Understanding GLP-1 Medications
Contact Hours: 2
Author(s):
R.E. Hengsterman MSN, RN
Course Highlights
- Discover how combining lifestyle modifications with GLP-1 receptor agonists can improve weight loss, glycemic control, and overall cardiometabolic health
- Examine the science behind GLP-1 receptor agonists, from their effects on insulin secretion and appetite regulation to next-generation agents like tirzepatide and orforglipron
- Learn best practices for patient education, dosing strategies, and interprofessional collaboration while addressing contraindications, adverse effects, and compounding warnings
Introduction
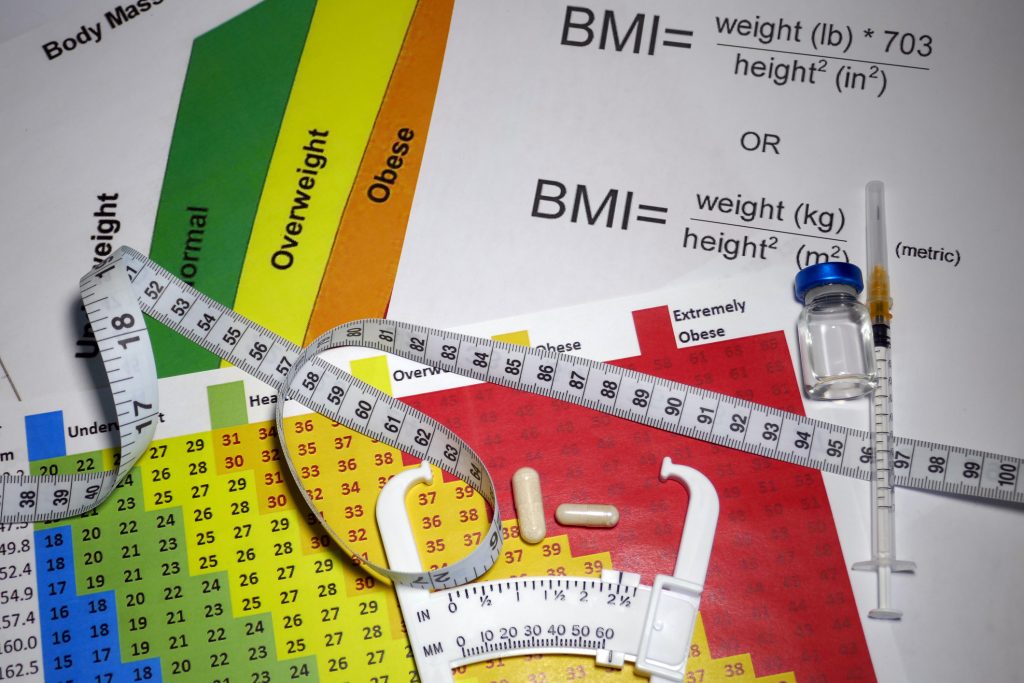
Obesity is a chronic and prevalent condition that impacts health, contributing to higher rates of morbidity and mortality. The body mass index (BMI) measures obesity as a weight-to-height ratio defined by the World Health Organization (WHO) as weight in kilograms divided by the square of height in meters (kg/m²) (1). The recommended levels follow the global WHO standard of 18.5–24.9 for a normal BMI (1). The World Health Organization classifies adults with a BMI of 25.0 to 29.9 kg/m² as overweight and those with a BMI of 30 kg/m² or higher as having obesity (2).
For children and adolescents aged 2 to 18, providers assess BMI using a percentile scale based on gender and age, defining obesity as a BMI at or above the 95th percentile and overweight as falling between the 85th and 94th percentiles (3). Increasing BMI has been correlated with higher mortality risks, including a 29% rise in overall mortality and over a 200% increase in diabetes-related mortality for every 5-unit BMI increase above 25 kg/m² (4).
While BMI is a valuable tool, measures of central adiposity, such as waist circumference, provide additional insight into cardiometabolic risks. In normal weight ranges, thresholds for abdominal obesity start at ≥80 cm for women and ≥90 cm for men (5).
Glucagon-like peptide-1 (GLP-1) agonists, designed for managing type 2 diabetes, now treat obesity due to their metabolic effects (6). These medications, known as GLP-1 receptor agonists (GLP-1-RAs), offer promising therapeutic options for type 2 diabetes and obesity-related conditions, including cardiovascular disease (CVD) and non-alcoholic fatty liver disease (NAFLD) (6).
Most often administered as subcutaneous injections, these medications help lower serum glucose levels, slow gastric emptying, reduce appetite, and promote weight loss (6). FDA-approved since 2005, GLP-1 agonists, including exenatide and newer formulations, have shown promise in improving glycemic control and aiding in sustained weight management (7). Despite their effectiveness, GLP-1 agonists are part of a broader treatment strategy requiring lifestyle and dietary modifications.
These drugs also exhibit additional benefits, such as cardio- and neuroprotection, anti-inflammatory properties, and modulation of reward and food intake behaviors. While well-tolerated, they require careful consideration of potential adverse effects and contraindications (6). An interprofessional team—including endocrinologists, primary care clinicians, nurses, and pharmacists—is critical in optimizing patient outcomes using GLP-1 receptor agonists.
Ask yourself...
- How might focusing on BMI as a measure of obesity and health overlook other key factors contributing to cardiometabolic risks, and what alternative metrics could provide a more comprehensive evaluation?
- What ethical considerations emerge when prescribing GLP-1 receptor agonists for obesity management, given the necessity of lifestyle and dietary modifications for long-term effectiveness?
From Insulin to GLP-1: Tracing the Path of Diabetes Innovation
Obesity is a complex condition that impacts overall health, contributing to increased morbidity and mortality. In the 1960s, manufacturers introduced short-term weight-loss medications such as phentermine, benzphetamine, and diethylpropion, but researchers did not collect long-term safety data (8).
In 1999, orlistat became the first drug approved for chronic weight management, acting by reducing intestinal fat absorption [9]. Modern research attributes excessive adiposity to dysfunctions in satiety hormone signaling and central nervous system (CNS) feeding centers (10). These interactions involve orexigenic hormones like ghrelin and anorexigenic hormones such as GLP-1, leptin, and peptide YY (PYY) (10). Post-weight-loss hormonal adaptations exacerbate challenges, with decreases in anorexigenic hormones and increases in orexigenic ones driving weight regain (10).
Regulatory authorities discontinued early appetite-suppressant drugs, including fenfluramine and sibutramine, because of safety concerns (11). Between 2012 and 2014, regulatory authorities approved phentermine/topiramate extended-release, liraglutide (3 mg/day), and naltrexone/bupropion extended-release, which achieved sustained weight loss (12). Semaglutide (2.4 mg/week), approved in 2021, delivers improved weight loss compared to the earlier treatments and represents considerable progress in obesity pharmacotherapy (13).
GLP-1 receptor agonists (GLP-1 RAs) have transformed obesity and Type 2 diabetes mellitus (T2DM) management, offering benefits in glucose regulation, weight loss, and cardiometabolic health (6). These drugs, derived from the hormone glucagon-like peptide-1, enhance insulin secretion, delay gastric emptying, reduce appetite, and promote weight loss [6]. The evolution of GLP-1 RAs began with the discovery of glucagon and its role in glucose metabolism (14).
