Course
Kentucky Pharmacology and Addiction Disorders
Course Highlights
- In this Kentucky Pharmacology and Addiction Disorders course, we will learn about statistics on addiction disorders in the United States and the state of Kentucky.
- You’ll also learn the impact of opioids and the underlying mechanisms of actions that contribute to addiction.
- You’ll leave this course with a broader understanding of the pharmacokinetics of buprenorphine and naltrexone.
About
Pharmacology Contact Hours Awarded: 1.5
Course By:
Abbie Schmitt
RN, MSN-Ed
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Individuals struggling with an addiction often feel powerless and hopeless in the battle. Thankfully, there are effective tools to empower and provide hope to these individuals, including initiatives for support, medications, counseling, behavioral therapies, as well as regulations to prevent inappropriate prescribing.
This course will provide pharmacology of opioids and drugs that can be an effective treatment tool, including buprenorphine and naltrexone. It is meaningful to explore the etiology of addiction disorders impacting the state of Kentucky and review laws and prescribing regulations.
Understanding Kentucky Addiction Disorders
The United States is facing an opioid crisis. The number of overdose deaths involving opioids, including prescription opioids, heroin, and synthetic opioids (like fentanyl), is currently roughly 10 times the number in 1999 (2). Opioid overdoses took the lives of 80,000 people in 2021 in the U.S., and nearly 88% of those deaths involved synthetic opioids (2).
Kentucky ranks in the top 10 states for drug overdoses in the U.S. (5). In 2022, there were 2,135 overdose deaths in Kentucky approximately 90% of those deaths involved opioids (5). Fentanyl causes more overdose fatalities than any other type of drug and in Kentucky, fentanyl was present in 70% of overdose deaths last year (5).
In 2021, a total of 12,946 Kentucky residents visited an ED for a nonfatal drug overdose (5).
Legislators in Kentucky have recognized the devastation of addiction disorders and created many initiates to prevent, identify, and treat addiction disorders, specifically opioid use disorders.
Opioid-Related Laws and Prescribing Regulations for APRNs in Kentucky
Several laws and policies are in place to mitigate the impact of increased opioid addiction and deaths. These include regulations for prescribers, legislation permitting the operation of syringe exchange programs, and Good Samaritan laws that provide legal protections to bystanders who seek help in the event of an overdose.
APRNs should be aware of the state laws, regulations, guidance, and policies related to oversight of opioid prescribing and monitoring of opioid use.
Kentucky’s Controlled Substances Act governs all controlled substances and has many provisions to be aware of (8).
- Kentucky’s Senate Bill 192, enacted in 2015, provided substance abuse treatment funds and amended KRS 218A.500 to permit communities to set up syringe exchange programs (8).
- Kentucky’s Good Samaritan Law (KRS 218A.133) protects individuals from prosecution if they seek medical attention while experiencing a drug overdose in certain circumstances (8). It also protects individuals from prosecution when they report a drug overdose if they stay with the individual who has overdosed until first responders arrive.
- Kentucky Administrative Regulations (KAR) KRS 218A.172 addresses prescribing and dispensing Schedule II controlled substances and Schedule III controlled substances containing hydrocodone.
- It requires prescribers to obtain the patient’s medical history and conduct a physical or mental health examination, obtain patient data in the prescription drug monitoring program (PDMP), make a written plan of treatment, discuss the risks with the patient, obtain written consent for treatment, and review data and modify treatment when needed (8).
- KRS 218A:205, limits the prescribing of a Schedule II controlled substance used for acute pain to a 3-day supply (8).
- Kentucky’s prescription drug monitoring program, Kentucky All Schedule Prescription Electronic Reporting (KASPER KRS 218A.172) requires prescribers to check KASPER.
- Required prior to the initial prescribing or dispensing of any Schedule II or Schedule III controlled substance containing hydrocodone
- Every three months thereafter
- Prescribers are required to complete continuing education relating to the use of KASPER, pain management, addiction disorders, or a combination of two or more of these subjects (KRS 218A.205).
The exceptions for an APRN to prescribe greater than a 3-day supply of hydrocodone combination products include only the following (4).
- In the professional judgment of the APRN, more than a 3-day supply is needed. The need must be thoroughly documented.
- Treating chronic pain.
- Treating cancer pain.
- Treating a patient at end of life or in Hospice.
