Course
West Nile Virus
Course Highlights
- In this West Nile Virus course, we will learn about the virus’s biology, transmission, and life cycle.
- You’ll also learn to identify clinical signs and symptoms of infection in humans and animals.
- You’ll leave this course with a broader understanding of the epidemiology of West Nile Virus and its global impact.
About
Contact Hours Awarded: 2
Author: R.E. Hengsterman MSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
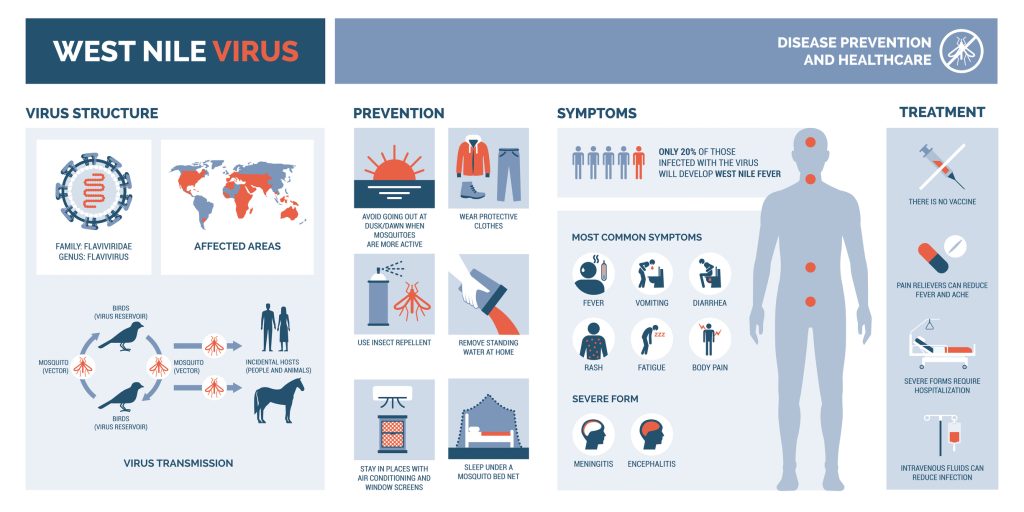
West Nile Virus (WNV) is a zoonotic, mosquito-borne flavivirus and a member of the Flaviviridae family, responsible for a spectrum of diseases ranging from mild febrile illness to severe, lethal neuroinvasive conditions (1)(3)(4). Researchers isolated WNV in 1937 from a febrile patient in the West Nile Province of Uganda, identifying it as causing mild, subclinical infections (2). WNV has emerged as a significant public health concern, causing outbreaks in humans, horses, birds, and other wildlife across all continents except Antarctica (2).
WNV infection displays diverse clinical manifestations. Most individuals show no symptoms, but 1 in 4 develops febrile symptoms resembling a viral syndrome (3). About 1 in 150 of those infected develop neuroinvasive diseases with severe neurological deficits, such as encephalitis (4).
Infected Culex mosquitoes transmit the virus through bites (5). Other modes of WNV transmission include blood transfusion, organ transplantation, intrauterine transfer, and breastfeeding (5). Researchers identify the virus in animals, including birds, horses, reptiles, and rodents, complicating its epidemiology (6).
The global spread of WNV began in the 1990s, expanding from its origins in Africa to Europe, Asia, and North America (1)(2)(7). The virus exists in many parts of the world, and globalization and climate change drive infectious disease outbreaks (10). Clinicians face challenges diagnosing WNV infection because of its variable clinical presentation and rarity. The disease can progress to devastating and fatal outcomes, making early recognition and supportive management crucial. In recent years, the spread of West Nile Virus (WNV) has emerged as a significant public health concern, with outbreaks reported in several European countries, including Italy, Hungary, Romania, and Greece.
During the 2023 transmission season, by December 2023, European countries reported 709 human WNV cases and 67 deaths, including cases in Italy, Greece, Romania, France, Hungary, Spain, and Germany (17)(18). Recent climate conditions and the introduction of new lineage 1 WNV genetic variants drive the increased spread of WNV in Europe (17) (19).
No specific antiviral treatment or licensed vaccine exists for human use, complicating efforts to control the virus (8). Preventative measures, such as insect repellents, permethrin-treated clothing, and mosquito control programs, remain the cornerstone of public health interventions (9). Effective management of WNV infections requires an interprofessional approach involving emergency medicine physicians, infectious disease specialists, critical care teams, pharmacists, and specialty-trained nurses.
Despite extensive research into the pathogenesis, epidemiology, and potential vaccine development, significant gaps in knowledge remain. This review provides an updated overview of the biology, pathobiology, epidemiology, diagnostic methods, control strategies, and “One Health” implications of WNV, highlighting the urgent need for continued efforts in prevention and management to mitigate the impact of this re-emerging zoonotic threat (2).

Self-Quiz
Ask Yourself...
- Given WNV’s diverse transmission routes—from mosquito bites to blood transfusions and organ transplants, how might public health strategies adapt to address these varied pathways, and what ethical considerations arise when balancing individual freedoms with broad prevention measures?
- Considering the global expansion of WNV in tandem with evolving climate conditions, how might healthcare teams collaborate across disciplines to improve early detection and diagnosis, and what barriers could impede a swift interprofessional response?
Transmission of West Nile Virus (WNV)
Biological vectors (mosquitoes) transmit West Nile Virus (WNV) and function as both vectors and intermediate hosts (11). Mosquitoes amplify the virus before transferring it to definitive hosts (11). These vectorial and intermediary roles underscore the critical contribution of mosquitoes to the WNV transmission cycle. Documented modes of transmission include organ transplants, needlestick injuries, hemodialysis, and blood transfusions (5).
Blood transfusions and solid organ transplants pose significant risks, as the virus can remain viable in solid organs even when serology results are negative (12) (13). Reports document WNV transmission through whole blood and components, including red blood cells, plasma, and platelets (14). Researchers identify other non-vectorial transmission beyond mosquito bites, including an oral-fecal route of WNV transmission in American alligators and saltwater crocodiles (2).
Contact transmission occurs in commercial geese farming through behaviors such as cannibalism and feather pecking among infected birds (15). Reports indicate rare cases of transplacental transmission in humans and possible breastfeeding transmission (2)(16). Experts hypothesize that aerosol transmission of West Nile Virus (WNV) may occur among animal handlers and laboratory workers exposed to infected materials.
Workers at higher risk include military personnel, veterinarians, agricultural workers, farmers, and laboratory staff in contact with infected fluids or aerosols (17). Identifying these high-risk groups enables occupational physicians to implement active surveillance, enhancing our understanding of WNV epidemiology and informing tailored preventive strategies to reduce disease burden in affected areas.
Behavioral and underlying health factors can influence the risk of West Nile Virus (WNV) infection and associated diseases. Research identifies alcohol abuse as a significant risk factor for West Nile Virus (WNV) infection (2)(20). Hypertension, cerebrovascular disease, chronic renal disease, and diabetes mellitus increase susceptibility (2)(20).
These diverse modes of transmission highlight the complexity of WNV spread and the importance of understanding biological and behavioral risk factors to manage and prevent outbreaks.

