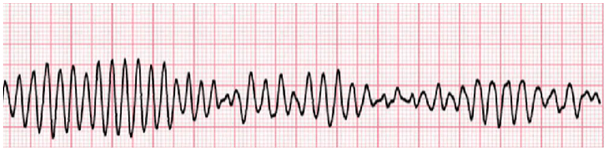
Figure 1. [40]

Self-Quiz
Ask Yourself...
- Considering the patient’s EKG results showing inverted T waves, prominent U waves, and a prolonged QTc, along with laboratory findings of significant electrolyte imbalances, what might be the underlying causes of these abnormalities, and how should the healthcare team prioritize and address these issues to prevent further episodes of Torsades de Pointes?
Therapeutic Intervention
After diagnosing Torsades de Pointes, the healthcare team administered 2 grams of magnesium and initiated aggressive repletion of potassium and phosphorus using both intravenous and oral routes, as the patient remained awake, oriented, and following commands. The medical team admitted the patient to the ICU for ongoing electrolyte repletion and continued evaluation.
Follow-up and Outcomes
During the patient’s hospital stay, he exhibited long QT syndrome and experienced several episodes of Torsades de Pointes and pulseless ventricular fibrillation, all of which required defibrillation. These episodes occurred in the intensive care unit despite the discontinuation of all QT-prolonging medications and the normalization of his normalization electrolytes. Each episode responded to defibrillation, resulting in a return to normal sinus rhythm. The team consulted interventional cardiology for further evaluation and treatment. Medical management failed, and persistent prolonged QT syndrome led to the diagnosis of congenital long QT syndrome exacerbated by significant diarrhea, electrolyte abnormalities, and QT-prolonging medications. The team implanted a cardioverter-defibrillator (ICD) on day 2 of his hospital stay and discharged him home on day 4.

Self-Quiz
Ask Yourself...
- Despite the normalization of electrolytes and discontinuation of QT-prolonging medications, the patient continued to experience episodes of Torsades de Pointes and ventricular fibrillation. What factors might contribute to the persistence of these arrhythmias, and how can the healthcare team address these underlying issues to prevent future occurrences?
- Given that medical management failed to control the patient’s arrhythmias, leading to the diagnosis of congenital long QT syndrome and the subsequent implantation of an ICD, what are the long-term implications for the patient’s management and lifestyle, and how should the healthcare team educate the patient about living with an ICD and congenital long QT syndrome?
Differential Diagnosis
The differential diagnosis for Torsades de Pointes includes a variety of conditions that can present similar symptoms. Ventricular tachycardia and ventricular fibrillation are primary considerations, as they can also manifest with rapid, irregular heart rhythms [11]. Evaluate dialysis-related complications in patients undergoing renal replacement therapy, as electrolyte imbalances associated with dialysis can precipitate arrhythmias [12]. Syncope, or sudden loss of consciousness, can be both a symptom and a differential diagnosis, necessitating a thorough assessment to distinguish it from arrhythmia-induced syncope [13].
Drug toxicity from medications such as antihistamines and antiarrhythmics is another crucial consideration [14]. These drugs can prolong the QT interval and lead to arrhythmogenic events [14]. Therefore, a detailed medication history and review of potential drug interactions are essential in the differential diagnosis of Torsades de Pointes [1].

Self-Quiz
Ask Yourself...
- When distinguishing Torsades de Pointes from ventricular tachycardia and ventricular fibrillation, what specific ECG characteristics and clinical signs should healthcare providers focus on to make an accurate diagnosis?
- How can the evaluation of dialysis-related complications and a detailed medication history help in differentiating between Torsades de Pointes and other potential causes of arrhythmias?
Assessment & Evaluation
Providers can diagnose Torsades de Pointes (TdP) through ECG, which shows an undulating QRS axis with the polarity of complexes shifting around the baseline [6]. Between episodes, the ECG can reveal a prolonged QT interval corrected for heart rate (QTc). Consider QTc values normal at 0.40 seconds for men and 0.41 seconds for women, and prolonged when exceeding 0.47 seconds for men or 0.48 seconds for women [6]. Family history may suggest congenital syndrome.
ECG warning signs of impending TdP VT, besides QT interval prolongation, include fusion of T-wave and U-wave (sometimes with giant TU waves), post-extra systolic changes in repolarization patterns, macroscopic T-wave alternans (visible alternation between two different T-wave appearances), frequent polymorphic ventricular premature beats representing single beats of TdP, often in bigeminy, and repetitive short runs of polymorphic ventricular tachycardia [15].
Although patients may have a normal QTc, it prolongs around the time of TdP VT. Just before TdP VT, the QT interval is often long and shows a giant TU wave. [16]. Abnormal, giant T-U waves distinguish TdP initiation in LQTS patients from PVCs in other heart diseases and other PVCs in LQTS patients [15]. These ECG analyses suggest that early after-depolarizations initiate TdP and, if present, may help identify an imminent risk for TdP [15][16].