- Treatment of pain after major surgery or significant trauma as defined by the licensing Board and the Office of Drug Control Policy.
- Administered directly to the patient in an inpatient setting.
- Scenarios authorized by the licensing board.

Self Quiz
Ask yourself...
- Can you describe the Good Samaritan law in Kentucky?
- How can the availability of syringe exchange programs impact communicable diseases?
- What are restrictions on opioid prescribing in Kentucky?
- What is the limit (in days) for supply of Schedule II and Schedule III controlled substance containing hydrocodone when prescribed for acute pain in Kentucky?
Basic Pharmacology of Opioids
Opioids are a group of analgesic agents commonly used in clinical practice. Opioid receptors are G-protein-coupled receptors which cause cellular hyperpolarization when bound to opioid agonists (3).
As long ago as 3000 BC the opium poppy, Papaver somniferum, was cultivated; followed by morphine being isolated from opium in 1806 by Serturner (3).
Opioids can also be classified according to their effect on opioid receptors and can be considered as agonists, partial agonists, antagonists, and agonist-antagonists (3).
- Agonists – interact with an opioid receptor to produce a maximal response from that receptor.
- Antagonists – bind to receptors but produce no functional response, while at the same time preventing an agonist from binding to that receptor (naloxone).
- Partial agonists – bind to receptors but elicit only a partial functional response regardless of the amount of drug administered
- Agonist-antagonists – act as agonist to a certain opioid receptor, but have antagonist activity to another opioid receptor
Naturally occurring opioid receptors are present throughout the body, both centrally (e.g., in the brain and spinal cord) and peripherally (e.g., in the heart and gut). The primary opioid receptors that have been identified are the mu (μ), kappa (κ), and delta (δ) subtypes (1). All of the opioid receptor subtypes are seven-transmembrane G protein–coupled receptors (GPCRs) that can exist in both active and inactive states.
Opioid agonists bind to inhibitory G proteins, which ultimately activate various signaling cascades. When opioid receptors are activated, release of the neurotransmitter γ-aminobutyric acid (GABA) is decreased (1). GABA produces a tonic inhibition of dopamine release, so this inhibition of GABA causes an increase in dopamine release. In the past few years, several important advances have occurred in our understanding of opioid receptor functioning, including biased signaling, allosteric regulation, and heteromerization (1).
Pain can be categorized as nociceptive, neuropathic or nociplastic pain (a combination of both that cannot be entirely explained as nociceptive or neuropathic). Nociceptive pain is generated as a warning signal transmitted to the brain about the possible damage of a non-neural tissue (9).
Neuropathic pain typically results from damage to neural tissue caused by a disease, toxin, or infection. The next type, called nociplastic pain, is a chronic and complex pain, not completely defined but probably caused by an alteration of neurons’ pain response and an increased sensitivity of the central nervous system (CNS). This pain sensations and is commonly observed in patients with cancer and other long-term chronic disorders (9).
The opioid system is a physiological control system that facilitates communication among a significant number of endogenous opioid peptides and several types of opioid receptors in the CNS and peripheral nervous system.
This system also significantly modulates numerous sensory, emotional, cognitive functions, as well as addictive behaviors (9). It is also involved in other physiological functions, including responses to stress, respiration, gastrointestinal transit, endocrine, and immune functions (9).