Subsequent research identified glucagon-like immunoreactive material in the intestine, secreted by L-cells, which stimulated insulin release but lacked hyperglycemic effects 914). This led to the identification of proglucagon and its derivatives, including GLP-1. The approval of GLP-1 RAs, starting with exenatide in 2005, followed by semaglutide and oral formulations, has expanded treatment options for patients seeking effective, long-term solutions for T2DM and obesity (15).
GLP-1 RAs also exhibit protective effects against cardiovascular and renal complications, making them integral to comprehensive metabolic disease management (6)(15). Ongoing research aims to refine these therapies further, ensuring maximum efficacy and safety for diverse patient populations.
Ask yourself...
- What mechanisms in the central nervous system (CNS) and satiety hormone signaling contribute to the challenges of maintaining weight loss after initial success, and how might GLP-1 receptor agonists address these issues?
- How does the evolution of obesity pharmacotherapy, from early appetite-suppressant drugs to modern GLP-1 receptor agonists, reflect advancements in our understanding of metabolic processes and patient safety concerns?
- What specific benefits do GLP-1 receptor agonists offer beyond weight loss and glucose regulation, and how might these benefits influence their role in comprehensive metabolic disease management?
GLP-1 Case Study
- Name: Richard J., a 58-year-old male
- Medical History:
- Type 2 Diabetes Mellitus (diagnosed 7 years ago)
- Obesity (BMI 35 kg/m²)
- Hypertension (well-controlled on an ACE inhibitor)
- Mild diabetic retinopathy
- Current Medications: Metformin 1000 mg twice daily, glimepiride 2 mg once daily, and lisinopril 20 mg once daily
Clinical Presentation
Richard presents a routine follow-up. Despite being on metformin and glimepiride, his diabetes control has worsened over the past year, with an HbA1c increasing from 7.5% to 8.6%. He reports a recent 5-kg weight gain and struggles to maintain dietary restrictions. He experiences occasional episodes of mild hypoglycemia with his current sulfonylurea (glimepiride) therapy but is more concerned about progressive weight gain and feeling fatigued.
Decision to Initiate GLP-1 Therapy
Richard’s endocrinologist is considering adding a GLP-1 receptor agonist to improve glycemic control and reduce weight. The clinician chooses semaglutide, drawing insights from landmark trials (including data from the LEAD study on liraglutide and parallel findings with semaglutide) (16).
- Proven Efficacy: In large-scale studies, semaglutide has demonstrated significant HbA1c reductions and cardiovascular benefits (17).
- Weight Loss Advantage: Clinical data show robust weight loss outcomes, aligning with Richard’s goal of achieving a healthier BMI (17).
- Long-Term Outcome Benefits: Similar to findings from the CORE diabetes model on liraglutide, semaglutide offers the potential for lower cardiovascular risk, reduced incidence of diabetes-related complications, and improved long-term survival (16)(17).
Initial Management and Education
- Starting Dose: Semaglutide 0.25 mg once weekly (to minimize gastrointestinal side effects), with a plan to titrate to 1.0 mg weekly over several weeks.
- Adjustment of Other Medications:
- Providers lower the glimepiride dose to 1 mg once daily to reduce the risk of hypoglycemia.
- Metformin remains unchanged, as Richard tolerates it well.
- Lifestyle Counseling:
- Nutrition: Recommends a moderate-calorie, balanced diet focusing on whole grains, lean protein, and vegetables.
- Physical Activity: Encourages 150 minutes of moderate-intensity exercise per week—Richard opts for brisk walking and occasional cycling.
- Self-Monitoring: Advises regular home glucose checks to detect potential hypoglycemia, especially during dose escalation.
- Follow-Up Visits and Outcomes
- Three-Month Check
- Weight: Down 3 kg (BMI 34 kg/m²)
- HbA1c: Decreased to 7.8% from 8.6%
- Blood Pressure: Controlled at 128/80 mmHg
- Side Effects: Mild nausea during the first few weeks, which subsided with slow dose titration
- Six-Month Check
- Weight: Down a total of 6 kg (BMI 33 kg/m²)
- HbA1c: Further improvement to 7.3%
- Cardiometabolic Indicators: Mild improvement in LDL cholesterol (from 110 mg/dL to 98 mg/dL); blood pressure remains controlled
- Medication Tolerability: Occasional early satiety reported, but no severe adverse events
- One-Year Visit
- Weight: Down 9 kg overall (BMI 31 kg/m²), approaching his target of losing 10–12 kg
- HbA1c: 6.9%, well within the personalized target range set by his endocrinologist
- Lifestyle: Continues brisk walking 3–4 times weekly and has enrolled in a weight management support group
- Quality of Life: Richard reports feeling more energetic, with fewer episodes of low blood sugar after the sulfonylurea dose adjustment
Discussion and Alignment with Research Findings
- Long-Term Projection: Based on modeling data akin to the CORE diabetes model, semaglutide may offer substantial long-term benefits, including lower risks of cardiovascular complications and improved survival (17)(18).
- Weight Maintenance Synergy: The benefits of combining medication therapy with increased physical activity mirror evidence from exercise-plus-liraglutide studies, highlighting that lifestyle interventions coupled with GLP-1 receptor agonists can enhance weight reduction and metabolic control.
- Limitations: As with all GLP-1 therapies, ongoing monitoring for gastrointestinal side effects and potential hypoglycemia (especially in combination with a sulfonylurea) remains crucial.
Conclusion
Richard J.’s experience with semaglutide illustrates how a popular GLP-1 receptor agonist can improve glycemic control, promote weight loss, and reduce long-term diabetes-related complications. His clinical journey aligns with evidence from pivotal GLP-1 studies—such as those in the LEAD series for liraglutide—and reinforces the value of integrative management strategies, which include medication titration, lifestyle modification, and vigilant follow-up (16).
Ask yourself...
- What factors should providers consider when initiating semaglutide therapy in a patient like Richard regarding his comorbid conditions and previous treatment outcomes?
- How does combining semaglutide therapy with lifestyle modifications such as a balanced diet and physical activity enhance the overall effectiveness of treatment, and what challenges might arise in maintaining these changes?
- Given Richard’s experience with mild nausea and occasional hypoglycemia, how should providers adjust his therapy and follow-up plan to ensure long-term tolerability and adherence while minimizing adverse effects?
List of the most common GLP-1 agonists include:
- Dulaglutide (Trulicity®).
- Exenatide (Byetta®).
- Exenatide extended-release (Bydureon®).
- Liraglutide (Victoza®).
- Lixisenatide (Adlyxin®).
- Semaglutide injection (Ozempic®).
- Semaglutide tablets (Rybelsus®).
Indications for Use
GLP-1 receptor agonists, also known as incretin mimetics or GLP-1 analogs, are a class of medications used to manage Type 2 diabetes mellitus (T2DM) and, in some cases, obesity. According to the American Diabetes Association (ADA), metformin remains the preferred first-line therapy for T2DM (19).. Providers recommend GLP-1 receptor agonists for patients who cannot tolerate metformin, exceed a hemoglobin A1c (HbA1c) target by more than 1.5%, or fail to achieve glycemic goals within three months of treatment (20).
These agents benefit individuals with atherosclerosis, heart failure, or chronic kidney disease (CKD) (6). The FDA approves high-dose Liraglutide and Semaglutide for obesity management and allows their use for overweight patients with comorbidities (21). While these medications show favorable outcomes in HbA1c reduction and weight loss, cost and tolerability remain significant barriers to widespread use.
