Course
SNRIs for Depression
Course Highlights
- In this SNRIs for Depression course, we will learn about the types and uses of Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs).
- You’ll also learn the mechanism of action, side effects, and contraindications of SNRIs.
- You’ll leave this course with a broader understanding of applying pharmacokinetics of SNRIs to individualized care planning and prescribing.
About
Pharmacology Contact Hours Awarded: 1.5
Course By:
Abbie Schmitt
MSN-Ed, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Depression can significantly interfere with daily activities and diminish quality of life among patients who experience it. Major depressive disorder (MDD) is one of the leading causes of the burden for worldwide diseases (5). At the present stage, the first-line treatment of MDD is selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). Research has found that SNRIs showed faster antidepressant effects than SSRIs (5).
The serotonin norepinephrine reuptake inhibitors (SNRIs) are a family of antidepressants that inhibit the reuptake of both serotonin and norepinephrine; however, it is important to recognize the differences among each specific drug in this class. In this course, we will examine pharmacological properties, clinical indications, mechanism of action, metabolism and excretion, dosing schedules, side effects, and warnings of each SNRI currently approved by the United States Food and Drug Administration (FDA) for the treatment of depression.
Forms and Symptoms of Depression
Depression can have crippling effects. Research suggests that genetic, biological, environmental, and psychological factors play a role in depression (7). Depression can be present with other comorbidities, such as mental disorders, diabetes, cancer, heart disease, and chronic pain. Depression can make these conditions worse, and vice versa. Certain medications have been shown to increase depressive symptoms.
Common forms of depression are: (7)
- Major depression - which includes symptoms of depression most of the time for at least two weeks that regularly interfere with work, sleep, eating, and quality of life.
- Persistent depressive disorder (dysthymia), which often includes less severe symptoms of depression that last much longer, typically for at least two years.
- Perinatal depression - occurs when a woman experiences major depression during pregnancy or after delivery (postpartum depression).
- Seasonal affective disorder – impacted by change in seasons, typically starting in late fall and early winter and improving during spring and summer.
- Depression with symptoms of psychosis - a severe form of depression in which the patient experiences psychosis symptoms, such as delusions or hallucinations
Common symptoms of depression include:
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness
- Irritability, frustration‚ or restlessness
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies or activities once enjoyed
- Fatigue or lack of energy
- Aches, musculoskeletal pain, or headaches without injury or obvious causation, and do not improve with medication.
- Gastrointestinal issues
- Difficulty concentrating, remembering, or making decisions
- Poor sleeping patterns
- Changes in appetite
- Suicide attempts or thoughts of death or suicide


Self Quiz
Ask yourself...
- Are you aware of certain biological, environmental, or psychological factors that play a role in depression?
- How would you describe the difference between occasional sadness and depression?
- Can you name common symptoms of depression?
- Which drug class has shown faster antidepressant effects in studies, SSRIs or SNRIs?
Pharmacokinetics of SNRIs
The major mechanism of action of SNRIs is the inhibition of presynaptic neuronal uptake of 5-HT (serotonin) and norepinephrine after release from the synaptic cleft (3). Blocking the reuptake prolongs the presence of monoamines in the synaptic cleft within the central nervous system (CNS). This causes an increase in postsynaptic receptor stimulation and additional post synaptic neuronal transmission (3).
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT), is a neurotransmitter that has an important role in regulating various activities, including behavior, mood, memory, and gastrointestinal homeostasis (1). It is often referred to as the “feel good hormone” and delivers messages between brain cells, contributing to well-being, mood, appetite, social behavior, as well as helping to regulate the body’s sleep-wake cycle.
Serotonin is synthesized in the raphe nuclei of the brainstem and the enterochromaffin cells of the intestinal mucosa (1). Serotonin is a primary treatment target for major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), and anxiety disorders.
Norepinephrine
Norepinephrine, also known as noradrenaline, is a neurotransmitter in the brain that plays an essential role in the regulation of arousal, attention, cognitive function, and stress reactions. It also functions as a hormone peripherally as part of the sympathetic nervous system in the “fight or flight” response.
During states of stress or anxiety, norepinephrine and epinephrine are released and bind to adrenergic receptors throughout the body which exert effects such as dilating pupils and bronchioles, increasing heart rate and constricting blood vessels, increasing renin secretion from the kidneys, and inhibiting peristalsis.