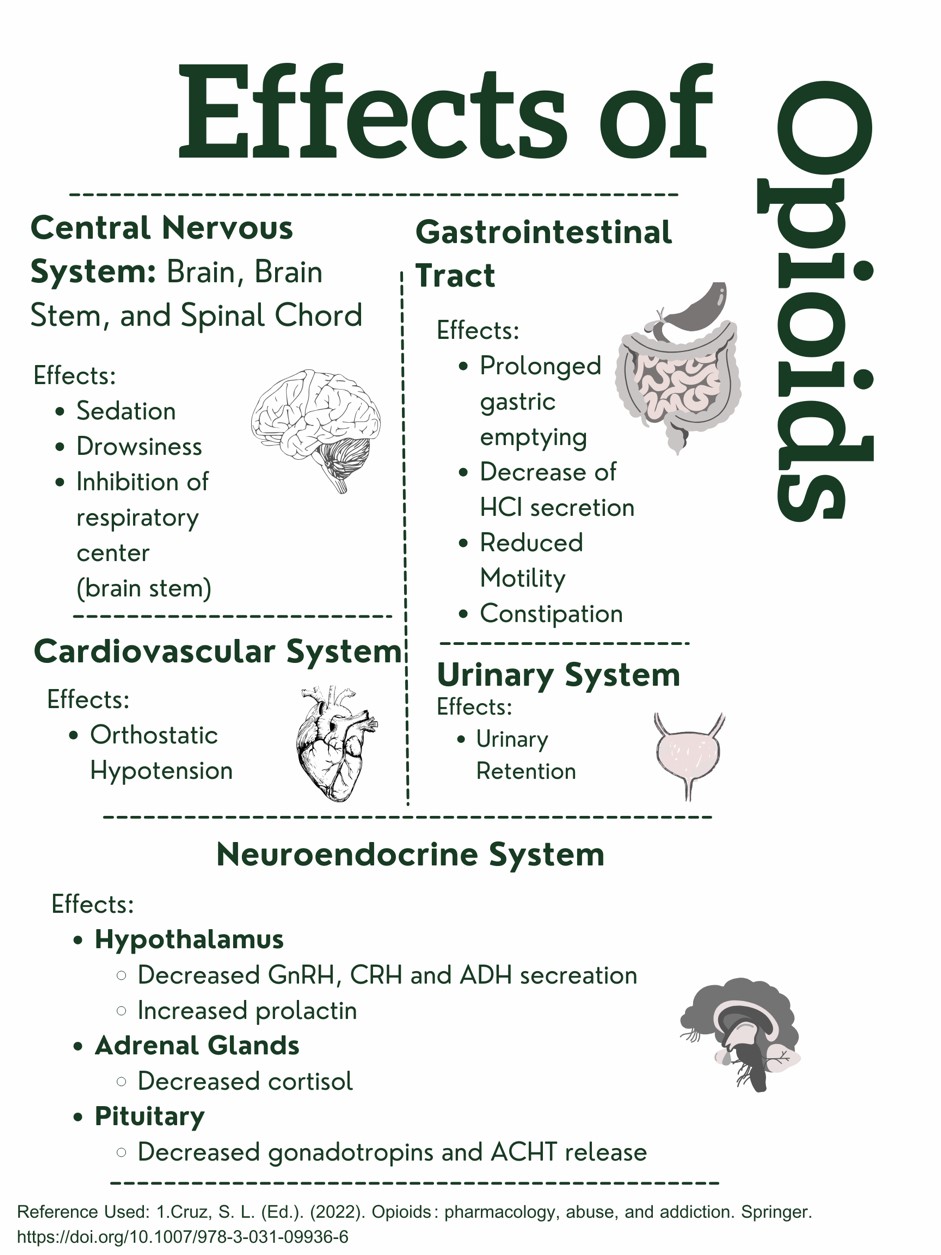 Figure 1. Effects of Opioids on the Body. Designed by Author. Information retrieved from (15).
Figure 1. Effects of Opioids on the Body. Designed by Author. Information retrieved from (15).
This design was created and copyrighted by Abbie Schmitt, RN, MSN and may not be reproduced without permission from Nursing CE Central.

Self Quiz
Ask yourself...
- Can you name the physiological effects of opioids on the different systems of the body?
- How are the actions of agonists and antagonists different in their interaction with opioid receptors?
- How does the activation and cascade of opioid receptors impact GABA and dopamine?
- Can you discuss the different categories of pain?
Medication-Assisted Treatment (MAT) for Opioid Use Disorder (OUD)
An opioid use disorder (OUD) is defined as a problematic pattern of opioid use that leads to serious impairment or distress (1). Medication-assisted treatment (MAT) is the use of medications, in combination with counseling and behavioral therapies, in the treatment of opioid use disorders (OUD). The goal is sustained recovery.
The U.S. Food and Drug Administration (FDA) has only approved three medication assisted treatments (MATs) for opioid use disorder (OUD): buprenorphine, methadone, and naltrexone.
FDA-approved buprenorphine products approved for the treatment of OUD include:
- Brixadi (buprenorphine) injection for subcutaneous use
- Bunavail (buprenorphine and naloxone) buccal film
- Cassipa (buprenorphine and naloxone) sublingual film
- Probuphine (buprenorphine) implant for subdermal administration
- Sublocade (buprenorphine extended release) injection for subcutaneous use
- Suboxone (buprenorphine and naloxone) sublingual film for sublingual or buccal use, or sublingual tablet.