Self-Quiz
Ask Yourself...
- How might recognizing non-mosquito transmission routes—such as organ transplantation, occupational exposure, and even potential aerosol spread—challenge traditional assumptions about WNV being exclusively vector-borne, and what implications could this have for public health surveillance and prevention strategies?
- In light of evidence suggesting that underlying health factors (e.g., hypertension, diabetes, alcohol abuse) can heighten susceptibility to WNV, how should healthcare providers and occupational health professionals adapt prevention efforts and patient education to account for these modifiable risk factors?
West Nile Virus (WNV) Life Cycle
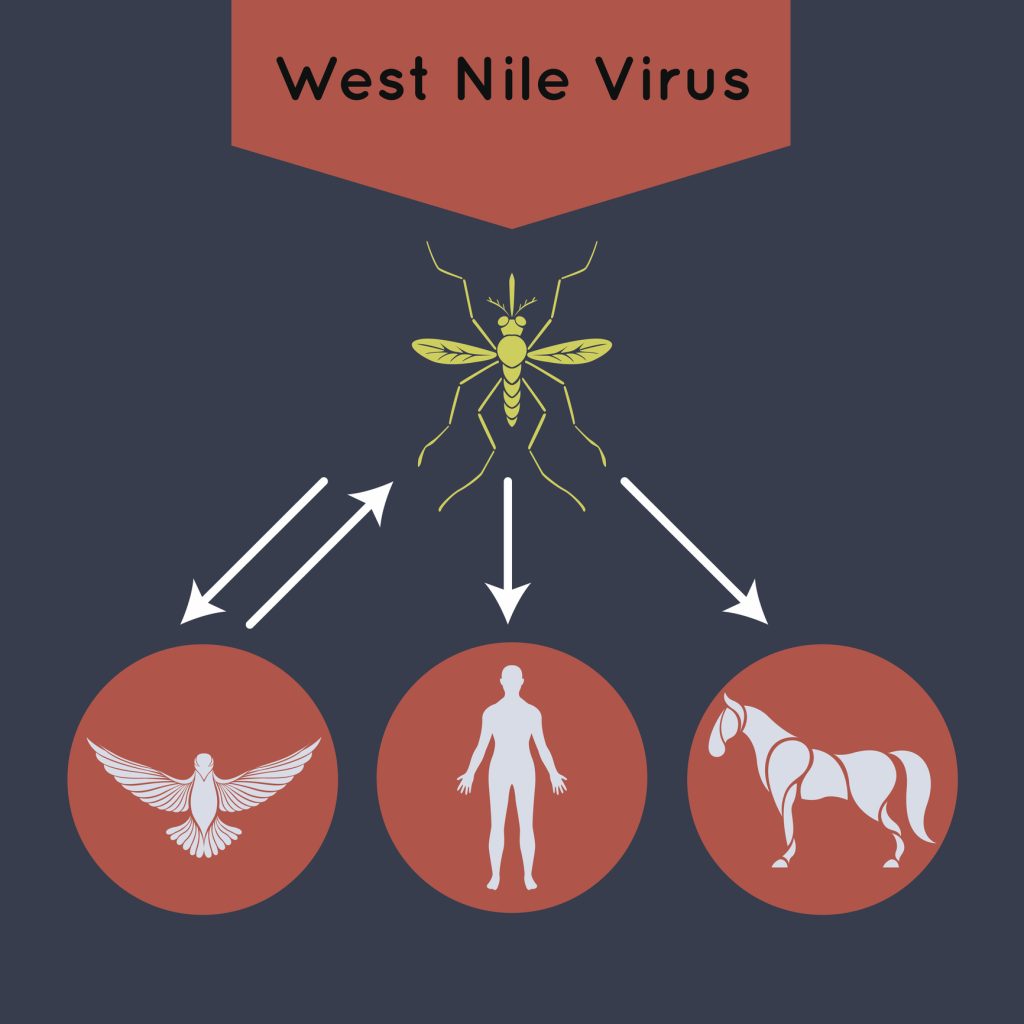
The life cycle of West Nile Virus (WNV) is complex, involving multiple key players: virus reservoirs, mosquito vectors, and final or incidental hosts. Birds serve as primary reservoirs, capable of harboring the virus without displaying clinical disease (4)(21). Mosquitoes serve as vectors and intermediate hosts, replicate the virus, and transmit it to final hosts, such as humans or other vertebrates, during blood meals (4)(5).

Self-Quiz
Ask Yourself...
- How might the virus‘s ability to remain asymptomatic in bird reservoirs complicate efforts to detect and interrupt WNV’s transmission cycle?
Dynamics of Transmission within the Mosquito Host
Competent mosquito vectors acquire the virus by feeding on a viremic vertebrate host (11). Once ingested, WNV reaches the mosquito’s midgut, replicating and spreading to the salivary glands (4)(24). During subsequent blood meals, mosquitoes transmit the virus to the final host when their saliva contains a viral concentration exceeding 10⁴ TCID50/mL (22). The saliva facilitates virus delivery and contains proteins that interfere with the host’s T-cell immune response, aiding initial immune evasion and viral dissemination (22)(23). WNV does not cause apparent disease in mosquitoes, ensuring their viability as effective vectors (4)(20).

Self-Quiz
Ask Yourself...
- How do the immune-evasive proteins in mosquito saliva influence the overall transmission and potential control strategies for West Nile Virus?
- How does the peritrophic matrix in the mosquito midgut influence the virus’s replication and spread to the salivary glands, and what implications does this have for controlling West Nile Virus transmission?
Infection of Vertebrate Hosts
Vertebrate hosts, including reservoirs and incidental hosts, are infected when mosquitoes inject saliva while probing for blood vessels (4)(21). In addition to its anticoagulant properties, mosquito saliva aids viral entry and immune evasion. Once inside the vertebrate host, WNV infects cells through receptor-mediated endocytosis. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin receptor (DC-SIGNR) is the primary receptor mediating WNV cell entry (22).
Mannose receptors and glycosaminoglycans also play roles. After binding to the receptor, the virus enters the host cell’s endosomal vesicles, where acidic conditions trigger conformational changes in the viral envelope protein (E protein), facilitating membrane fusion and release of the viral nucleocapsid and RNA into the cytoplasm (25).

Self-Quiz
Ask Yourself...
- How does mosquito saliva facilitate West Nile Virus entry and immune evasion beyond its anticoagulant function, and why might understanding these mechanisms be pivotal in developing preventive strategies?
- What role does the acidic environment in endosomal vesicles play in triggering West Nile Virus membrane fusion, and how might interrupting this process alter the host’s overall outcome of viral infection?
Viral Replication and Release
WNV RNA undergoes replication in the host cell cytoplasm. Synthesized viral particles acquire a lipid envelope by budding into the lumen of the endoplasmic reticulum (ER) (25). During exocytosis, furin cleaves prM proteins on immature virions, producing mature infectious particles that leave the cell and infect additional cells (22).

Self-Quiz
Ask Yourself...
- How does the prM protein cleavage by furin during exocytosis illustrate the critical interplay between the host cell’s machinery and the virus’s capacity to produce mature, infectious particles?
Amplification in Crocodilians
Most final hosts, such as humans and horses, cannot sustain sufficient viremia for further transmission, but crocodilians sustain viremia and contribute to transmission (4)(5). Crocodilians amplify the virus, contributing to its transmission dynamics in their ecosystems (26).

Self-Quiz
Ask Yourself...
- How do you think crocodilians‘ unique biology or ecological niche enables them to sustain viremia and contribute to West Nile Virus transmission, unlike other final hosts such as humans and horses?
Behavioral and Immune Considerations
During vector-mediated infection, WNV infects cutaneous macrophages and dendritic cells through DC-SIGN receptors, while its systemic spread in the host occurs independently of these receptors (4)(27). This multifaceted life cycle highlights WNV’s evolutionary adaptations for efficient replication, immune evasion, and transmission across diverse hosts.