The 2023 ADA guidelines recommend GLP-1 receptor agonists to reduce cardiovascular risk in individuals with a history of atherosclerotic cardiovascular disease (ASCVD), including myocardial infarction and stroke (22). Agents with proven cardiovascular benefits include Liraglutide, subcutaneous Semaglutide, and Dulaglutide (6)(12)(22). These medications have also shown potential in slowing CKD progression and reducing hypoglycemia risks (6). Experts classify GLP-1 receptor agonists into two types: human GLP-1 backbone agents (e.g., Liraglutide, Dulaglutide, Semaglutide) and exendin-4 backbone agents (e.g., exenatide) (23).
Emerging agents like Tirzepatide, which activates both GLP-1 and GIP receptors, and Orforglipron, a nonpeptide oral GLP-1 agonist in phase 2 trials, further expand the therapeutic potential of this class (23)(24). Companies discontinued agents like Lixisenatide and Albiglutide, while the approval of combination therapies like Liraglutide/insulin-degludec highlights the role of GLP-1 receptor agonists in diabetes and obesity management (23)(25).
Ask yourself...
- What clinical circumstances make GLP-1 receptor agonists better than metformin for managing Type 2 diabetes, and how do these circumstances influence patient outcomes?
- How do GLP-1 receptor agonists reduce cardiovascular risks in patients with a history of atherosclerotic cardiovascular disease (ASCVD), and why might these benefits be significant for long-term patient care?
- What factors could limit the widespread adoption of GLP-1 receptor agonists despite their proven benefits in HbA1c reduction, weight loss, and cardiovascular risk management, and how might healthcare systems address these barriers?
Mechanism of Action
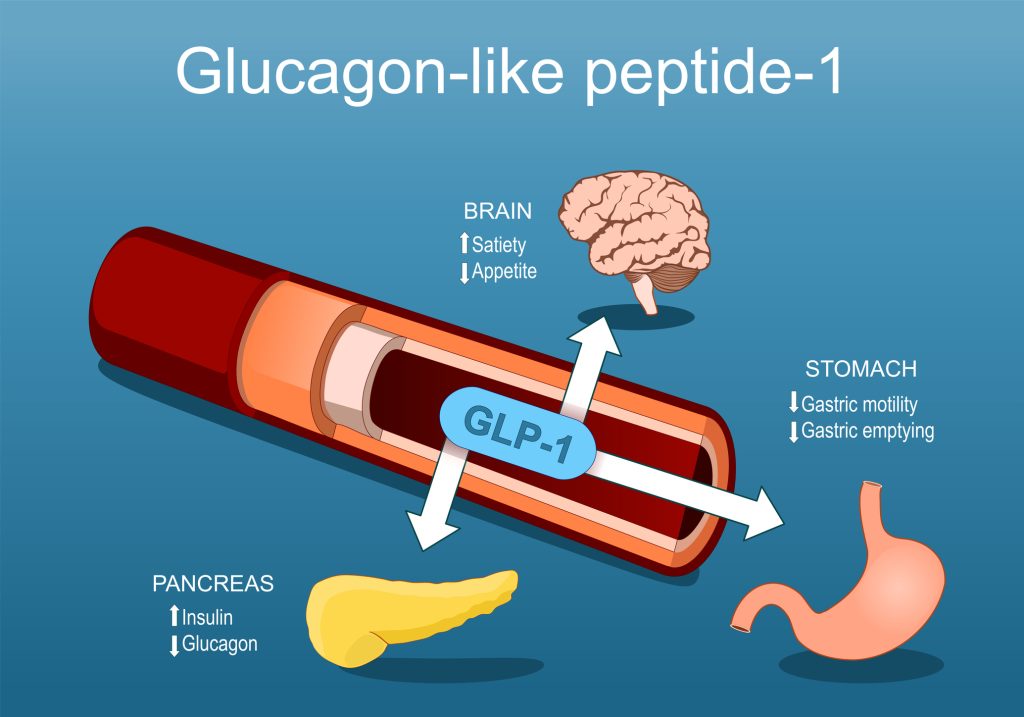
Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1 RAs) are a class of medications that mimic the natural hormone GLP-1, produced by the small intestine (23). GLP-1 plays several critical roles in glucose and appetite regulation: it stimulates insulin release from the pancreas, inhibits glucagon secretion to prevent excess glucose production, slows gastric emptying to moderate post-meal blood sugar spikes, and promotes satiety by acting on hunger centers in the brain (6)(23).
Pharmacological GLP-1 RAs replicate these actions, aiding in managing Type 2 diabetes mellitus (T2DM) and promoting weight loss (23). The higher the GLP-1 RA dose, the more pronounced these effects. These medications are effective in lowering HbA1c levels, delaying β-cell apoptosis, and enhancing β-cell proliferation, thereby improving long-term glycemic control (6)(23)(26).
GLP-1 RAs have evolved since the approval of exenatide in 2005. Earlier formulations required frequent dosing, but newer agents like Dulaglutide and Semaglutide provide once-a-week dosing, and regulators approved an oral Semaglutide formulation (18)(21)(27).
These medications also show cardiovascular and renal benefits, such as reducing blood pressure, improving endothelial function, and lowering the risk of cardiovascular events like myocardial infarction and stroke [6][16]. Studies demonstrate an average weight loss of 2.9 kg compared to placebo, alongside improved cholesterol levels and left ventricular function (23).
The satiety-inducing effects of GLP-1 RAs, combined with their ability to address hyperglycemia, make them a valuable tool for managing obesity and metabolic disorders. Emerging therapies, such as Tirzepatide, a dual GLP-1/GIP agonist, and Orforglipron, a nonpeptide oral GLP-1 RA, continue to expand the potential of this medication class in treating metabolic diseases (23)(24).
Ask yourself...
- How might the ability of GLP-1 receptor agonists to promote satiety and delay gastric emptying influence both short-term and long-term management strategies for obesity and Type 2 diabetes?
- What are the potential advantages and challenges of transitioning from earlier GLP-1 receptor agonist formulations requiring frequent dosing to newer options like Dulaglutide, Semaglutide, and oral formulations?
- Given the cardiovascular and renal benefits of GLP-1 receptor agonists, how do these effects expand their use beyond glycemic control in patients with metabolic disorders?
Absorption
Clinicians administer GLP-1 receptor agonists, such as Exenatide, Liraglutide, and Semaglutide, for rapid absorption and peak plasma concentrations within hours (16)(23). The oral formulation of Semaglutide uses a design that facilitates gastrointestinal absorption and overcomes challenges like first-pass metabolism and bioavailability (23)(28). Food intake and potential interactions with other medications influence effective dosing regimens for oral Semaglutide, making its administration unique among GLP-1 Ras (23).
Ask yourself...
- How does the unique design of oral Semaglutide address the challenges of first-pass metabolism and bioavailability compared to injectable GLP-1 receptor agonists?
- How do factors like food intake and interactions with other medications impact the dosing regimen and effectiveness of oral Semaglutide?
Distribution
Post-absorption, GLP-1 RAs exhibit a low volume of distribution, remaining within the bloodstream (23). These agents bind to GLP-1 receptors in tissues critical for glucose regulation, including pancreatic β-cells (29). This selective targeting underpins their efficacy in enhancing insulin secretion, inhibiting glucagon release, and promoting metabolic control in diabetes management (29).
Ask yourself...
- How does the selective binding of GLP-1 receptor agonists to pancreatic β-cells influence their role in regulating insulin secretion and glucagon release?