Self-Quiz
Ask Yourself...
- How do the specific ECG characteristics, such as an undulating QRS axis and prolonged QT interval, aid in diagnosing Torsades de Pointes, and what other ECG warning signs should healthcare providers look for to identify an imminent risk of TdP?
- Considering the role of early after-depolarizations in initiating TdP, what factors in a patient’s family history and clinical presentation help distinguish between congenital long QT syndrome and other causes of prolonged QT intervals?
Epidemiology
The prevalence of congenital prolonged QT syndrome suggests that between 1 in 2,000 and 1 in 20,000 people have the genetic mutation for QT prolongation [17]. Torsades de Pointes is 2-3 times more common in women than in males, with women tending to have longer QT intervals and experiencing more QT prolongation as a result of drug therapy [17]. Several European centers estimate the annual reporting rate of drug-induced Torsades de Pointes to range from 0.8 to 1.2 cases per million person-years [3].

Self-Quiz
Ask Yourself...
- Given that congenital prolonged QT syndrome affects between 1 in 2,000 and 1 in 20,000 people and is more common in males, how should healthcare providers approach screening and prevention strategies when considering the annual reporting rate of drug-induced Torsades de Pointes?
Pathophysiology
The suspected mechanism for Torsades de Pointes involves inhibiting the delayed rectifier potassium current, leading to an accumulation of positive ions within the cellular membrane and causing a prolonged repolarization phase [1]. If an ectopic beat occurs during this phase, known as an R on T phenomenon, it can trigger Torsades de Pointes [1] [24]. Both congenital and drug-induced QT prolongation affect the cellular membrane by blocking the potassium channel [1]. Unlike ventricular fibrillation, Torsades de Pointes can resolve, but if left untreated, it can progress into ventricular fibrillation. The risk of TdP increases when the QTc prolongation exceeds 0.50 seconds [6].
The most common factor in acquired TdP is a QT interval–prolonging Class Ia, Ic, or Class III antiarrhythmic drugs [6]. Other drugs that can induce TdP include tricyclic antidepressants, phenothiazines, and certain antivirals and antifungals [18]. Other predisposing causes often coexist, such as female sex, older age, hypokalemia, hypomagnesemia, hypothyroidism, slow or irregular ventricular rates, acute intracranial events (e.g., bleeding, stroke, traumatic brain injury), eating disorders, organophosphate poisoning, and structural heart disease (especially acute ischemia, myocarditis, and ventricular hypertrophy) [1] [6] [19].

Self-Quiz
Ask Yourself...
- How do the inhibition of the delayed rectifier potassium current and the accumulation of positive ions within the cellular membrane contribute to the development of Torsades de Pointes, and what implications does this mechanism have for the prevention and treatment of this arrhythmia?
- Considering the various drugs and predisposing conditions that can lead to acquired Torsades de Pointes, what strategies should healthcare providers implement to identify and mitigate these risk factors in patients at higher risk for developing this arrhythmia?
Treatment & Management
The initial step in managing Torsades de Pointes (TdP) involves preventing its onset by addressing modifiable risk factors [20]. This includes discontinuing any QT-prolonging medications and optimizing the patient’s electrolyte levels. Correcting hypokalemia, hypomagnesemia, and hypocalcemia can help prevent TdP. Some studies suggest a potential prophylactic benefit of oral or IV magnesium for patients with drug-induced prolonged QT, though its overall benefit is not well established, and there is little evidence that magnesium affects the QT interval [1] [20].