- Subutex (buprenorphine) sublingual tablet
- Zubsolv (buprenorphine and naloxone) sublingual tablets
FDA-approved methadone products for the treatment of OUD include:
- Dolophine (methadone hydrochloride) tablets
- Methadose (methadone hydrochloride) oral concentrate
FDA-approved naltrexone products for the treatment of OUD include:
- Vivitrol (naltrexone for extended-release injectable suspension) intramuscular
The FDA requires that prescribing information for medicines that are intended for use in the outpatient setting include how to safely decrease the dose. Prescribers should not abruptly discontinue opioids in a patient who is physically dependent, but slowly decrease the dose of the opioid and continue to manage pain therapeutically.

Self Quiz
Ask yourself...
- Are medications intended to be used independently of other therapeutic measures in the treatment of OUD?
- Can you discuss the medications that are approved by the FDA for the treatment of OUD?
- Is it appropriate to abruptly quit these medications?
- Have you had experience administering methadone or similar drugs?
Buprenorphine
Buprenorphine should be used as part of a comprehensive treatment program to include counseling and psychosocial support.
Definition
Buprenorphine is a synthetic opioid developed in the late 1960s and is used to treat opioid use disorder. This drug is a synthetic analog of thebaine, which is an alkaloid compound derived from the poppy flower (6).
Buprenorphine is categorized as a Schedule III drug. This schedule includes drugs that have a moderate-to-low potential for physical dependence or a high potential for psychological dependence (6).
Buprenorphine is approved by the U.S. Food and Drug Administration (FDA) to treat acute and chronic pain and opioid use disorder.
Drug Class
- Analgesic, Opioid
- Analgesic, Opioid Partial Agonist
Uses
Buprenorphine is used to treat opioid use disorder (OUD) and to manage pain that is severe enough to require long-term opioid treatment, and in patients for which alternative treatment options (e.g., nonopioid analgesics) are ineffective, not tolerated, or inadequate enough to provide sufficient management of pain (13).
Buprenorphine should be used as part of a complete treatment program to include counseling and psychosocial support.
Mechanism of Action
Buprenorphine has an analgesic effect by binding to mu opiate receptors in the CNS. Due to it being a partial mu agonist, its analgesic effects plateau at higher doses and it then behaves like an antagonist (13). This is a meaningful attribute, and this plateauing of its analgesic effects at higher doses, causes it to have ceiling, or limited, effects on respiratory depression (6). This is a positive attribute, signifying its safety superiority.
Buprenorphine exhibits high-affinity binding to the mu-opioid receptors and slow-dissociation kinetics. In this way, it differs from other full-opioid agonists such as morphine and fentanyl, which results in milder and less uncomfortable withdrawal symptoms for the patient (6).
Essentially, the benefits of this drug include: (1) higher doses do not lead to greater analgesic effects, thus respiratory depression; and (2) the withdrawal symptoms from buprenorphine are not as intense as full-opioid antagonists.
The extended-release formulation is injected subcutaneously as a liquid (13).
Note on absorption: When administered orally, buprenorphine has poor bioavailability due to the first-pass effect, in which the liver and intestine metabolize the drug.
The preferred route of administration is sublingual, so it can have rapid absorption and circumvent the first-pass effect. Placing the tablet under the tongue results in a slow onset of action, with the peak effect occurring approximately 3 to 4 hours after administration (6).
Pharmacodynamics/Kinetics
Pharmacodynamics/Kinetics of buprenorphine include the following (13).
- Onset of action: Immediate-release IM: ≥15 minutes
- Peak effect: Immediate-release IM: ~1 hour
- Duration: Immediate-release IM: ≥ 6 hours; Extended-release Sub-Q: 28 days
- Absorption: Immediate-release IM and Sub-Q: 30% to 40%.
- Application of a heating pad may increase blood concentrations of buprenorphine 26% to 55%.