Self-Quiz
Ask Yourself...
- How might West Nile Virus‘s initial reliance on DC-SIGN receptors for local infection, yet independence from these receptors once disseminated, reflect an evolutionary strategy that optimizes immune evasion and transmission across various hosts?
Epidemiology
Researchers first identified West Nile Virus (WNV) in Uganda in 1937. It caused mild illnesses until 1999, when cases appeared in the Western Hemisphere, revealing its global impact (2). The New York outbreak caused 62 cases of encephalitis and seven deaths (28). Earlier outbreaks involved minor illnesses, but the virus has caused severe neurological diseases since the mid-1990s (1)(2)(7).

Self-Quiz
Ask Yourself...
- How does West Nile Virus’s shift from causing mild illness before the late 1990s to more severe neurological disease afterward illustrate the interplay between viral adaptation, environmental changes, and public health surveillance?
Clinical Presentation in Humans
The clinical spectrum of WNV infection varies. 80% of individuals experience no symptoms, while 20% develop West Nile fever, characterized by fever above 38°C, headache, lethargy, depression, and maculopapular or petechial rashes (4). While most individuals with West Nile virus-related febrile illness recover, fatigue and weakness may persist for weeks or months (4). 1 in 150 individuals infected with West Nile virus develops severe illness involving the central nervous system, such as encephalitis or meningitis (2)(4).
Symptoms of neuroinvasive disease include nuchal rigidity, photophobia, flaccid paralysis, and myasthenia, with a mortality rate approaching 10% (4)(29). Patients experience gastrointestinal symptoms, including nausea, vomiting, diarrhea, and anorexia, along with lymphadenopathy and hepatosplenomegaly (2)(4). Children, the elderly, and individuals with chronic conditions are at higher risk for severe disease.

Self-Quiz
Ask Yourself...
- How do the varied clinical manifestations of West Nile virus infection—from asymptomatic cases to severe neuroinvasive disease—challenge clinicians in diagnosing and managing the disease?
- How might individual factors such as age and chronic health conditions influence the prognosis of West Nile virus infection, and how should these insights shape public health strategies?
Clinical Presentation in Animals
- Birds: Susceptible avian species show lethargy, ataxia, head tremors, seizures, leg paralysis, and weight loss (30).
- Reptiles: Symptoms in reptiles range from subclinical in saltwater crocodiles to severe gastrointestinal and neurological diseases in American alligators. Neurological signs include unbalanced swimming, head tilt, and muscle tremors, while gastrointestinal symptoms include bloating and anorexia (2)(31).
- Snakes: Common signs include aggression, cachexia, immobility of the caudal body, and weakness (2)(32).

Self-Quiz
Ask Yourself...
- How do the varying clinical signs of West Nile Virus across birds, reptiles, and snakes challenge our understanding of disease progression and inform our strategies for timely detection and control measures in diverse animal populations?
Case Study: West Nile Virus (WNV) Neuroinvasive Disease
Patient Information:
- Name: John Doe
- Age: 64 years
- Sex: Male
- Occupation: Retired teacher, recreational gardener
- History of Travel: A recent visit to a rural area in the Midwest United States during late summer
- Presenting Complaint: Fever, confusion, and severe headache
Clinical Presentation:
John presented to the emergency department with complaints of fever (39.4°C), severe headache, nausea, and confusion for three days. His family reported a recent history of fatigue, lethargy, and decreased appetite. He also experienced mild muscle weakness and photophobia. His wife noted that he had spent considerable time gardening outdoors without insect repellent.
Vital Signs:
- Temperature: 39.4°C
- Heart Rate: 129 beats/min (tachycardia)
- Respiratory Rate: 22 breaths/min
- Blood Pressure: 128/86 mmHg
- Oxygen Saturation: 96% on room air
Physical Examination:
- General Appearance: Alert but disoriented to time and place
- Neurological Exam:
- Positive for nuchal rigidity
- Decreased motor strength in bilateral lower extremities (4/5)
- Hyperreflexia in the lower extremities
- No focal deficits were observed
- Positive Brudzinski’s and Kernig’s signs
- Skin Examination: Maculopapular rash noted on the trunk
- Ophthalmologic Exam: Mild conjunctival injection with no evidence of multifocal chorioretinitis
Laboratory Findings:
- Complete Blood Count (CBC):
- WBC: 12.8 x 10⁹/L (elevated)
- Hemoglobin: 14.1 g/dL
- Platelets: 180 x 10⁹/L (normal)
- Basic Metabolic Panel (BMP):
- Sodium: 129 mmol/L (hyponatremia)
- Potassium: 4.2 mmol/L
- Creatinine: 1.0 mg/dL
- Cerebrospinal Fluid (CSF) Analysis:
- WBC: 78/mm³ (lymphocytic predominance)
- Protein: 120 mg/dL (elevated)
- Glucose: 58 mg/dL (normal)
- IgM antibodies for WNV: Positive by ELISA
- Viral PCR: Positive for WNV RNA
Imaging Studies:
- CT Brain (Initial): Normal, no acute findings
- MRI Brain (Day 3): Hyperintense signals in the basal ganglia and thalamus, consistent with neuroinvasive WNV (33)
Diagnosis:
- Neuroinvasive West Nile Virus (WNV) infection presenting as meningoencephalitis.
Treatment Plan:
- Supportive Care:
- Intravenous fluids to correct hyponatremia
- Antipyretics for fever management
- Pain management for headache
- Neurologic Monitoring:
- Admitted to the ICU for close observation and prevention of complications
- Prophylaxis for deep vein thrombosis (DVT) with subcutaneous heparin
- Regular repositioning to prevent pressure sores
- Rehabilitation Plan:
- Initiated physical and occupational therapy to address motor deficits
- Cognitive treatment for improving memory and orientation
Outcome and Follow-Up:
After 14 days in the hospital, the team discharged John to a rehabilitation center for physical and cognitive therapy. At his one-month follow-up, he showed weakness in his lower extremities and attention deficits. His care team emphasized continued rehabilitation and scheduled further neurological evaluations to monitor for long-term sequelae.

Self-Quiz
Ask Yourself...
- Given John’s presenting symptoms and lab findings, what thought process might lead providers to consider WNV meningoencephalitis over other causes of meningitis or encephalitis, and what evidence do you find most compelling in supporting this diagnosis?
- How might John’s recent travel to a rural area and his gardening habits without insect repellent have contributed to WNV transmission, and what broader public health strategies could mitigate these risk factors?
- Considering John’s prolonged hospitalization and ongoing rehabilitation needs, what roles do different healthcare professionals (e.g., physicians, nurses, therapists) play in managing both the acute neuroinflammatory phase and the long-term sequelae of WNV neuroinvasive disease?
Factors Influencing Susceptibility to West Nile Virus
Intrinsic and extrinsic factors play critical roles in determining the risk of West Nile Virus (WNV) infection. Age is a significant inherent factor, with susceptibility increasing by 1.5 times for every decade of life. Individuals over 75 years are vulnerable due to age-related declines in immune function, such as reduced macrophage activity and impaired type I interferon responses (34).
Gender also influences susceptibility, with males at higher risk (35). Pre-existing conditions like cancer, cardiovascular disease, diabetes, and immunosuppression elevate the risk of severe outcomes (34). Immunosuppressed individuals are vulnerable, facing a 40-fold higher likelihood of severe complications (1)(2).
Environmental and ecological influences also contribute to WNV transmission. Urban settings are associated with outbreaks due to the behavior of mosquito species such as Cx. pipiens and Cx. quinquefasciatus, which thrive in peri-domestic environments (2)(36). In rural areas, agricultural practices like irrigation create breeding grounds for mosquitoes, while habitat destruction forces reservoirs and vectors into closer contact with humans (2)(37).