Self-Quiz
Ask Yourself...
- What are the most effective strategies for preventing the onset of Torsades de Pointes by addressing modifiable risk factors, and how can healthcare providers balance the use of prophylactic magnesium in patients with drug-induced prolonged QT despite limited evidence of its overall benefit?
Assessment and Immediate Management
Management of Torsades begins with assessing the patient’s hemodynamic stability. While most episodes are self-limiting, the risk is higher in patients who may develop ventricular fibrillation. Perform electrical cardioversion in cases of hypotension or cardiac arrest due to TdP [21]. Recommend synchronized cardioversion for unstable patients with a pulse (100J monophasic, 50J biphasic), and defibrillate pulseless TdP [1] [21].

Self-Quiz
Ask Yourself...
- How should healthcare providers prioritize and implement interventions for managing Torsades de Pointes in patients with varying degrees of instability?
First-Line Therapy
Intravenous magnesium is the first-line pharmacologic therapy for TdP [22]. Magnesium stabilizes the cardiac membrane, though the exact mechanism is unknown. The recommended initial dose is a slow 2 g IV push, followed by an infusion of 1 g to 4 g per hour to maintain magnesium levels above 2 mmol/L [1] [23]. Stop the infusion once levels exceed 3 mmol/L. Severe magnesium toxicity, characterized by confusion, respiratory depression, coma, and cardiac arrest, occurs at levels above 3.5 mmol/L [1]. It is also important to correct any hypokalemia, maintaining serum potassium between 4.5 mmol/L and 5 mmol/L.
Correcting electrolyte hypokalemia is crucial as these imbalances can increase the risk of ventricular arrhythmias [29]. Use lidocaine, a class Ib antiarrhythmic drug, to shorten the QT interval in cases of drug-induced TdP, and avoid class Ia, Ic, and III antiarrhythmics [30].


Self-Quiz
Ask Yourself...
- How should healthcare providers balance the administration of intravenous magnesium for treating Torsades de Pointes, ensuring effective stabilization of the cardiac membrane while avoiding the risks of magnesium toxicity?
For Intermittent Runs
For patients experiencing intermittent runs of TdP despite magnesium treatment, increasing the heart rate may help. Providers can achieve this through pharmacological interventions with isoproterenol, a non-selective beta-agonist that increases heart rate and shortens the QT interval, reducing the likelihood of an R-on-T phenomenon leading to TdP [24]. Administer isoproterenol as a 10 mcg to 20 mcg IV push or titrate an infusion to maintain a heart rate of 100 bpm [26]. However, it is contraindicated in patients with congenital prolonged QT, as it can lengthen the QT interval [25] [26].

Self-Quiz
Ask Yourself...
- How can healthcare providers use isoproterenol to manage patients experiencing intermittent runs of Torsades de Pointes despite magnesium treatment, and what considerations are the considerations for patients with congenital prolonged QT to avoid exacerbating their condition?
Overdrive Pacing
Use overdrive pacing as another option for terminating TdP, effective for both frequent runs of TdP and TdP refractory to magnesium [27]. Recommend overdrive pacing for drug-induced TdP, as it terminates drug-induced prolonged QT intervals and consequent TdP by shortening the QT interval [27] [28]. Temporary transvenous overdrive pacing at rates of around 100 bpm has proven effective in terminating TdP [28].
Addressing Precipitating Factors
- Hypokalemia: Treat and target a high-normal potassium level (>4.5 mEq/L).
- Hypocalcemia: Treat if present, as it may promote TdP.
- Hypothermia: Reverse.
Common Medications Linked to TdP
Antiarrhythmics
- Class IA: Quinidine, disopyramide, procainamide [30].
- Class IC: Flecainide [30].
- Class III: Dofetilide, ibutilide, sotalol, dronedarone [30].
Psychotropics
- Haloperidol, droperidol, chlorpromazine, pimozide [31].
- Citalopram, escitalopram [31].
- Tricyclic antidepressants [31].
Antibiotics
- Clarithromycin, erythromycin [32].
- Fluoroquinolones [32].
- Fluconazole, itraconazole, voriconazole, posaconazole [32].
- Pentamidine [32].
Other
- Methadone [35].
- Cocaine, loperamide (when abused in massive doses) [33] [34].
- Ondansetron (via rapid administration) [36].
- Propofol [37].
- Arsenic trioxide, sunitinib, vandetanib [38].
Long-Term Management
For long-term management, avoiding conditions and medications that prolong the QT interval is essential. Some patients with an acquired long QT-interval syndrome may have an underlying subclinical congenital long QT-interval syndrome, as suggested by persistent QTc interval prolongation even after removing exogenous QTc-prolonging factors.