- Distribution: Cerebral spinal fluid (CSF) concentrations are 15% to 25% of plasma concentrations
- Protein binding: High (~96%, primarily to alpha- and beta globulin)
- Metabolism: Primarily hepatic via N-dealkylation by CYP3A4 to norbuprenorphine (active metabolite), and to a lesser extent via glucuronidation by UGT1A1 and 2B7 to buprenorphine 3-O-glucuronide; the major metabolite, norbuprenorphine, also undergoes glucuronidation via UGT1A3; extensive first-pass effect
- Bioavailability (relative to IV administration): Buccal film: 46% to 65%; Immediate-release IM: 70%; Sublingual tablet: 29%; Transdermal patch: ~15%
- Half-life elimination in adults:
- IV: 2.2 to 3 hours
- Buccal film: 27.6 ± 11.2 hours
- Sublingual tablet: ~37 hours
- Transdermal patch: ~26 hours
- Time to peak, plasma:
- Buccal film: 2.5 to 3 hours
- Extended-release Sub-Q: 24 hours, with steady state achieved after 4 to 6 months
- Subdermal implant: 12 hours after insertion, with steady state achieved by week four
- Sublingual: 30 minutes to 1 hour
- Transdermal patch: Steady state achieved by day three
- Excretion: Most of the drug and its metabolite are eliminated through feces, with less than 20% excreted by the kidneys (6)
- Clearance: Related to hepatic blood flow
- Adults: 0.78 to 1.32 L/hour/kg
Adverse Effects
Buprenorphine has anticholinergic-like effects and may cause CNS depression, dry mouth, dizziness, hypotension, drowsiness, QT prolongation, and lower seizure threshold (6).
Additional adverse effects of buprenorphine include:
- Nausea
- Vomiting
- Headache
- Memory loss
- Orthostatic hypotension
- Urinary retention
Following buprenorphine treatment, a patient's tolerance to opioids decreases, increasing risk for harm if they resume their previous opioid dosage. Patients should be strongly advised against using opioids without prior consultation with their healthcare provider.
Warnings
Prescribers should exercise caution when prescribing buprenorphine to patients with hepatic impairment, morbid obesity, thyroid dysfunction, a history of ileus or bowel obstruction, prostatic hyperplasia or urinary stricture, CNS depression or coma, delirium tremens, depression, anxiety disorders, posttraumatic stress disorder, and toxic psychosis (6).
Concerns related to adverse effects:
Hepatic Impairment
- In individuals with hepatic impairment, such as patients with hepatitis B and C, the dose of buprenorphine has to be modified to prevent toxicity (6). As buprenorphine metabolism takes place in the liver, individuals with liver impairment should undergo close monitoring of their liver function and drug levels. Clinicians should educate patients with hepatitis about the correlation between IV use of buprenorphine and hepatotoxicity (6).
- For buccal film and sublingual tablets in patients with severe hepatic impairment, it is advisable to reduce the dose by 50% and closely monitor for signs and symptoms of toxicity.
- Subcutaneous injections are not recommended.
CNS Depression
- Due to side effects and CNS depression, patients must be cautioned about performing tasks that require mental alertness (e.g., operating machinery, driving).
Hypersensitivity Reactions
- Hypersensitivity, including bronchospasm, angioneurotic edema, and anaphylactic shock, have been reported. The most common symptoms include rash, hives, and pruritus.
Hypotension
- Clinicians must be aware of possible hypotension (including orthostatic hypotension and syncope); use with caution in patients with hypovolemia, cardiovascular disease, or if patient takes drugs that may exaggerate hypotensive effects (including phenothiazines or general anesthetics).
- Monitor for symptoms of hypotension following initiation or dose titration.
Infection from Subdermal Implant
- Infection may occur at the site of insertion or removal (6).
Figure 2. Buprenorphine Injectable (13)

Self Quiz
Ask yourself...
- Can you describe the mechanisms of action for Buprenorphine?
- What are some comorbidities to be careful with when prescribing this drug?
- How are the mechanisms of actions for Buprenorphine different than the mechanisms of actions for opioids?
- Can you describe the analgesic effects of higher doses of Buprenorphine?
Naltrexone
Naltrexone is a pure opioid antagonist, it acts as a competitive antagonist at opioid receptor sites, showing the highest affinity for mu receptors (14).
Naltrexone was developed in 1963 and patented in 1967 and is used for treatment of alcohol use disorders (6). In 1984, naltrexone received approval for medical use in the U.S.
Drug Class
- Antidote
- Opioid Antagonist
Uses
- Alcohol use disorder: FDA-approved
- Opioid use disorder: For the blockade of the effects of exogenously administered opioids; FDA-approved
- A fixed-dose combination of naltrexone and bupropion is FDA-approved for obesity (6)
- Researchers are studying its use in patients with stimulant use disorder, particularly patients with polydrug dependence on opioids, heroin, and amphetamine (6)
Mechanism of Action
Naltrexone (and its active metabolite 6-beta-naltrexone) is pharmacologically effective against opioids by blocking the mu-opioid receptor.