Metabolism
The metabolism of common GLP-1 RAs varies across agents:
- Exenatide undergoes hydrolysis in the kidneys and liver, producing smaller inactive peptides excreted via the kidneys (23).
- Liraglutide follows a similar proteolytic cleavage pathway in various tissues, akin to the metabolism of other large proteins. Enzymes such as Dipeptidyl Peptidase-4 (DPP-4) and Neutral Endopeptidase (NEP) contribute to this process, yielding inactive biological fragments (23)(30)
- Semaglutide, Serum, and tissue proteases metabolize the polypeptide into amino acids, clearing it from the system and preventing accumulation (23)(21).
Ask yourself...
- How does the metabolic process of exenatide in the kidneys and liver differ from the pathways utilized by liraglutide and semaglutide, and what implications might these differences have for patients with renal impairment?
- Considering the role of enzymes like DPP-4 and NEP in the metabolism of liraglutide, how might inhibitors of these enzymes impact the drug’s efficacy or duration of action?
Excretion
Renal elimination is the primary route for GLP-1 RA clearance (23). The kidneys play a critical role in excreting these agents, with the rate of renal elimination influencing their duration of action and dosing frequency. Semaglutide’s extended-release (ER) formulation provides a prolonged half-life compared to short-acting GLP-1 RAs, supporting once-weekly dosing regimens and enhancing patient adherence (23)(32).
Ask yourself...
- How might impaired renal function affect the clearance, dosing frequency, and efficacy of GLP-1 receptor agonists, such as semaglutide?
GLP-1 Agonist Use Overview
GLP-1 agonists are FDA-approved medications for managing Type 2 diabetes (T2D) because they lower blood sugar levels (15)(23). While metformin is the first-line oral medication for T2D, healthcare providers may recommend GLP-1 agonists in specific situations. These include cases where metformin is ineffective, contraindicated or when a client’s A1C remains above target levels despite three months of treatment if they also have conditions like atherosclerosis, heart failure, or chronic kidney disease (6)(16).
Effective T2D management involves a combination of therapies, including lifestyle and dietary changes, exercise, and medications. Clients and healthcare providers collaborate to create treatment plans, using GLP-1 agonists with other medications to achieve blood glucose control.
The use of GLP-1 agonists in Type 1 diabetes (T1D) is still under investigation (33). Preliminary studies suggest potential benefits, such as lowering A1C and promoting weight loss (33). Despite the spike in GLP-1 prescriptions for T1D, the FDA has not approved GLP-1 agonists for T1D treatment (33)(34).
Some healthcare providers may prescribe these medications off-label, so individuals with T1D should consult their provider to explore this option. Obesity management often requires a multifaceted approach, including dietary modifications, exercise, medications, behavior therapy, and, in some cases, bariatric surgery.
Ask yourself...
- What factors might healthcare providers consider when prescribing GLP-1 agonists for a client with Type 2 diabetes, and how do these factors influence the overall treatment plan?
- How could the ongoing investigation into the use of GLP-1 agonists for Type 1 diabetes shape future treatment options, and what ethical considerations might arise from prescribing these medications off-label?
Dosing Frequency Overview of Common GLP-1 Receptor Agonists
Liraglutide
Liraglutide treats Type 2 diabetes mellitus (T2DM) as the first daily injectable GLP-1 receptor agonist (GLP-1 RA) (15)(23).. The LEAD clinical trials, which began in 2006 and included thousands of participants worldwide, demonstrated liraglutide’s ability to improve glycemic control and promote weight loss (35). These trials compared liraglutide with other diabetes treatments, including glimepiride, metformin, and insulin glargine.
Results showed that liraglutide provided glycemic control comparable to or better than these alternatives, with additional benefits such as reduced weight gain and fewer hypoglycemic episodes (36). The LEAD-3 study found that liraglutide monotherapy reduced glycosylated hemoglobin (A1C), body weight, and blood pressure compared to glimepiride (36). The LEAD-6 trial demonstrated liraglutide’s superior glycemic control and tolerability compared to exenatide [37]. These findings solidified liraglutide as a valuable therapy for T2DM for individuals seeking weight management and a lower risk of hypoglycemia (35).
Beyond its role in diabetes management, liraglutide has proven effective in treating obesity, even among non-diabetic individuals. Clinical trials revealed significant weight loss with liraglutide at doses of up to 3.0 mg per day, with participants losing an average of 4–6 kg over 20 weeks, outperforming placebo and orlistat (38). Long-term studies confirmed sustained weight loss over two years, alongside reductions in prediabetes prevalence and cardiovascular risk factors (38).
While gastrointestinal side effects such as nausea were common, they were mild and temporary. Combining liraglutide therapy with exercise further enhanced outcomes, leading to more significant weight loss, improved insulin sensitivity, and better cardiovascular fitness than either intervention alone (36). Liraglutide causes nausea and rare cases of pancreatitis but demonstrates safety and effectiveness for most individuals, supporting its role in managing obesity and reducing related risks (35).
Ask yourself...
- What mechanisms of action allow liraglutide to manage Type 2 diabetes and support weight loss, and how do these mechanisms compare to other treatments like metformin or insulin glargine?
- Given the findings from the LEAD clinical trials, how would you evaluate the trade-offs between liraglutide’s benefits (e.g., glycemic control, weight loss) and its potential side effects, such as nausea or pancreatitis?
- How might combining liraglutide therapy with exercise amplify its benefits, and what implications does this have for developing comprehensive treatment plans for patients with obesity or Type 2 diabetes?
Semaglutide

Semaglutide, a GLP-1 receptor agonist (GLP-1 RA), has demonstrated remarkable efficacy in managing weight and improving glycemic control in individuals with Type 2 diabetes mellitus (T2DM) and obesity (23)(39). The STEP clinical trials, particularly at 2.4 mg a week, revealed significant weight loss in both diabetic and non-diabetic populations, with body weight reductions ranging from 10% to 15% over extended periods (6)(23)(39).
These trials, including STEP-1 through STEP-6, highlighted semaglutide’s superiority over placebo and other treatments, such as liraglutide, in promoting weight loss and enhancing cardiometabolic parameters, including blood pressure and lipid profiles [6][15]. Furthermore, the STEP-TEENS trial demonstrated semaglutide’s effectiveness in reducing BMI and cardiometabolic risks in obese adolescents, suggesting its potential for use in younger populations (40). Long-term studies confirmed that semaglutide supports sustained weight loss and improves physical and metabolic health, solidifying its role in managing obesity and related disorders (6)(23).
In addition to its effects on weight and glycemic control, semaglutide has shown renal protective benefits in individuals with T2DM and chronic kidney disease (23). Pooled analyses from trials such as SUSTAIN-6 and LEADER reported reductions in albuminuria and slower declines in estimated glomerular filtration rates (eGFR), with pronounced benefits observed in those with lower baseline eGFR (41). Exercise caution in conditions such as diabetic retinopathy, pancreatitis, and gallbladder dysfunction (23)(41).
While meta-analyses suggest a slight association between semaglutide and cholelithiasis, they do not indicate an increased risk of pancreatitis or pancreatic cancer (42). Semaglutide has also shown promise in reducing liver fat content and improving liver injury indices, offering potential therapeutic benefits for individuals with non-alcoholic steatohepatitis (NASH) and hepatic fibrosis (23)(41). Its broad range of therapeutic effects, combined with its established safety profile, underscores its versatility and value in addressing complex metabolic and endocrine disorders.
Ask yourself...