Self-Quiz
Ask Yourself...
- How does overdrive pacing function to terminate Torsades de Pointes, particularly in cases refractory to magnesium treatment, and what are the key considerations for its use in drug-induced TdP?
- What strategies should healthcare providers employ to address precipitating factors such as hypokalemia, hypocalcemia, and hypothermia in patients with Torsades de Pointes, and how can the avoidance of specific medications help in the long-term management of QT interval prolongation?
- Given that QTc prolongation characterizes Torsades de Pointes (TdP) and poses a significant risk of degenerating into ventricular fibrillation, how can healthcare providers recognize and manage the key ECG warning signs, and what strategies should be employed to address modifiable risk factors and ensure long-term management?
Conclusion
Torsades de Pointes (TdP), characterized by oscillatory changes in the QRS complex amplitude on an electrocardiogram, is a type of polymorphic ventricular tachycardia associated with QTc prolongation [1]. This prolonged QT interval reflects delayed ventricular repolarization, predisposing the myocardium to early after-depolarizations and triggers re-entrant tachycardias such as TdP [2]. While TdP can sometimes resolve without intervention, it poses a significant risk of degenerating into ventricular fibrillation, leading to sudden death if not promptly recognized and treated [1][24]. The ECG is diagnostic, revealing key warning signs such as giant T-U waves and a prolonged QT interval [15][16].
Management of TdP involves addressing modifiable risk factors, discontinuing QT-prolonging medications, and correcting electrolyte imbalances [1]. Intravenous magnesium is the first-line treatment, stabilizing the cardiac membrane to prevent further episodes [20][22]. For patients experiencing recurrent TdP despite magnesium therapy, increasing the heart rate through isoproterenol or overdrive pacing can be effective [27]. Healthcare providers must train to recognize ECG signs of TdP and use temporary pacing modalities.
Long-term management includes avoiding conditions and medications that prolong the QT interval and considering underlying congenital long QT syndrome in persistent cases. Vigilant monitoring and rapid interventions can mitigate the risks associated with TdP.
References + Disclaimer
- Cohagan, B., & Brandis, D. (2023, August 8). Torsade de Pointes. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK459388/
- Jankelson, L., Karam, G., Becker, M. L., Chinitz, L. A., & Tsai, M. (2020). QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: A systematic review. Heart Rhythm, 17(9), 1472–1479. https://doi.org/10.1016/j.hrthm.2020.05.008
- Cabahug, M. C., & Vempati, A. (2022). Torsade de Pointes Due to Hypokalemia and Hypomagnesemia. PubMed, 7(4), S27–S51. https://doi.org/10.21980/j8jp8g
- Chen, C. H., De Souza, A., Franciosi, S., Harris, K. C., & Sanatani, S. (2022). Physical activity in pediatric long QT syndrome patients. CJC Pediatric and Congenital Heart Disease, 1(2), 80–85. https://doi.org/10.1016/j.cjcpc.2021.12.001
- Schnell, F., Behar, N., & Carré, F. (2018). Long-QT syndrome and competitive sports. Arrhythmia & Electrophysiology Review, 7(3), 187. https://doi.org/10.15420/aer.2018.39.3
- Mitchell, L. B. (2023, January 10). Torsades de Pointes Ventricular Tachycardia. Merck Manual Professional Edition. https://www.merckmanuals.com/professional/cardiovascular-disorders/specific-cardiac-arrhythmias/torsades-de-pointes-ventricular-tachycardia
- Qiu, H., Li, H., Zhang, S., Zhou, X., & Li, W. (2021). Torsades de pointes episode in a woman with high-grade fever and inflammatory activation: A case report. World Journal of Clinical Cases, 9(12), 2899–2907. https://doi.org/10.12998/wjcc.v9.i12.2899