Naltrexone blocks the effect of opioids and prevents opioid intoxication and physiologic dependence on opioid users. Naltrexone helps with alcohol dependency because it modifies the hypothalamic-pituitary-adrenal axis to suppress ethanol consumption (14) .
Opioids act mainly via the mu receptor, although they affect mu, delta, and kappa-opioid receptors. Naltrexone competes for opiate receptors and displaces opioid drugs from these receptors, thus reversing their effects (14). It is capable of antagonizing all opiate receptors. (14). Exogenous opioids include the commonly prescribed pain relievers such as hydrocodone, oxycodone, and heroin. These typically induce euphoria at much higher doses than those prescribed by medical providers to relieve pain. If naltrexone occupies the receptors, the opioids are not going to provide these euphoric effects.
According to guidelines by the American Society of Addiction Medicine (ASAM), a combination of buprenorphine and low doses of oral naltrexone is effective for opioid use disorder for managing withdrawal (14).
Pharmacodynamics/Kinetics
- Duration: Oral: 50 mg: 24 hours; 100 mg: 48 hours; 150 mg: 72 hours; IM: 4 weeks
- Absorption: Oral: Almost complete
- Distribution: Vd: ~1350 L; widely throughout the body but considerable interindividual variation exists
- Metabolism: Extensively metabolized via noncytochrome-mediated dehydrogenase conversion to 6-beta-naltrexol (primary metabolite) and related minor metabolites; glucuronide conjugates are also formed from naltrexone and its metabolites
- Oral: Extensive first-pass effect
- Protein binding: 21%
- Bioavailability: Oral: Variable range (5% to 40%)
- Half-life elimination:
- Oral: 4 hours; 6-beta-naltrexol: 13 hours
- IM: naltrexone and 6-beta-naltrexol: 5 to 10 days (dependent upon erosion of polymer)
- Time to peak, serum:
- Oral: ~60 minutes
- IM: Biphasic: ~2 hours (first peak), ~2 to 3 days (second peak)
- Excretion: Primarily urine (as metabolites and small amounts of unchanged drug)
Side Effects
Commonly reported side effects of naltrexone include:
- Abdominal pain
- Gastrointestinal Distress
- Constipation
- Nausea and vomiting
- Diarrhea
- Insomnia
- Joint and muscle pain
- Fatigue
- Loss of strength and energy
- Tooth pain
- Dry mouth
- Increased thirst
Warnings
Patients should be opioid-free for a minimum of 7 to 10 days prior to taking naltrexone (14)
Prescribers must be aware that patients who had been treated with naltrexone may respond to lower opioid doses than previously used, which could result in potentially life-threatening or fatal opioid intoxication. Patients should be educated that they may be more sensitive to lower doses of opioids after naltrexone treatment is discontinued, after a missed dose, or near the end of the dosing interval (14).
Opioid withdrawal may be noted in patients, and symptoms include pain, hypertension, sweating, agitation, and irritability; in neonates: shrill cry, failure to feed (14).
Cases of eosinophilic pneumonia have been reported and should be assessed in patients presenting with progressive hypoxia and dyspnea.
Hepatotoxicity can occur, and clinicians should note that elevated transaminases may be a result of alcoholic liver disease, hepatitis B and/or C infection, or concomitant use of other hepatotoxic drugs; abrupt opioid withdrawal may also lead to acute liver injury. Clinicians should discontinue this drug if any signs or symptoms of hepatotoxicity are found.
Drug-Drug Interactions:
- Bremelanotide- Contraindicated to administer with naltrexone due to reduced therapeutic effect of naltrexone (10).
- Thioridazine – Contraindicated to administer with naltrexone due to the risk of lethargy and somnolence (10).
- Methylnaltrexone: May enhance the adverse/toxic effect of opioid antagonists; the risk for opioid withdrawal may be increased (14).
- Naldemedine: Opioid Antagonists may enhance the adverse/toxic effect of naldemedine; the risk for opioid withdrawal may be increased (14).
Treatment Resources
The KY HELP Call Center is available to those with a substance use disorder, or their friends or family members, as a resource for information on treatment options and treatment providers.