- How does semaglutide’s mechanism of action contribute to both its weight loss effects and its ability to improve glycemic control in individuals with Type 2 diabetes?
- What might be the implications of semaglutide’s renal protective benefits and its ability to reduce liver fat content for patients with multiple metabolic disorders, such as chronic kidney disease and non-alcoholic steatohepatitis?
- Given the potential risks associated with semaglutide, such as its effects on gallbladder dysfunction and diabetic retinopathy, how should clinicians balance these risks against its demonstrated benefits in managing obesity and Type 2 diabetes?
Tirzepatide
Tirzepatide, a novel dual agonist of GIP and GLP-1 receptors, has demonstrated exceptional efficacy in improving glycemic control and promoting weight loss in individuals with Type 2 diabetes mellitus (T2DM) and obesity (43). The extensive SURPASS clinical trials, encompassing eight completed studies, have evaluated tirzepatide as both monotherapy and in combination with other treatments (4). For instance, SURPASS-1 highlighted its effectiveness as a standalone therapy, showing significant reductions in HbA1c levels and notable weight loss [43]. SURPASS-2 further established tirzepatide’s superiority over semaglutide in glycemic control and weight management (44).
Trials such as SURPASS-3 and SURPASS-4 compared tirzepatide to insulin therapies, demonstrating superior weight reduction and glycemic outcomes (45). SURPASS-5 underscored its benefits for patients already on insulin glargine, while studies conducted in Japan, including SURPASS J-mono and SURPASS J-combo, reinforced its safety and efficacy in managing T2DM (46). Across these trials, tirzepatide delivered dose-dependent improvements in glycemic control and body weight, often surpassing comparators like semaglutide and dulaglutide (43)(44).
Regarding weight loss, the SURMOUNT-1 trial revealed meaningful results, with participants achieving body weight reductions of up to 20.9% when treated with 15 mg doses over 72 weeks [46]. Due to its significant weight loss effects, tirzepatide has also shown potential in managing conditions like non-alcoholic fatty liver disease (NAFLD), though further research is necessary (43)(46).
While approved for the treatment of T2DM, tirzepatide’s safety profile warrants caution in specific populations. It is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 (MEN 2) due to risks observed in animal studies (47). Providers take additional precautions for individuals with gallbladder disease, diabetic retinopathy, or a history of hypersensitivity reactions (47).
Tirzepatide does not treat Type 1 diabetes or NAFLD, and clinical trials explore its therapeutic applications, including cardiovascular benefits for individuals with T2DM and obesity (43). These promising findings position tirzepatide as a transformative therapy for metabolic and endocrine disorders.
The availability of injectable medications, such as GLP-1 receptor agonists, varies based on the specific drug prescribed. These medications may come in single- or multi-dose pens, and clients may require a separate prescription for needles, which are available in various gauge sizes. Pharmaceutical companies now offer combination therapies that pair GLP-1 analogs with long-acting insulin in a single injection.
This approach leverages the complementary actions of insulin, which lowers fasting and post-absorptive blood sugars, and GLP-1 agonists, which target postprandial blood sugars (6). This strategy may reduce the risk of hypoglycemia by decreasing reliance on bolus and basal insulin and mitigating weight gain often associated with insulin use.
Renal pathways excrete GLP-1 analogs, and clinicians do not adjust dosages for clients with mild renal or hepatic impairment (6). To maintain safety, follow the product monograph for dose modifications for older clients. Researchers lack sufficient data on their use in breastfeeding or pediatric populations, and no recommendations address these groups. GLP-1 analogs remain contraindicated during pregnancy, requiring alternative treatment approaches.
Ask yourself...
- How does tirzepatide’s dual action on GIP and GLP-1 receptors enhance its efficacy compared to other treatments like semaglutide and dulaglutide in managing Type 2 diabetes and obesity?
- What might be the physiological or clinical reasons behind tirzepatide’s contraindications in individuals with medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, and how could this impact its broader application?
- Considering the promising results of the SURPASS and SURMOUNT trials, what additional factors or challenges must researchers address before adopting tirzepatide for conditions beyond Type 2 diabetes, such as non-alcoholic fatty liver disease (NAFLD)?
Risk & Benefits
GLP-1 agonists, used to lower blood sugar levels and promote weight loss, offer additional potential benefits (4)(6). Studies suggest these medications may help lower blood pressure, improve lipid profiles, manage fatty liver disease, and reduce risks of heart and kidney disease (4)(6)(10). They may also slow the progression of diabetes-related nephropathy, making them valuable in comprehensive diabetes management (10).
The most common side effects associated with GLP-1 agonists include appetite loss, nausea, vomiting, and diarrhea, which are often more pronounced when starting the medication or increasing the dosage (4)(10)(23). Other side effects include dizziness, mild tachycardia, infections, headaches, and indigestion.
With longer-acting formulations, injection sites may show mild redness or itchiness (23).To ensure safety for older clients, follow the product monograph for dose modifications. Researchers have not gathered sufficient data on use in breastfeeding or pediatric populations, and no guidance exists for these groups. Avoid GLP-1 analogs during pregnancy and consider alternative treatment approaches (23).
Allergic reactions with exenatide may result in injection site irritation or, in rare cases, anaphylaxis (23). Symptoms of anaphylaxis, such as shortness of breath, rash, or chest tightness, require immediate medical attention. These medications are contraindicated in pregnancy due to evidence of fetal developmental abnormalities in animal studies (4)(48). Providers advise birth control for individuals of childbearing potential while using GLP-1 agonists (48).
Hypoglycemia occurs when GLP-1 agonists combine with other glucose-lowering drugs like sulfonylureas or insulin (4). Immunogenicity with exenatide may lead to the formation of anti-drug antibodies, reducing the medication’s efficacy and causing injection site reactions (49). A meta-analysis also suggests an increased risk of gallbladder or biliary disorders with prolonged or high-dose use (50).
Clinicians avoid combining therapy with dipeptidyl peptidase-4 inhibitors due to minimal glycemic benefits and increased hypoglycemic risk (30). Providers escalate the dosage to minimize side effects like nausea and counsel clients on satiety to prevent overconsumption. Despite these potential risks, discontinuation rates due to the adverse impacts remain low, highlighting the tolerability and effectiveness of GLP-1 agonists for most clients.
Ask yourself...
- What factors should providers consider when prescribing GLP-1 agonists to patients, and how can their medical history, such as pregnancy or concurrent medications, influence the treatment plan?
- How do the potential benefits of GLP-1 agonists in reducing risks of heart and kidney disease compare with the risks of side effects like hypoglycemia or gallbladder disorders, and how should a clinician balance these considerations?
- Why are GLP-1 agonists contraindicated in specific populations, such as pregnant individuals or those with severe gastrointestinal disorders, and what alternative treatment approaches could providers consider in these cases?
Contraindications and Precautions
GLP-1 agonists are contraindicated in individuals with hypersensitivity to these medications and those who are pregnant (48). Women of childbearing potential should use reliable contraception if they receive GLP-1 agonists, given the potential risks to fetal development (48). People with severe gastrointestinal (GI) disorders, such as gastroparesis or inflammatory bowel disease, should also avoid GLP-1 agonists due to the possibility of exacerbating GI symptoms (23).
Concerns focus on the impact of GLP-1 agonists on thyroid C-cells. In rodent models, liraglutide stimulates calcitonin release and causes C-cell hyperplasia and tumor formation (51). Clinicians avoid prescribing GLP-1 agonists for individuals with a personal or family history of medullary thyroid cancer, multiple endocrine neoplasia type 2A (MEN 2A), or type 2B (MEN 2B) (51)(53).