Self-Quiz
Ask Yourself...
- How do intrinsic factors (e.g., age, gender, immunosuppression) intersect with external environmental conditions (urban versus rural habitats, agricultural practices) to influence an individual’s overall vulnerability to West Nile Virus?
- Given the higher risk for severe disease in older adults and immunosuppressed populations, what considerations might public health professionals integrate into West Nile Virus’s targeted prevention and response strategies?
Transmission and Outbreak Trends
The mosquito life cycle and bird-mosquito amplification cycles drive outbreaks, with cases peaking in late summer and fall (2)(38). In warmer climates, infections may occur year-round (38). From 1999 to 2015, the U.S. reported 44,000 cases of WNV, with over 20,000 involving neuroinvasive disease (39). Neuroinvasive cases vary by year, ranging from 386 to 2,946 per annum (40). Surveys and blood donor screening suggest a neuroinvasive disease rate of 0.5% among infected individuals and an infection rate of 10% in outbreak areas, extrapolating to 3 to 5 million estimated cases worldwide (41).
WNV remains endemic in many regions, including Africa, the Middle East, Australia, Europe, and Asia. Endemic and non-endemic areas report outbreaks, highlighting the virus’s growing global reach. The interplay of environmental, demographic, and biological factors continues to shape the dynamics of WNV transmission and its impact on public and animal health

Self-Quiz
Ask Yourself...
- How do you think environmental, demographic, and biological factors influence West Nile Virus transmission patterns in both endemic and non-endemic regions?
- How does understanding the bird-mosquito amplification cycle help us predict—and help mitigate—the seasonal and geographic spread of West Nile Virus?
Pathophysiology
Mosquitoes transmit West Nile Virus (WNV) during blood meals, introducing infected saliva into the host (5). Following transmission, the virus undergoes an initial replication phase in dermal dendritic cells and keratinocytes at the entry site (24). The early phase transitions to a visceral-organ dissemination phase (24). The virus replicates in draining lymph nodes, enters the bloodstream, and spreads to visceral organs (24).
The final phase involves the virus reaching the central nervous system (CNS) (43). These include direct crossing of the blood-brain barrier, passive transport through endothelial cells, migration of infected macrophages across the blood-brain barrier, or retrograde axonal transport (42)(43). Once in the CNS, the virus induces significant inflammation, leading to neuronal loss in the gray matter of the spinal cord and brainstem (42)(43).

Self-Quiz
Ask Yourself...
- What factors enable West Nile Virus to move from the initial skin infection site into the central nervous system, and why might these pathways be critical to developing neuroinvasive disease?
- How might the virus’s replication in dermal dendritic cells and keratinocytes influence the host’s immune response, and what implications could this have for disease progression?
Etiology
Infected mosquitoes from the Culex species transmit West Nile Virus (WNV) to humans through bites (1)(4). In addition to humans, WNV can infect many hosts, including birds, horses, dogs, and other mammals (11). Wild birds serve as the primary reservoirs, facilitating viral amplification and perpetuation of the transmission cycle (11). Humans are incidental dead-end hosts because their low and transient viremia fails to sustain further transmission (44).
Mosquito bites transmit the virus, but infected donor blood, organ transplants, breast milk, and transplacental routes also transmit it in rare cases. Around 1% of infected individuals develop severe symptoms, with neurological complications being the most common (5)(11).

Self-Quiz
Ask Yourself...
- How might recognizing wild birds as the primary reservoirs of West Nile Virus challenge providers‘ assumptions and strategies for controlling mosquito populations and human infection rates?
History & Physical
The West Nile Virus (WNV) infection incubation period ranges from 2 to 14 days (3). Symptoms last 5 to 7 days, with fever present in one-quarter of cases (3). The clinical presentation is often mild, characterized by symptoms such as myalgia, malaise, and low-grade fever. Additional symptoms may include headache, eye pain, vomiting, anorexia, and, in up to 50% of cases, a maculopapular rash on the trunk that appears during defervescence (1)(2).
In rare cases, WNV can cause severe neurologic complications, including significant muscle weakness, altered mental status, seizures, and flaccid paralysis (1)(2). These patients often present with signs of encephalitis or meningitis, which can progress, necessitating intensive care unit (ICU) management (45). Despite aggressive supportive care, the mortality rate for neuroinvasive WNV infection remains high.


Self-Quiz
Ask Yourself...
- How might the often mild and often nonspecific presentation of West Nile Virus in many patients affect clinicians‘ ability to detect the infection early, and what strategies could they employ to differentiate it from other common viral illnesses?
Epidemiology and Typical Course of Illness
Most individuals with WNV have a history of travel to an area where the virus is endemic. Endemic regions span multiple continents, as discussed in the epidemiology section. The typical infection course includes fever lasting around 5 days, headache up to 10 days, and fatigue that can persist for a month or longer (3).

Self-Quiz
Ask Yourself...
- How might travel patterns, environmental factors, and local population characteristics influence the duration and severity of West Nile Virus symptoms in different endemic regions?
Laboratory Findings and Diagnosis
Routine laboratory tests in WNV infection may show leukocytosis and other nonspecific findings (46). Serological testing detects WNV IgM antibodies in blood or cerebrospinal fluid (CSF) using enzyme-linked immunosorbent assay (ELISA) for definitive diagnosis (46). The plaque reduction neutralization test (PRNT) distinguishes serologic cross-reactions with other flaviviruses (47).
In neuroinvasive cases, lumbar puncture can reveal findings consistent with viral meningitis, including elevated protein and leukocyte levels in the CSF (46). Neutrophils dominate early in the disease and transition to lymphocyte predominance, while glucose levels stay normal (46)(47). Hyponatremia is another common finding in CNS involvement (46).

Self-Quiz
Ask Yourself...
- How might understanding the shifts in CSF findings—such as early neutrophil predominance transitioning to lymphocyte predominance—guide clinicians in differentiating WNV neuroinvasive disease from other viral or bacterial CNS infections?
Imaging Studies
Neuroimaging is unremarkable in the initial stages of neuroinvasive WNV. Computed tomography (CT) of the brain often shows no acute abnormalities (33). However, magnetic resonance imaging (MRI) may reveal abnormalities associated with neuroinvasive disease, often weeks after the acute phase (33).
This comprehensive approach to understanding WNV clinical presentation, diagnostic tools, and testing highlights the importance of early recognition and targeted diagnostics in cases with potential neuroinvasive involvement.

Self-Quiz
Ask Yourself...
- How might the often unremarkable findings on initial neuroimaging for neuroinvasive West Nile Virus (WNV) influence clinicians‘ decisions about diagnostic testing and interventions?
Differential Diagnosis
Diagnosing West Nile Virus (WNV) infection in clinical settings relies on clinical examination, laboratory testing, and, in some cases, post-mortem examination. However, diagnosis can be challenging due to the absence of pathognomonic clinical signs and the overlap of symptoms with other diseases.

Self-Quiz
Ask Yourself...
- In the absence of pathognomonic signs for West Nile Virus, how might providers distinguish WNV infection from other neurologic or febrile illnesses with similar clinical features?
Clinical Examination
In humans, clinical diagnosis is based on symptoms such as acute fever, anorexia, nausea, vomiting, eye pain, headache, myalgia, rash, lymphadenopathy, and arthralgia (48). Multifocal chorioretinitis identifies severe WNV infection, prompting ophthalmological examination in suspected cases (49).
However, clinical diagnosis alone is often presumptive in non-endemic regions, where other infectious diseases with similar clinical presentations may be more common. A travel history to endemic areas plays a vital role in diagnosing regions where diseases like malaria mimic one another (1)(4).