- Ambhore, A., Teo, S., Omar, A., & Poh, K. (2014). Importance of QT interval in clinical practice. Singapore Medical Journal/Singapore Medical Journal, 55(12), 607–612. https://doi.org/10.11622/smedj.2014172
- Gray, B., Baruteau, A., Antolin, A. A., Pittman, A., Sarganas, G., Molokhia, M., Blom, M. T., Bastiaenen, R., Bardai, A., Priori, S. G., Napolitano, C., Weeke, P. E., Shakir, S. A., Haverkamp, W., Mestres, J., Winkel, B., Witney, A. A., Chis-Ster, I., Sangaralingam, A., . . . Behr, E. R. (2022). Rare variation in drug metabolism and long QT genes and the genetic susceptibility to acquired long QT syndrome. Circulation. Genomic and Precision Medicine, 15(1). https://doi.org/10.1161/circgen.121.003391
- Padilla, O., & Abadie, J. (2021, December 2). Normal laboratory values. Merck Manual Professional Edition. https://www.merckmanuals.com/professional/resources/normal-laboratory-values/normal-laboratory-values
- Desai, D. S., & Hajouli, S. (2023, June 5). Arrhythmias. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK558923/
- Akhtar, Z., Leung, L. W., Kontogiannis, C., Chung, I., Waleed, K. B., & Gallagher, M. M. (2022). Arrhythmias in chronic kidney disease. European Cardiology, 17. https://doi.org/10.15420/ecr.2021.52
- Pascual, J. F., Marchite, P. J., Silva, J. R., & Gándara, N. R. (2023). Arrhythmic syncope: From diagnosis to management. World Journal of Cardiology, 15(4), 119–141. https://doi.org/10.4330/wjc.v15.i4.119
- Li, M., & Ramos, L. G. (2017, July 1). Drug-Induced QT Prolongation And Torsades de Pointes. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5481298/
- Sridharan, A., Han, J. K., & Feliciano, Z. (2021). An ominous ECG. Circulation, 144(3), 246–248. https://doi.org/10.1161/circulationaha.121.055225
- Kahlon, S. S., Sikandar, R., Tejovath, S., Nair, S., Hassan, D., Patel, K. K., Peddemul, A., & Mostafa, J. A. (2022). Diagnosing Torsades de pointes based on correlation to QT Interval: A Systematic review. Curēus. https://doi.org/10.7759/cureus.27833
- Galić, E. (2021). Congenital Long QT Syndrome: a Systematic Review. Acta Clinica Croatica. https://doi.org/10.20471/acc.2021.60.04.22
- Das, B., Ramasubbu, S. K., Agnihotri, A., Kumar, B., & Rawat, V. S. (2021). Leading 20 drug–drug interactions, polypharmacy, and analysis of the nature of risk factors due to QT interval prolonging drug use and potentially inappropriate psychotropic use in elderly psychiatry outpatients. Therapeutic Advances in Cardiovascular Disease, 15, 175394472110588. https://doi.org/10.1177/17539447211058892
- Gregory, T., & Smith, M. (2012). Cardiovascular complications of brain injury. Continuing Education in Anaesthesia, Critical Care & Pain, 12(2), 67–71. https://doi.org/10.1093/bjaceaccp/mkr058
- Khatib, R., Sabir, F. R. N., Omari, C., Pepper, C., & Tayebjee, M. H. (2020). Managing drug-induced QT prolongation in clinical practice. Postgraduate Medical Journal, 97(1149), 452–458. https://doi.org/10.1136/postgradmedj-2020-138661
- Goyal, A., Sciammarella, J. C., Chhabra, L., & Singhal, M. (2023, March 27). Synchronized Electrical cardioversion. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482173/
- Tzivoni, D., Banai, S., & Keren, A. (2024). Story of magnesium for torsade de pointes. Heart Rhythm, 21(4), 360–361. https://doi.org/10.1016/j.hrthm.2024.01.013
- Farkas, J. (2018, November 27). PulmCrit- A better approach to Torsade de Pointes. EMCrit Project. https://emcrit.org/pulmcrit/tdp-magnesium/
- Mahanta, D. S., Barik, R. C., & Acharya, D. (2023). R on T phenomenon, a dangerous ECG sign. Visual Journal of Emergency Medicine, 32, 101790. https://doi.org/10.1016/j.visj.2023.101790