Ask yourself...
- How does the potential impact of GLP-1 agonists on thyroid C-cells influence prescribing decisions for patients with a family history of medullary thyroid cancer or multiple endocrine neoplasia?
- What are the ethical considerations in prescribing GLP-1 agonists to women of childbearing potential, given the risks to fetal development and the necessity of contraception?
Pancreatitis Risks
Researchers have not determined whether GLP-1 agonists cause pancreatitis or increase the risk of pancreatic cancer, but some users report experiencing acute pancreatitis, including hemorrhagic and necrotizing forms (15)(52). Clinicians avoid prescribing GLP-1 agonists for people with a history of pancreatitis and stop treatment if pancreatitis occurs during use.

Ask yourself...
- What factors should clinicians consider when weighing the benefits of prescribing GLP-1 agonists against the potential risks of pancreatitis in patients with a history of this condition?
Compounded Semaglutide Warnings
The U.S. Food and Drug Administration (FDA) issues advisories about the compounding of semaglutide formulations, emphasizing that these products lack evaluation for safety, efficacy, or quality (54). Compounded semaglutide use links to adverse events, including cases where patients took doses exceeding the prescribed amount, causing nausea, vomiting, and abdominal discomfort (54).
Unlike prefilled pens, compounded semaglutide products lack standardized safety features, and the use of incorrect syringes can introduce dosing variability and confusion. Proper counseling, labeling, and dispensing protocols are essential to reduce the risk of overdoses and associated hospitalizations. Healthcare professionals should educate patients on these dangers and discourage them from purchasing or using compounded semaglutide.
Ask yourself...
- What are compounded semaglutide products’ potential risks and consequences compared to FDA-approved formulations, and how might healthcare professionals communicate these dangers to patients?
Overdose Considerations
Researchers have conducted limited studies on GLP-1 analog overdoses. Reported cases include gastrointestinal symptoms such as nausea, vomiting, diarrhea, belching, and abdominal pain (23). Published reports of GLP-1 agonist overdoses do not document severe complications such as pancreatitis or hypoglycemia (23). The treatment focuses on managing nausea and vomiting with antiemetics to ensure comfort and prevent complications.
Ask yourself...
- What factors might influence the severity of symptoms in a GLP-1 agonist overdose, and how can understanding these factors improve the management of such cases?
Outcomes
To investigate the long-term clinical and economic outcomes of various anti-diabetic treatments, researchers utilized the CORE diabetes (Centre for Outcomes and Resource Evaluation) model, which simulates morbidity, mortality, and associated healthcare costs based on epidemiological data from extended clinical trials (16)(17). The LEAD-1 study provided clinical input and compared glimepiride with two doses of liraglutide (1.2 mg and 1.8 mg) and rosiglitazone (4 mg) in patients with type 2 diabetes mellitus (T2DM) (16).
Researchers calibrated the CORE model with baseline characteristics from LEAD-1 and projected outcomes, including survival rates, cardiovascular, ophthalmic, and renal events incidence, and healthcare expenditures over 10-, 20-, and 30-year intervals (16)(17).. The analysis shows that liraglutide 1.2 mg and 1.8 mg increase 30-year survival rates compared to rosiglitazone (15.0% and 16.0% vs. 12.6%) and reduce cardiovascular, renal, and ocular complications in a cohort of 5000 patients per treatment (55).
After 30 years, cardiovascular mortality rates for liraglutide 1.2 mg, 1.8 mg, and rosiglitazone reached 69.7%, 68.4%, and 72.5%. Data from LEAD-1 and the CORE model show that liraglutide with glimepiride reduces mortality and diabetes-related complications more than rosiglitazone with glimepiride (55).
Ask yourself...
- How might the differences in long-term survival rates and complication reductions between liraglutide and rosiglitazone influence treatment decisions for patients with type 2 diabetes mellitus in managing comorbid conditions like cardiovascular disease?
Conclusion
The understanding and utilizing GLP-1 receptor agonists (GLP-1 RAs) underscore their transformative impact on managing type 2 diabetes mellitus (T2DM), obesity, and related cardiometabolic conditions. By targeting multiple mechanisms—such as enhancing insulin secretion, suppressing glucagon release, slowing gastric emptying, and promoting satiety—GLP-1 RAs provide significant improvements in glycemic control, weight reduction, and long-term cardiovascular and renal health (6)(16)(23).
Advancements in GLP-1 RA formulations, including once-a-week injectables and oral options, have enhanced patient convenience and adherence. Robust clinical evidence supports the inclusion of agents like semaglutide and liraglutide in reducing HbA1c levels, achieving substantial weight loss, and mitigating complications associated with T2DM and obesity (16)(39). These agents also offer additional benefits, such as neuroprotection, anti-inflammatory properties, and reductions in NAFLD-related risks, further broadening their clinical utility (23)(41).
While GLP-1 RAs present promising therapeutic outcomes, clinicians must address potential challenges, including gastrointestinal side effects, contraindications (e.g., pregnancy, severe GI disorders), and the risks associated with improper compounding practices (48)(540. Ongoing monitoring, education, and collaboration among interprofessional team members remain crucial to optimizing patient outcomes and minimizing adverse events.
Emerging agents like tirzepatide and orforglipron demonstrate the expanding horizon of GLP-1-based therapies, offering enhanced efficacy and novel mechanisms of action. The future of GLP-1 RAs lies in their potential to address a wider array of metabolic disorders, supported by ongoing research and innovation (43)(47).GLP-1 receptor agonists, when integrated into comprehensive treatment plans, serve as tools in improving the lives of patients with T2DM and obesity.