Self-Quiz
Ask Yourself...
- How can healthcare professionals in non-endemic regions leverage travel history and ophthalmological findings to differentiate West Nile Virus from other infectious diseases with similar symptoms?
Laboratory-Based Diagnostics
Serological diagnosis for West Nile Virus (WNV) involves detecting IgM and IgG antibodies, which often appear 3–7 days post-exposure (46). IgM antibodies can persist for extended periods, up to two years in some cases (e.g., horses), limiting their diagnostic utility in chronic cases (46).
Blocking ELISA is a reliable and species-independent tool that measures the ability of patient serum antibodies to inhibit the binding of monoclonal antibodies against NS1 and E protein epitopes (46). However, this method may exhibit cross-reactivity with other flaviviruses, such as the Murray Valley encephalitis and Alfuy virus (50).
Virus neutralization tests (VNT) are the gold standard due to their specificity. They enable the detection and quantification of neutralizing antibodies (51). However, VNTs require considerable time, often up to a week, and prohibitive costs limit their widespread use.
Molecular diagnostics, including reverse transcription polymerase chain reaction (RT-PCR) and quantitative RT-PCR (qRT-PCR), are sensitive and specific methods for detecting WNV (46). These techniques target conserved genome regions, such as the NS5 or E protein, to identify WNV strains. qRT-PCR offers an additional advantage of quantifying viral genomes through fluorescence monitoring (52).
However, RT-PCR may detect RNA from killed-virus vaccines, necessitating complementary methods like virus isolation or primers targeting specific viral regions to distinguish vaccine RNA from active infections (52). Molecular testing focuses on analyzing serum or cerebrospinal fluid (CSF) samples.
Pathological examination remains a valuable diagnostic tool in research and post-mortem investigations. This approach identifies macroscopic and microscopic lesions and detects viral antigens in tissue sections using immunohistochemistry (IHC). IHC targeting NS1 or NS3 proteins provides definitive evidence of viral replication in tissues (46)(52). The utility of this method decreases due to the invasive sample collection process and the lack of specific antibodies.
Rapid diagnostic tools like the immunochromatographic assay RapidWN™ detect WNV IgM antibodies in human plasma or serum (46). This cost-effective, user-friendly test is well-suited for low-resource settings, as it does not require specialized equipment or extensive training. Similar rapid diagnostic tools are not yet available for veterinary use, highlighting the need for affordable and accessible diagnostic options across human and animal health domains (46).
The clinical presentation of WNV infection overlaps with many other conditions, necessitating a broad differential diagnosis that includes:
- Viral Infections: Varicella-zoster virus, herpes simplex virus type 1, St. Louis encephalitis (cross-reactivity with flavivirus serology), enteroviruses, dengue virus, and Japanese encephalitis (20).
- Bacterial Infections: Bacterial meningitis (20).
- Tickborne Diseases: Lyme disease, ehrlichiosis, anaplasmosis, Rocky Mountain spotted fever, and Powassan virus (53).
Careful travel history, clinical evaluation, and comprehensive testing are essential for distinguishing WNV from these conditions. Despite advancements in serological and molecular diagnostics, limitations persist in the availability of rapid, affordable tools for early detection. Developing user-friendly diagnostic methods for human and veterinary use remains a priority in improving WNV management and control.

Self-Quiz
Ask Yourself...
- Considering the various diagnostic methods for WNV—ranging from serological to molecular—how might cost, turnaround time, and cross-reactivity influence clinical decision-making and public health strategies for disease surveillance?
- Given that IgM antibodies can persist for extended periods and molecular diagnostics sometimes detect vaccine-derived RNA, what are the key considerations for interpreting test results in acute and chronic WNV cases, especially in settings with limited resources?
Treatment
The treatment of West Nile Virus (WNV) infection is primarily supportive, as no specific antiviral therapy has demonstrated apparent efficacy (8). Researchers have explored various agents, including interferon, ribavirin, and intravenous immunoglobulin, but the evidence remains limited (54).
Management is often outpatient for individuals with mild cases and focuses on symptom relief. These patients have an excellent prognosis. In contrast, patients with severe neurologic symptoms usually require intensive care unit (ICU) management and may face a prolonged recovery, high mortality, and early disability (55).
Long-term physical and occupational therapy at rehabilitation centers can address residual neurologic deficits. These deficits may involve gross motor, fine motor, and cognitive impairments, some of which can take months or years to recover, while others may be permanent (55).
In central nervous system (CNS) involvement cases, patients are at substantial risk for complications due to prolonged immobility. West Nile Virus (WNV) affects anterior horn cells, leading to a poliomyelitis-like syndrome, and is the most common underlying cause of paralysis associated with WNV infection (56). Resulting issues include pressure sores, aspiration pneumonia, and deep vein thrombosis (DVT). Prophylactic measures to prevent these complications, such as regular repositioning, aspiration precautions, and DVT prophylaxis, are essential components of comprehensive care.
Documented sequelae include Parkinsonian tremors, poliomyelitis, meningitis, and cognitive impairments, affecting more than one-third of confirmed cases (55). The virus’s vestibular tropism causes hearing loss, leading to chronic vestibulocochlear neuritis and functional impairment (57).
Experimental studies in mice have demonstrated WNV infection in spiral ganglion neurons of the inner ear, highlighting its potential for long-term cranial nerve damage (57). Persistent damage to other cranial nerves, such as the ocular nerve, occurs for up to three years post-recovery (55). Chronic retinopathy, contractures of extremities, severe dysphonia, and movement disorders are other reported complications (2). Neurologic damage to the cerebral cortex is linked to attention deficits, memory loss, concentration issues, anxiety, depression, and abnormal gait (55)(58)(59). Further research must explore potential links between WNV infection and chronic neurological disorders, including Alzheimer’s disease, dementia, multiple sclerosis, and Parkinson’s disease (60).

Self-Quiz
Ask Yourself...
- How does the current reliance on supportive care for West Nile Virus infection—amid limited evidence for specific antiviral therapies—shape immediate management strategies (e.g., ICU support, prophylaxis, and rehabilitation) and long-term outcomes for patients with severe neurologic involvement?
Persistence of WNV Infection
Evidence suggests that WNV can persist in host species, including humans, birds, and mammals (2). Experimental studies have demonstrated persistent viruria in hamsters for up to 52 days post-infection, with viral antigens detected in kidney epithelial cells (2)(61). Infected hamsters have also shown WNV persistence in the brain for up to 89 days, leading to neuron dysfunction and poliomyelitis-like syndromes (2)(61).
Infectious WNV and its RNA remain detectable in mice for four and six months after infection (2)(62). In monkeys, intracranial infection resulted in prolonged viral persistence, while human studies have detected WNV RNA in urine samples for over six years post-infection (2)(63).
The persistence of WNV in hosts beyond the viremic period poses significant epidemiological and public health concerns, necessitating further investigation into its mechanisms and implications.

Self-Quiz
Ask Yourself...
- What underlying biological mechanisms might enable West Nile virus to persist in host tissues for extended periods, and how could this long-term viral presence influence our strategies to prevent and manage West Nile virus infections in human and animal populations?
Immunity and Reinfection
There have been no reported cases of WNV reinfection in clinical or experimental settings (2). Primary infection confers lifelong immunity (2)(4). Studies confirm the persistence of WNV IgM and IgG antibodies for up to three years post-infection, with neutralizing antibodies present in 94.4% of seropositive blood donors five years after infection (2)(64). These findings suggest that long-lasting immunity protects against reinfection (2)(64).

Self-Quiz
Ask Yourself...
- What mechanisms or evidence would you examine to confirm whether West Nile virus infection confers long-lasting immunity, and how might providers account for the absence of documented reinfections in clinical and experimental contexts?
Coinfections with Other Pathogens
Coinfections with WNV and other pathogens are known in areas with high mosquito activity (2)(65). Coinfections can result from overlapping geographic distributions and shared vectors. Researchers documented coinfections of WNV with malaria in birds and humans (65). While some studies suggest an inverse association between WNV and avian malaria, others report a direct positive correlation between WNV and human Plasmodium falciparum cases (65).
Natural coinfections of WNV with other arboviruses, including dengue virus (DENV), chikungunya virus (CHIKV), and Japanese encephalitis virus (JEV), occurred (2)(66). Reports confirm WNV and JEV coinfections in encephalitis patients, and WNV and Toscana virus (TOSV) coinfections occur in febrile patients in Türkiye (2)(67). Researchers have noted a coinfection of poxviruses in American crows and Halicephalobus gingivalis in humans (2)(68).