- Szymanski, M. W., & Singh, D. P. (2023, May 1). Isoproterenol. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK526042/
- Suarez, K., Mack, R., Hardegree, E. L., Chiles, C., Banchs, J. E., & Gonzalez, M. D. (2018). Isoproterenol suppresses recurrent torsades de pointes in a patient with long QT syndrome type 2. HeartRhythm Case Reports, 4(12), 576–579. https://doi.org/10.1016/j.hrcr.2018.08.013
- Koh, E. J., Yee, X. Q., Chin, M. L., & Latib, N. L. B. A. (2024). Overdrive Pacing for Persistent Torsades de Pointes and Pulseless Ventricular Tachycardia. PubMed, 14(1), 42–47. https://doi.org/10.6705/j.jacme.202403_14(1).0006
- Zhang, Y., Wang, X., Pan, Y., Du, B., Nanthakumar, K., & Yang, P. (2022). Overdrive pacing in the acute management of osimertinib-induced ventricular arrhythmias: A case report and literature review. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.934214
- Kyaw, M. T., & Maung, Z. M. (2022). Hypokalemia-Induced Arrhythmia: A case series and literature review. Curēus. https://doi.org/10.7759/cureus.22940
- King, G. S., Goyal, A., Grigorova, Y., Patel, P., & Hashmi, M. F. (2024, February 28). Antiarrhythmic medications. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482322/
- Sicouri, S., & Antzelevitch, C. (2020). Mechanisms underlying the actions of antidepressant and antipsychotic drugs that cause sudden cardiac arrest. Radcliffe Cardiology. https://www.aerjournal.com/articles/mechanisms-underlying-actions-antidepressant-and-antipsychotic-drugs-cause-sudden-cardiac
- Teng, C., Walter, E. A., Gaspar, D. K. S., Obodozie-Ofoegbu, O. O., & Frei, C. R. (2019). Torsades de pointes and QT prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. International Journal of Medical Sciences, 16(7), 1018–1022. https://doi.org/10.7150/ijms.34141
- Isang, E., Shali, L., Morris, C. B., & Mahlow, J. (2021). Loperamide-Induced Torsades de Pointes. Curēus. https://doi.org/10.7759/cureus.20299
- Noori, M. a. M., Fichadiya, H., Jesani, S., Abid, F., Sachdeva, N., Saeed, H., Jawed, Q., Elkattawy, S., & Joshi, M. (2022). A rare yet morbid complication of cocaine use: Brugada Type 1 on electrocardiogram. Curēus. https://doi.org/10.7759/cureus.24309
- Khalesi, S., Shemirani, H., & Dehghani-Tafti, F. (2014, November 1). Methadone induced torsades de pointes and ventricular fibrillation: A case review. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4354087/
- Patel, E., Rosemond, D., & Afzal, A. (2019). Ondansetron induced torsades de pointes. Clinical Case Reports, 7(8), 1557–1558. https://doi.org/10.1002/ccr3.2251
- Abrich, V. A., Ramakrishna, H., Mehta, A., Mookadam, F., & Srivathsan, K. (2017). The possible role of propofol in drug-induced torsades de pointes: A real-world single-center analysis. International Journal of Cardiology, 232, 243–246. https://doi.org/10.1016/j.ijcard.2017.01.011
- Porta‐Sánchez, A., Gilbert, C., Spears, D., Amir, E., Chan, J., Nanthakumar, K., & Thavendiranathan, P. (2017). Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic review. Journal of the American Heart Association. Cardiovascular and Cerebrovascular Disease, 6(12). https://doi.org/10.1161/jaha.117.007724
- Tisdale, J. E., Chung, M. K., Campbell, K. B., Hammadah, M., Joglar, J. A., Leclerc, J., & Rajagopalan, B. (2020). Drug-Induced Arrhythmias: A scientific statement from the American Heart Association. Circulation, 142(15). https://doi.org/10.1161/cir.0000000000000905
- Chrysant, S. G. (2019). Proton pump inhibitor-induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Review of Cardiovascular Therapy, 17(5), 345–351. https://doi.org/10.1080/14779072.2019.1615446
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