Individuals may call 833-8KY-HELP (833-859-4357) to speak one-on-one with a specialist who will connect them with treatment as quickly as possible (5).
The Kentucky Injury Prevention and Research Center (KIPRC) at the University of Kentucky College of Public Health manages a vital website, www.findhelpnowky.org, for Kentucky health care providers, court officials, families and individuals seeking options for substance abuse treatment and recovery (5).
The Kentucky State Police (KSP) Angel Initiative is a proactive program designed to help those who battle addiction. There are 16 posts located throughout the commonwealth that will connect individuals with a local officer who will assist with locating an appropriate treatment program. The Angel Initiative is completely voluntary, and individuals will not be arrested or charged with any violations if they agree to participate in treatment.

Figure 3. Substance Use Disorder Call Center Contact Information (5)
Conclusion
A common theme among individuals who struggle with OUD is a loss of power to this addictive substance. There is significant opportunity to help this population gain back control, including legislation and supportive initiative, and appropriate combined use of medications, counseling, and behavioral therapies. Knowledge on the pharmacology of opioids, as well as buprenorphine and naltrexone is critical. Although the opioid crisis is substantial, there is hope on the horizon.

References + Disclaimer
- Brady, K., Levin, F. R., Galanter, M., & Kleber, H. D. (Eds.). (2021). The American Psychiatric Association Publishing textbook of substance use disorder treatment (Sixth edition.). American Psychiatric Association Publishing.
- Centers for Disease Control and Prevention (CDC). (2023). The drug overdose epidemic: Behind the numbers. Retrieved from https://www.cdc.gov/opioids/data/index.html
- James, A., & Williams, J. (2020). Basic Opioid Pharmacology – An Update. British journal of pain, 14(2), 115–121. https://doi.org/10.1177/2049463720911986.
- Kentucky Board of Pharmacy. (2023). APRN and PA Prescribing. Ky.gov. Commonwealth of Kentucky. https://pharmacy.ky.gov/Pages/APRN-and-PA-Prescribing.aspx
- Kentucky Office of Drug Control Policy Commonwealth of Kentucky Justice & Public Safety Cabinet. (2023). 2022 Overdose fatality report. Team Kentucky. Retrieved from https://odcp.ky.gov/Reports/2022%20Overdose%20Fatality%20Report.pdf
- Kumar R, Viswanath O, Saadabadi A. Buprenorphine. [Updated 2023 Nov 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459126/
- National, Academies of Sciences, Engineering, and Medicine, et al. , edited by Michelle Mancher, and Alan I. Leshner. (2019). Medications for opioid use disorder save lives National Academies Press, 2019. ProQuest Ebook Central, https://ebookcentral.proquest.com/lib/liberty/detail.action?docID=5774508.
- Oversight.gov. (2020). Factsheet: Kentucky’s oversight of opioid prescribing and monitoring of opioid use. Retrieved from https://www.oversight.gov/sites/default/files/oig-reports/41902022_Factsheet.pdf
- Paul AK, Smith CM, Rahmatullah M, Nissapatorn V, Wilairatana P, Spetea M, Gueven N, Dietis N. (2021). Opioid analgesia and opioid-induced adverse effects: a review. Pharmaceuticals. 2021; 14(11):1091. https://doi.org/10.3390/ph14111091
- Singh D, Saadabadi A. Naltrexone. (2023) In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534811/
- Spencer MR, Miniño AM, Warner M. (2022). Drug overdose deaths in the United States, 2001–2021. NCHS Data Brief, no 457. Hyattsville, MD: National Center for Health Statistic. DOI: https://dx.doi. org/10.15620/cdc:122556.
- Strickler, G. K., Zhang, K., Halpin, J. F., Bohnert, A. S. B., Baldwin, G. T., & Kreiner, P. W. (2019). Effects of mandatory prescription drug monitoring program (PDMP) use laws on prescriber registration and use and on risky prescribing. Drug and Alcohol Dependence, 199, 1-9. https://doi.org/10.1016/j.drugalcdep.2019.02.010
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Buprenorphine. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426498#monoNumber=426498§ionID=241825553&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Naltrexone. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426798#monoNumber=426798§ionID=239566147&tab=tab0
- Cruz, S. L., & Granados-Soto, V. (2022). Opioids: Pharmacology, abuse, and addiction. Springer Cham. https://doi.org/10.1007/978-3-031-09936-6
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