Self-Quiz
Ask Yourself...
- How might recognizing the potential for West Nile virus coinfections with other mosquito-borne pathogens—such as malaria, dengue, chikungunya, and Japanese encephalitis—influence how providers diagnose, treat, and develop public health strategies for these diseases?
Vector and Host Interactions
Interactions between WNV and endosymbionts, such as Wolbachia, can influence vector competence. While Wolbachia reduces the transmission of DENV and CHIKV in Aedes aegypti, its impact on WNV varies. Studies have shown that Wolbachia can enhance WNV infection in Culex tarsalis mosquitoes but impair transmission in Culex quinquefasciatus (69). Insect-specific flaviviruses (ISFs), such as Palm Creek and Bamaga viruses, suppress WNV replication and provide potential options for biologically controlling pathogenic flaviviruses (70).

Self-Quiz
Ask Yourself...
- What factors might explain why the same endosymbiont, Wolbachia, enhances WNV infection in one mosquito species while impairing it in another, and how might insect-specific flaviviruses fit into this dynamic?
Public Health Implications
The persistence, immunity, and coinfection dynamics of WNV raise significant public health concerns. Understanding these phenomena and their interactions with other pathogens and vectors could inform strategies for controlling WNV and other flaviviruses. Further research into the long-term impacts of WNV infection, including its potential link to chronic neurological conditions, remains a critical public health priority.