References + Disclaimer
- World Health Organization: WHO. (2010, May 6). A healthy lifestyle – WHO recommendations. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle—who-recommendations
- World Health Organization: WHO. (2020, February 21). Obesity. https://www.who.int/health-topics/obesity#tab=tab_1
- Child and Teen BMI categories. (2024, June 28). BMI. https://www.cdc.gov/bmi/child-teen-calculator/bmi-categories.html
- Di Angelantonio, E., Bhupathiraju, S. N., Wormser, D., Gao, P., Kaptoge, S., De Gonzalez, A. B., Cairns, B. J., Huxley, R., Jackson, C. L., Joshy, G., Lewington, S., Manson, J. E., Murphy, N., Patel, A. V., Samet, J. M., Woodward, M., Zheng, W., Zhou, M., Bansal, N., . . . Hu, F. B. (2016). Body-mass index and all-cause mortality: an individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet, 388(10046), 776–786. https://doi.org/10.1016/s0140-6736(16)30175-1
- Ross, R., Neeland, I. J., Yamashita, S., Shai, I., Seidell, J., Magni, P., Santos, R. D., Arsenault, B., Cuevas, A., Hu, F. B., Griffin, B. A., Zambon, A., Barter, P., Fruchart, J., Eckel, R. H., Matsuzawa, Y., & Després, J. (2020). Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nature Reviews Endocrinology, 16(3), 177–189. https://doi.org/10.1038/s41574-019-0310-7
- Hamed, K., Alosaimi, M. N., Ali, B. A., Alghamdi, A., Alkhashi, T., Alkhaldi, S. S., Altowarqi, N. A., Alzahrani, H., Alshehri, A. M., Alkhaldi, R. K., Alqahtani, K. W., Alharbi, N. H., Alhulayfi, H. F., Sharifi, S. Y., & Dighriri, I. M. (2024). Glucagon-like Peptide-1 (GLP-1) Receptor Agonists: Exploring their impact on diabetes, obesity, and cardiovascular health through a comprehensive literature review. Cureus. https://doi.org/10.7759/cureus.68390
- Bendicho-Lavilla, C., Seoane-Viaño, I., Otero-Espinar, F. J., & Luzardo-Álvarez, A. (2021). Fighting type 2 diabetes: Formulation strategies for peptide-based therapeutics. Acta Pharmaceutica Sinica B, 12(2), 621–636. https://doi.org/10.1016/j.apsb.2021.08.003
- Gadde, K. M., & Atkins, K. D. (2020). The limits and challenges of antiobesity pharmacotherapy. Expert Opinion on Pharmacotherapy, 21(11), 1319–1328. https://doi.org/10.1080/14656566.2020.1748599
- Dragano, N. R., Fernø, J., Diéguez, C., López, M., & Milbank, E. (2020). Recent updates on obesity treatments: available drugs and future directions. Neuroscience, 437, 215–239. https://doi.org/10.1016/j.neuroscience.2020.04.034
- Yeung, A. Y., & Tadi, P. (2023, January 3). Physiology, obesity, neurohormonal appetite, and satiety control. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK555906/
- Müller, T. D., Blüher, M., Tschöp, M. H., & DiMarchi, R. D. (2021). Anti-obesity drug discovery: advances and challenges. Nature Reviews Drug Discovery, 21(3), 201–223. https://doi.org/10.1038/s41573-021-00337-8
- Kim, B., Kang, S. M., Kang, J., Kim, K. K., Kim, B., Kim, S. J., Kim, Y., Kim, J., Kim, J. H., Nam, G. E., Park, J. Y., Son, J. W., Shin, H., Oh, T. J., Lee, H., Jeon, E., Chung, S., Hong, Y. H., & Kim, C. H. (2020). Current Long-Term pharmacotherapies for the management of obesity. Journal of Obesity & Metabolic Syndrome, 29(2), 99–109. https://doi.org/10.7570/jomes20010
- Colin, I. M., & Gérard, A. (2022). Once-weekly 2.4 mg Semaglutide for Weight Management in Obesity: A Game Changer? touchREVIEWS in Endocrinology, 18(1), 35. https://doi.org/10.17925/ee.2022.18.1.35
- Drucker, D. J. (2024). The GLP-1 journey: from discovery science to therapeutic impact. Journal of Clinical Investigation, 134(2). https://doi.org/10.1172/jci175634
- Nauck, M. A., Quast, D. R., Wefers, J., & Meier, J. J. (2020). GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism, 46, 101102. https://doi.org/10.1016/j.molmet.2020.101102
- Marre, M., Shaw, J., Brändle, M., Bebakar, W. M. W., Kamaruddin, N. A., Strand, J., Zdravkovic, M., Thi, T. D. L., & Colagiuri, S. (2009). Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabetic Medicine, 26(3), 268–278. https://doi.org/10.1111/j.1464-5491.2009.02666.x
- Lingvay, I., Deanfield, J., Kahn, S. E., Weeke, P. E., Toplak, H., Scirica, B. M., Rydén, L., Rathor, N., Plutzky, J., Morales, C., Lincoff, A. M., Lehrke, M., Jeppesen, O. K., Gajos, G., Colhoun, H. M., Cariou, B., & Ryan, D. (2024). Semaglutide and cardiovascular outcomes by baseline HBA1C and change in HBA1C in people with overweight or obesity but without diabetes in SELECT. Diabetes Care, 47(8), 1360–1369. https://doi.org/10.2337/dc24-0764
- Berry, S., Chubb, B., Acs, A., Falla, E., Verma, A., Malkin, S. J. P., Hunt, B., & Palmer, A. J. (2023). Calibration of the IQVIA Core Diabetes Model to the stroke outcomes from the SUSTAIN 6 cardiovascular outcomes trial of once-weekly semaglutide. Journal of Medical Economics, 26(1), 1019–1031. https://doi.org/10.1080/13696998.2023.2240957
- Baker, C., Retzik-Stahr, C., Singh, V., Plomondon, R., Anderson, V., & Rasouli, N. (2021). Should metformin remain the first-line therapy for treatment of type 2 diabetes? Therapeutic Advances in Endocrinology and Metabolism, 12. https://doi.org/10.1177/2042018820980225
- King, A., & Miller, E. M. (2022). Glucagon-like peptide 1 receptor agonists have the potential to revolutionize the attainment of target A1C levels in Type 2 Diabetes—So why is their uptake so low? Clinical Diabetes, 41(2), 226–238. https://doi.org/10.2337/cd22-0027
- Deng, Y., Park, A., Zhu, L., Xie, W., & Pan, C. Q. (2022). Effect of semaglutide and liraglutide in individuals with obesity or overweight without diabetes: a systematic review. Therapeutic Advances in Chronic Disease, 13. https://doi.org/10.1177/20406223221108064
- Marx, N., Husain, M., Lehrke, M., Verma, S., & Sattar, N. (2022). GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation, 146(24), 1882–1894. https://doi.org/10.1161/circulationaha.122.059595
- Collins, L., & Costello, R. A. (2024, February 29). Glucagon-like peptide-1 receptor agonists. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK551568/
- Wharton, S., Blevins, T., Connery, L., Rosenstock, J., Raha, S., Liu, R., Ma, X., Mather, K. J., Haupt, A., Robins, D., Pratt, E., Kazda, C., & Konig, M. (2023). Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. New England Journal of Medicine, 389(10), 877–888. https://doi.org/10.1056/nejmoa2302392
- Rosenstock, J., Nino, A., Soffer, J., Erskine, L., Acusta, A., Dole, J., Carr, M. C., Mallory, J., & Home, P. (2020). Impact of a weekly Glucagon-Like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in Type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabetes Care, 43(10), 2509–2518. https://doi.org/10.2337/dc19-2316
- Delrue, C., & Speeckaert, M. M. (2024). Mechanistic pathways and clinical implications of GLP-1 receptor agonists in Type 1 diabetes management. International Journal of Molecular Sciences, 25(17), 9351. https://doi.org/10.3390/ijms25179351
- Andersen, A., Knop, F. K., & Vilsbøll, T. (2021). A Pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs, 81(9), 1003–1030. https://doi.org/10.1007/s40265-021-01499-w
- Aroda, V. R., Blonde, L., & Pratley, R. E. (2022). A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. Reviews in Endocrine and Metabolic Disorders, 23(5), 979–994. https://doi.org/10.1007/s11154-022-09735-8