Self-Quiz
Ask Yourself...
- How might exploring the interplay between WNV persistence, immunity, and coinfection with other pathogens prompt a deeper reevaluation of current public health strategies and priorities in preventing chronic neurological complications?
Conclusion
West Nile Virus (WNV) represents a complex and growing public health concern characterized by diverse transmission pathways, clinical presentations, and global distribution. The virus’s ability to spread through mosquito vectors, blood transfusions, organ transplants, and rare non-vectorial modes underscores the multifaceted nature of its transmission.
Clinical manifestations range from asymptomatic infections to severe neuroinvasive diseases, which can lead to long-term neurological deficits and significant morbidity (2). The emergence of new genetic variants and environmental changes further complicates the epidemiological landscape, necessitating an integrated approach to surveillance, prevention, and management (2)(10)(38).
Despite advancements in understanding WNV pathogenesis, significant gaps remain in diagnostic capabilities, treatment options, and vaccine development. Preventive measures like vector control and personal protection remain the cornerstone of public health efforts. Collaboration among healthcare professionals, researchers, and policymakers is essential to address these challenges.
Future efforts should focus on enhancing diagnostic tools, exploring therapeutic interventions, and investigating the virus’s long-term health impacts to mitigate the burden of this re-emerging zoonotic threat.
Teaching Points:
- Neuroinvasive WNV is a rare but severe complication of WNV infection, requiring prompt diagnosis and supportive care.
- Early recognition of symptoms such as fever, headache, and neurologic changes is crucial for initiating appropriate management.
- Prevention strategies, including insect repellents and protective clothing, are critical, particularly for individuals in endemic regions.
References + Disclaimer
- Factsheet about West Nile virus infection. (2023, December 15). European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/west-nile-fever/facts
- Hamburger, G., Suen, W. W., Hobson-Peters, J., Hall, R. A., & Bielefeldt-Ohmann, H. (2020). West Nile Virus: An update on pathobiology, epidemiology, diagnostics, control and “One Health” implications. Pathogens, 9(7), 589. https://doi.org/10.3390/pathogens9070589
- Clinical signs and symptoms of West Nile virus disease. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/hcp/clinical-signs/index.html
- West Nile: Symptoms, diagnosis, & treatment. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/symptoms-diagnosis-treatment/index.html
- Transmission of West Nile virus. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/php/transmission/index.html
- Agliani, G., Giglia, G., Marshall, E. M., Gröne, A., Rockx, B. H., & Van Den Brand, J. M. (2023). A comparative review of the pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and humans. One Health, 16, 100525. https://doi.org/10.1016/j.onehlt.2023.100525
- Petersen, L. R., & Nett, R. J. (2022). West Nile virus: From Africa to Europe, America, and beyond. In Springer eBooks (pp. 1–44). https://doi.org/10.1007/978-3-030-85877-3_38-1
- Treatment and prevention of West Nile virus disease. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/hcp/treatment-prevention/index.html
- Preventing West Nile. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/prevention/index.html
- Baker, R. E., Mahmud, A. S., Miller, I. F., Rajeev, M., Rasambainarivo, F., Rice, B. L., Takahashi, S., Tatem, A. J., Wagner, C. E., Wang, L., Wesolowski, A., & Metcalf, C. J. E. (2021). Infectious disease in an era of global change. Nature Reviews Microbiology, 20(4), 193–205. https://doi.org/10.1038/s41579-021-00639-z
- Ferraguti, M., Martins, A. D., & Artzy-Randrup, Y. (2023). Quantifying the invasion risk of West Nile virus: Insights from a multi-vector and multi-host SEIR model. One Health, 17, 100638. https://doi.org/10.1016/j.onehlt.2023.100638
- Soto, R. A., McDonald, E., Annambhotla, P., Velez, J. O., Laven, J., Panella, A. J., Machesky, K. D., White, J. L., Hyun, J., Freuck, E., Habel, J., Oh, D., Levi, M., Hasz, R., Eidbo, E., Staples, J. E., Basavaraju, S. V., & Gould, C. V. (2021). West Nile Virus transmission by solid organ transplantation and Considerations for organ donor screening Practices, United States. Emerging Infectious Diseases, 28(2), 403–406. https://doi.org/10.3201/eid2802.211697
- Abbas, A., Qiu, F., Sikyta, A., Fey, P. D., & Florescu, D. F. (2022). Neuroinvasive West Nile virus infections after solid organ transplantation: Single center experience and systematic review. Transplant Infectious Disease, 24(6). https://doi.org/10.1111/tid.13929
- Pealer, L. N., Marfin, A. A., Petersen, L. R., Lanciotti, R. S., Page, P. L., Stramer, S. L., Stobierski, M. G., Signs, K., Newman, B., Kapoor, H., Goodman, J. L., & Chamberland, M. E. (2003). Transmission of West Nile Virus through Blood Transfusion in the United States in 2002. New England Journal of Medicine, 349(13), 1236–1245. https://doi.org/10.1056/nejmoa030969
- Reemtsma, H., Holicki, C. M., Fast, C., Bergmann, F., Eiden, M., Groschup, M. H., & Ziegler, U. (2022). Pathogenesis of West Nile Virus Lineage 2 in Domestic Geese after Experimental Infection. Viruses, 14(6), 1319. https://doi.org/10.3390/v14061319
- Hinckley, A. F., O’Leary, D. R., & Hayes, E. B. (2007). Transmission of West Nile virus through human breast milk seems to be rare. PEDIATRICS, 119(3), e666–e671. https://doi.org/10.1542/peds.2006-2107
- Odigie, A. E., Stufano, A., Schino, V., Zarea, A. a. K., Ndiana, L. A., Mrenoshki, D., Ugochukwu, I. C. I., Lovreglio, P., Greco, G., Pratelli, A., Camero, M., & Tempesta, M. (2024). West Nile Virus Infection in Occupational Settings—A Systematic Review. Pathogens, 13(2), 157. https://doi.org/10.3390/pathogens13020157
- Epidemiological update: West Nile virus transmission season in Europe, 2023. (2024, February 20). European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2023-0
- Klingelhöfer, D., Braun, M., Kramer, I. M., Reuss, F., Müller, R., Groneberg, D. A., & Brüggmann, D. (2023). A virus becomes a global concern: research activities on West Nile virus. Emerging Microbes & Infections, 12(2). https://doi.org/10.1080/22221751.2023.2256424
- Guidelines for West Nile Virus Surveillance and Control. (2024, July 18). West Nile Virus. https://www.cdc.gov/west-nile-virus/php/surveillance-and-control-guidelines/index.html
- Vidaña, B., Busquets, N., Napp, S., Pérez-Ramírez, E., Jiménez-Clavero, M. Á., & Johnson, N. (2020). The role of Birds of Prey in West Nile virus epidemiology. Vaccines, 8(3), 550. https://doi.org/10.3390/vaccines8030550
- Martin, M., & Nisole, S. (2020). West Nile Virus Restriction in Mosquito and Human Cells: A Virus under Confinement. Vaccines, 8(2), 256. https://doi.org/10.3390/vaccines8020256
- Saiz, J., Martín-Acebes, M. A., Blázquez, A. B., Escribano-Romero, E., Poderoso, T., & De Oya, N. J. (2021). Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation. Virulence, 12(1), 1145–1173. https://doi.org/10.1080/21505594.2021.1908740
- Fiacre, L., Pagès, N., Albina, E., Richardson, J., Lecollinet, S., & Gonzalez, G. (2020). Molecular determinants of West Nile virus virulence and pathogenesis in vertebrate and invertebrate hosts. International Journal of Molecular Sciences, 21(23), 9117. https://doi.org/10.3390/ijms21239117
- Verhaegen, M., & Vermeire, K. (2024). The endoplasmic reticulum (ER): a crucial cellular hub in flavivirus infection and potential target site for antiviral interventions. Npj Viruses, 2(1). https://doi.org/10.1038/s44298-024-00031-7
- Andersen, D. K., Fischer, G. A., & Combrink, L. (2024). The Alligator and the Mosquito: North American crocodilians as amplifiers of West Nile virus in changing climates. Microorganisms, 12(9), 1898. https://doi.org/10.3390/microorganisms12091898
- Bai, F., Thompson, E. A., Vig, P. J. S., & Leis, A. A. (2019). Current understanding of West Nile virus clinical manifestations, immune responses, neuroinvasion, and immunotherapeutic implications. Pathogens, 8(4), 193. https://doi.org/10.3390/pathogens8040193
- Epidemiology and pathogenesis of West Nile virus infection – UpToDate. (2024). UpToDate. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-west-nile-virus-infection/print
- Burden, Z., Fasen, M., Judkins, B. L., & Isache, C. (2019). A case of West Nile virus encephalitis accompanied by diabetic ketoacidosis and rhabdomyolysis. IDCases, 15, e00505. https://doi.org/10.1016/j.idcr.2019.e00505
- Nemeth, N. M., & Kunkel, M. R. (2024, March 6). West Nile virus in birds. Merck Veterinary Manual. https://www.merckvetmanual.com/poultry/viral-encephalitides-in-birds/west-nile-virus-in-birds
- Byas, A. D., Gallichotte, E. N., Hartwig, A. E., Porter, S. M., Gordy, P. W., Felix, T. A., Bowen, R. A., Ebel, G. D., & Bosco-Lauth, A. M. (2022). American alligators can amplify the West Nile virus and mosquito infection and transmission. Virology, 568, 49–55. https://doi.org/10.1016/j.virol.2022.01.009
- Dahlin, C., Hughes, D., Meshaka, W., Coleman, C., & Henning, J. (2016). Wild snakes harbor the West Nile virus. One Health, 2, 136–138. https://doi.org/10.1016/j.onehlt.2016.09.003
- Moreno-Reina, C., Martínez-Moya, M., De La Peña, P. P., & Caro-Domínguez, P. (2022). Neuroinvasive disease due to West Nile virus: Clinical and imaging findings associated with a re-emerging pathogen. Radiología (English Edition), 64(5), 473–483. https://doi.org/10.1016/j.rxeng.2021.06.007
- Montgomery, R. R. (2016). Age-related alterations in immune responses to West Nile virus infection. Clinical & Experimental Immunology, 187(1), 26–34. https://doi.org/10.1111/cei.12863
- Hoffman, K. W., Lee, J. J., Foster, G. A., Krysztof, D., Stramer, S. L., & Lim, J. K. (2019). Sex differences in cytokine production following West Nile virus infection: Implications for symptom manifestation. Pathogens and Disease, 77(2). https://doi.org/10.1093/femspd/ftz016
- Haba, Y., & McBride, L. (2022). Origin and status of Culex pipiens mosquito ecotypes. Current Biology, 32(5), R237–R246. https://doi.org/10.1016/j.cub.2022.01.062
- Ferraguti, M., La Puente, J. M., & Figuerola, J. (2021). Ecological effects on the dynamics of West Nile virus and Avian plasmodium: the importance of mosquito communities and landscape. Viruses, 13(7), 1208. https://doi.org/10.3390/v13071208
- Dunphy, B.M., Kovach, K.B., Gehrke, E.J. et al. Long-term surveillance defines spatial and temporal patterns implicating Culex tarsalis as the primary vector of the West Nile virus. Sci Rep 9, 6637 (2019). https://doi.org/10.1038/s41598-019-43246-y
- Brazier, Y. (2017, June 29). What is West Nile virus? https://www.medicalnewstoday.com/articles/187839
- Petersen, L. R. (2019). Epidemiology of West Nile virus in the United States: Implications for arbovirology and public health. Journal of Medical Entomology, 56(6), 1456–1462. https://doi.org/10.1093/jme/tjz085
- Betsem, E., Kaidarova, Z., Stramer, S. L., Shaz, B., Sayers, M., LeParc, G., Custer, B., Busch, M. P., & Murphy, E. L. (2016). Correlation of West Nile Virus Incidence in Donated Blood with West Nile Neuroinvasive Disease Rates, United States, 2010–2012. Emerging Infectious Diseases, 23(2), 212–219. https://doi.org/10.3201/eid2302.161058
- Winkelmann, E. R., Luo, H., & Wang, T. (2016). West Nile virus infection in the central nervous system. F1000Research, 5, 105. https://doi.org/10.12688/f1000research.7404.1
- Stonedahl, S., Clarke, P., & Tyler, K. L. (2020). The Role of Microglia during West Nile Virus Infection of the Central Nervous System. Vaccines, 8(3), 485. https://doi.org/10.3390/vaccines8030485
- Pierson, T. C., & Diamond, M. S. (2020). The continued threat of emerging flaviviruses. Nature Microbiology, 5(6), 796–812. https://doi.org/10.1038/s41564-020-0714-0
- McDonald, E., Mathis, S., Martin, S. W., Staples, J. E., Fischer, M., & Lindsey, N. P. (2021). Surveillance for West Nile Virus Disease — United States, 2009–2018. MMWR Surveillance Summaries, 70(1), 1–15. https://doi.org/10.15585/mmwr.ss7001a1
- Clinical testing and diagnosis for West Nile virus disease. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/hcp/diagnosis-testing/index.html
- Chan, K. R., Ismail, A. A., Thergarajan, G., Raju, C. S., Yam, H. C., Rishya, M., & Sekaran, S. D. (2022). Serological cross-reactivity among common flaviviruses. Frontiers in Cellular and Infection Microbiology, 12. https://doi.org/10.3389/fcimb.2022.975398
- Sewgobind, S., McCracken, F., & Schilling, M. (2023). JMM Profile: West Nile virus. Journal of Medical Microbiology, 72(7). https://doi.org/10.1099/jmm.0.001730
- Moussa, K., Jeng-Miller, K. W., Kim, L. A., & Eliott, D. (2021). West Nile virus chorioretinitis in the presence of negative cerebrospinal fluid polymerase chain reaction results. Journal of VitreoRetinal Diseases, 5(6), 513–519. https://doi.org/10.1177/2474126420979254
- Chan, K. R., Ismail, A. A., Thergarajan, G., Raju, C. S., Yam, H. C., Rishya, M., & Sekaran, S. D. (2022). Serological cross-reactivity among common flaviviruses. Frontiers in Cellular and Infection Microbiology, 12. https://doi.org/10.3389/fcimb.2022.975398
- Savini, G., Bonfini, B., & Spedicato, M. (2024). Virus Neutralization Test for detecting and quantifying Serum-Neutralizing antibodies to Epizootic Hemorrhagic Disease Virus (EHDV) (Serotypes 1, 2, and 4–8). Methods in Molecular Biology, 123–136. https://doi.org/10.1007/978-1-0716-4035-7_7
- Tomar, P. S., Kumar, J. S., Patel, S., & Sharma, S. (2020). Polymerase spiral reaction assay for rapid and real-time detection of West Nile virus from clinical samples. Frontiers in Cellular and Infection Microbiology, 10. https://doi.org/10.3389/fcimb.2020.00426
- Kemenesi, G., & Bányai, K. (2018). Tickborne Flaviviruses, with a Focus on Powassan Virus. Clinical Microbiology Reviews, 32(1). https://doi.org/10.1128/cmr.00106-17
- West Nile Virus Disease Therapeutics: Review of the Literature. (2024, May 15). West Nile Virus. https://www.cdc.gov/west-nile-virus/hcp/treatment-prevention/therapeutics-review.html
- Santini, M., Haberle, S., Židovec-Lepej, S., Savić, V., Kusulja, M., Papić, N., Višković, K., Župetić, I., Savini, G., Barbić, L., Tabain, I., Kutleša, M., Krajinović, V., Potočnik-Hunjadi, T., Dvorski, E., Butigan, T., Kolaric-Sviben, G., Stevanović, V., Gorenec, L., . . . Vilibić-Čavlek, T. (2022). Severe West Nile Virus Neuroinvasive Disease: Clinical Characteristics, Short- and Long-Term Outcomes. Pathogens, 11(1), 52. https://doi.org/10.3390/pathogens11010052
- Sejvar, J. J., Bode, A. V., Marfin, A. A., Campbell, G. L., Ewing, D., Mazowiecki, M., Pavot, P. V., Schmitt, J., Pape, J., Biggerstaff, B. J., & Petersen, L. R. (2005). West Nile virus-associated flaccid paralysis. Emerging Infectious Diseases, 11(7), 1021–1027. https://doi.org/10.3201/eid1107.040991
- Parrino, D., Brescia, G., Trimarchi, M. V., Tealdo, G., Sasset, L., Cattelan, A. M., Bovo, R., & Marioni, G. (2019). Cochlear-Vestibular Impairment due to West Nile Virus Infection. Annals of Otology Rhinology & Laryngology, 128(12), 1198–1202. https://doi.org/10.1177/0003489419866219
- Bhandari, T. (2018, January 11). Memory loss from West Nile virus may be preventable | WashU Medicine. WashU Medicine. https://medicine.washu.edu/news/memory-loss-from-west-nile-virus-may-be-preventable/
- Fulton, C. D., Beasley, D. W., Bente, D. A., & Dineley, K. T. (2020). Long-term, West Nile virus-induced neurological changes: A comparison of patients and rodent models. Brain Behavior & Immunity – Health, 7, 100105. https://doi.org/10.1016/j.bbih.2020.100105
- Vittor, A. Y., Long, M., Chakrabarty, P., Aycock, L., Kollu, V., & DeKosky, S. T. (2020). West Nile Virus-Induced Neurologic Sequelae—Relationship to neurodegenerative cascades and dementias. Current Tropical Medicine Reports, 7(1), 25–36. https://doi.org/10.1007/s40475-020-00200-7
- Saxena, V., Xie, G., Li, B., Farris, T., Welte, T., Gong, B., Boor, P., Wu, P., Tang, S., Tesh, R., & Wang, T. (2013). A Hamster-Derived West Nile virus isolate induces persistent renal infection in mice. PLoS Neglected Tropical Diseases, 7(6), e2275. https://doi.org/10.1371/journal.pntd.0002275
- Garcia, M. N., Hasbun, R., & Murray, K. O. (2014). Persistence of West Nile virus. Microbes and Infection, 17(2), 163–168. https://doi.org/10.1016/j.micinf.2014.12.003
- Murray, K., Walker, C., Herrington, E., Lewis, J. A., McCormick, J., Beasley, D. W. C., Tesh, R. B., & Fisher‐Hoch, S. (2009). Persistent Infection with West Nile Virus Years after Initial Infection. The Journal of Infectious Diseases, 201(1), 2–4. https://doi.org/10.1086/648731
- West Nile virus IgM and IgG antibodies three years post-infection. (2015, March 1). PubMed. https://pubmed.ncbi.nlm.nih.gov/26435644/
- Gallichotte, E. N., Fitzmeyer, E. A., Williams, L., Spangler, M. C., Bosco-Lauth, A. M., & Ebel, G. D. (2024). WNV and SLEV coinfection in avian and mosquito hosts: impact on viremia, antibody responses, and vector competence. Journal of Virology. https://doi.org/10.1128/jvi.01041-24
- Khan, E., Barr, K. L., Farooqi, J. Q., Prakoso, D., Abbas, A., Khan, Z. Y., Ashi, S., Imtiaz, K., Aziz, Z., Malik, F., Lednicky, J. A., & Long, M. T. (2018). Human West Nile virus disease outbreak in Pakistan, 2015–2016. Frontiers in Public Health, 6. https://doi.org/10.3389/fpubh.2018.00020
- Ayhan, N., Eldin, C., & Charrel, R. (2024). Toscana virus infection clinical characterization. medRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2024.07.31.24310805
- Brault, A. C., Huang, C. Y., Langevin, S. A., Kinney, R. M., Bowen, R. A., Ramey, W. N., Panella, N. A., Holmes, E. C., Powers, A. M., & Miller, B. R. (2007). A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nature Genetics, 39(9), 1162–1166. https://doi.org/10.1038/ng2097
- Minwuyelet, A., Petronio, G. P., Yewhalaw, D., Sciarretta, A., Magnifico, I., Nicolosi, D., Di Marco, R., & Atenafu, G. (2023). Symbiotic Wolbachia in mosquitoes and its role in reducing the transmission of mosquito-borne diseases: updates and prospects. Frontiers in Microbiology, 14. https://doi.org/10.3389/fmicb.2023.1267832
- Colmant, A. M. G., Hobson-Peters, J., Bielefeldt-Ohmann, H., Van Den Hurk, A. F., Hall-Mendelin, S., Chow, W. K., Johansen, C. A., Fros, J., Simmonds, P., Watterson, D., Cazier, C., Etebari, K., Asgari, S., Schulz, B. L., Beebe, N., Vet, L. J., Piyasena, T. B. H., Nguyen, H., Barnard, R. T., & Hall, R. A. (2017). A New Clade of Insect-Specific Flaviviruses from Australian Anopheles Mosquitoes Displays Species-Specific Host Restriction. mSphere, 2(4). https://doi.org/10.1128/msphere.00262-17
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