- Thomas, M. C., Coughlan, M. T., & Cooper, M. E. (2023). The postprandial actions of GLP-1 receptor agonists: The missing link for cardiovascular and kidney protection in type 2 diabetes. Cell Metabolism, 35(2), 253–273. https://doi.org/10.1016/j.cmet.2023.01.004
- Malm-Erjefält, M., Bjørnsdottir, I., Vanggaard, J., Helleberg, H., Larsen, U., Oosterhuis, B., Van Lier, J. J., Zdravkovic, M., & Olsen, A. K. (2010). Metabolism and excretion of the Once-Daily human Glucagon-Like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metabolism and Disposition, 38(11), 1944–1953. https://doi.org/10.1124/dmd.110.034066
- Papakonstantinou, I., Tsioufis, K., & Katsi, V. (2024). Spotlight on the mechanism of action of semaglutide. Current Issues in Molecular Biology, 46(12), 14514–14541. https://doi.org/10.3390/cimb46120872
- Mariam, Z., & Niazi, S. K. (2023). Glucagon‐like peptide agonists: A prospective review. Endocrinology Diabetes & Metabolism, 7(1). https://doi.org/10.1002/edm2.462
- Redondo, M. J., & Bacha, F. (2020). GLP-1 receptor agonist as adjuvant therapy in Type 1 diabetes: No apparent benefit for Beta-Cell function or glycemia. The Journal of Clinical Endocrinology & Metabolism, 105(8), e3000–e3002. https://doi.org/10.1210/clinem/dgaa314
- Manalac, T. (2024, October 24). GLP-1 prescriptions for Type 1 diabetes spike despite lack of FDA approval: study. BioSpace. https://www.biospace.com/drug-development/glp-1-prescriptions-for-type-1-diabetes-spike-despite-lack-of-fda-approval-study
- Jackson, S. H., Martin, T. S., Jones, J. D., Seal, D., & Emanuel, F. (2010, September 1). Liraglutide (Victoza): the first Once-Daily Incretin mimetic injection for type-2 diabetes. https://pmc.ncbi.nlm.nih.gov/articles/PMC2957743/
- Garber, A., Henry, R., Ratner, R., Garcia-Hernandez, P. A., Rodriguez-Pattzi, H., Olvera-Alvarez, I., Hale, P. M., Zdravkovic, M., & Bode, B. (2008). Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. The Lancet, 373(9662), 473–481. https://doi.org/10.1016/s0140-6736(08)61246-5
- Schmidt, W. E., Christiansen, J. S., Hammer, M., Zychma, M. J., & Buse, J. B. (2011). Patient‐reported outcomes are superior in patients with Type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: results from a randomized, open‐label study. Diabetic Medicine, 28(6), 715–723. https://doi.org/10.1111/j.1464-5491.2011.03276.x
- Garvey, W. T., Birkenfeld, A. L., Dicker, D., Mingrone, G., Pedersen, S. D., Satylganova, A., Skovgaard, D., Sugimoto, D., Jensen, C., & Mosenzon, O. (2020). Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care, 43(5), 1085–1093. https://doi.org/10.2337/dc19-1745
- Kurtzhals, P., Kreiner, F. F., & Bindra, R. S. (2023). The role of weight control in the management of type 2 diabetes mellitus: Perspectives on semaglutide. Diabetes Research and Clinical Practice, 203, 110881. https://doi.org/10.1016/j.diabres.2023.110881
- Weghuber, D., Barrett, T., Barrientos-Pérez, M., Gies, I., Hesse, D., Jeppesen, O. K., Kelly, A. S., Mastrandrea, L. D., Sørrig, R., & Arslanian, S. (2022). Once-Weekly Semaglutide in Adolescents with Obesity. New England Journal of Medicine, 387(24), 2245–2257. https://doi.org/10.1056/nejmoa2208601
- Shaman, A. M., Bain, S. C., Bakris, G. L., Buse, J. B., Idorn, T., Mahaffey, K. W., Mann, J. F., Nauck, M. A., Rasmussen, S., Rossing, P., Wolthers, B., Zinman, B., & Perkovic, V. (2021). Effect of the Glucagon-Like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with Type 2 Diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation, 145(8), 575–585. https://doi.org/10.1161/circulationaha.121.055459
- Smits, M. M., & Van Raalte, D. H. (2021). Safety of semaglutide. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.645563
- Dutta, P., Kumar, Y., Babu, A. T., Ravindran, S. G., Salam, A., Rai, B., Baskar, A., Dhawan, A., & Jomy, M. (2023). Tirzepatide: a promising drug for type 2 diabetes and beyond. Cureus. https://doi.org/10.7759/cureus.38379
- Frías, J. P., Davies, M. J., Rosenstock, J., Manghi, F. C. P., Landó, L. F., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine, 385(6), 503–515. https://doi.org/10.1056/nejmoa2107519
- Giorgino, F., Franco, D. R., Nicolay, C., Hemmingway, A., Rodríguez, Á., & Wiese, R. J. (2024). Effects of tirzepatide versus basal insulins in people with type 2 diabetes and different baseline glycemic patterns: post hoc analyses of the SURPASS-3 and SURPASS-4 trials. Diabetes Care, 47(6), 1020–1027. https://doi.org/10.2337/dc23-2366
- Dahl, D., Onishi, Y., Norwood, P., Huh, R., Bray, R., Patel, H., & Rodríguez, Á. (2022). Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes. JAMA, 327(6), 534. https://doi.org/10.1001/jama.2022.0078
- Farzam, K., & Patel, P. (2024, February 20). Tirzepatide. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK585056/
- Drummond, R. F., Seif, K. E., & Reece, E. A. (2024). Glucagon-Like peptide-1 receptor agonist use in pregnancy: a review. American Journal of Obstetrics and Gynecology. https://doi.org/10.1016/j.ajog.2024.08.024
- Hinnen, D. (2017). Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum, 30(3), 202–210. https://doi.org/10.2337/ds16-0026
- He, L., Wang, J., Ping, F., Yang, N., Huang, J., Li, Y., Xu, L., Li, W., & Zhang, H. (2022). Association of Glucagon-Like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases. JAMA Internal Medicine, 182(5), 513. https://doi.org/10.1001/jamainternmed.2022.0338
- Knudsen, L. B., Madsen, L. W., Andersen, S., Almholt, K., De Boer, A. S., Drucker, D. J., Gotfredsen, C., Egerod, F. L., Hegelund, A. C., Jacobsen, H., Jacobsen, S. D., Moses, A. C., Mølck, A., Nielsen, H. S., Nowak, J., Solberg, H., Thi, T. D. L., & Zdravkovic, M. (2010). Glucagon-Like peptide-1 receptor agonists activate rodent thyroid C-Cells causing calcitonin release and C-Cell proliferation. Endocrinology, 151(4), 1473–1486. https://doi.org/10.1210/en.2009-1272
- Dankner, R., Murad, H., Agay, N., Olmer, L., & Freedman, L. S. (2024). Glucagon-like peptide-1 receptor agonists and pancreatic cancer risk in patients with type 2 diabetes. JAMA Network Open, 7(1), e2350408. https://doi.org/10.1001/jamanetworkopen.2023.50408
- Do, D., Lee, T., Peasah, S. K., Good, C. B., Inneh, A., & Patel, U. (2024). GLP-1 receptor agonist discontinuation among patients with obesity and/or type 2 diabetes. JAMA Network Open, 7(5), e2413172. https://doi.org/10.1001/jamanetworkopen.2024.13172
- Ashraf, A. R., Mackey, T. K., Vida, R. G., Kulcsár, G., Schmidt, J., Balázs, O., Domián, B. M., Li, J., Csákó, I., & Fittler, A. (2024). Multifactor quality and safety analysis of semaglutide products sold by online sellers without a prescription: market surveillance, content analysis, and product purchase Evaluation study. Journal of Medical Internet Research, 26, e65440. https://doi.org/10.2196/65440
- Sullivan, S. D., Alfonso-Cristancho, R., Conner, C., Hammer, M., & Blonde, L. (2009). Long-term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone. Cardiovascular Diabetology, 8(1), 12. https://doi.org/10.1186/1475-2840-8-12
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
Complete Survey
Give us your thoughts and feedback!