It works closely with dopamine and serotonin systems and is thought to help mobilize the brain for action, increasing alertness, focus, and the retrieval of memory (4).
The increased availability of serotonin and norepinephrine in the nerve synapse means that information can be transmitted easier from one nerve to another.
There are currently five SNRIs approved by the FDA for use in the U.S.:
- Venlafaxine (Effexor XR)
- Duloxetine (Cymbalta, Irenka)
- Desvenlafaxine (Pristiq, Khedezla)
- Levomilnacipran (Fetzima)
- Milnacipran (Savella)
- Milnacipran (Savella TM) is not approved by the FDA to treat depression, but rather only fibromyalgia. For this reason, this course will not review this SNRI.
As mentioned, all SNRIs regulate serotonin and norepinephrine, but there are subtle differences in how much absorption they prevent and the side effects of each type.

Self Quiz
Ask yourself...
- Do all SNRIs have the same uses?
- Can you define serotonin and norepinephrine in your own words?
- Are you familiar with these SNRIs? If so, have you known patients who have benefited from use?
- How would you describe the relationship between sleep cycles, nutrition, and mood?
Venlafaxine
Venlafaxine (Effexor ™) is approved by the FDA and was the first SNRI approved in the U.S. It is available in an extended-release formula and works by regulating levels of serotonin and norepinephrine.
Uses
- Major depressive disorder
- Generalized anxiety disorder
- Social anxiety disorder
- Panic disorder
Mechanism of Action
Venlafaxine increases serotonin, norepinephrine, and dopamine in the brain by blocking transport proteins and preventing their reuptake at the presynaptic terminal (10). This action leads to more neurotransmitters available at the synapse, which ultimately increases the stimulation of postsynaptic receptors.
Venlafaxine is a bicyclic phenylethylamine compound; it is a more potent inhibitor of serotonin reuptake than norepinephrine reuptake (8). Venlafaxine acts as a selective serotonin reuptake inhibitor at 75 mg, but when a higher dose is given, such as 225 mg/day, it has significant effects on the norepinephrine transporter as well (10). Venlafaxine does not have MAO-inhibitory properties.
Pharmacodynamics
The pharmacodynamics of venlafaxine is as follows: (9)
Absorption: 92-100% absorbed after oral administration.
Distribution: Extensive distribution into body tissues.
Metabolism and Excretion: Extensively metabolized in first pass through the liver. 5% of venlafaxine is excreted unchanged in urine; 30% of the active metabolite is excreted in urine.
Half-life: 3-5 hr.; ODV: 9-11 hr.
Contraindications
- Hypersensitivity
- Precaution advised for use in patients with:
- Cardiovascular disease
- Hepatic or renal impairment
- History of seizures or neurologic impairment
- History of mania
- History of drug abuse
- Angle-closure glaucoma
Obstetrics (OB): Use during pregnancy only if risks are closely examined. There is a potential for discontinuation syndrome or toxicity in the neonate when venlafaxine is taken during the 3rd trimester (9). Venlafaxine is a category C pregnancy drug and can potentially pass into breast milk (8).
Venlafaxine is contraindicated if it causes worsening suicidal ideation, depression, anxiety, and psychosis. Precaution is needed for patients with heart failure patients, hyperthyroidism, and those with recent myocardial infarctions, as it can raise blood pressure and increase heart rate. Venlafaxine raises the risk of seizures, and prescribers should avoid the drug in patients with a seizure disorder (8).
Adverse Reactions / Side Effects
Adverse reactions / side effects of venlafaxine include: (10)
CV: Chest pain, hypertension, palpitations, tachycardia.
Neuro: Abnormal dreams, anxiety, dizziness, headache, insomnia, nervousness, paresthesia.
Dermatology: ecchymoses, itching, photosensitivity, skin rash.
EENT: rhinitis, visual disturbances.
GI: abdominal pain, altered taste, anorexia, constipation, diarrhea, dry mouth, dyspepsia, nausea, vomiting, weight loss.
GU: Decreased libido, erectile dysfunction, urinary frequency, urinary retention.
Hematological: Bleeding.
Serotonin syndrome – a condition of building up high levels of serotonin in the body due to medication use. Although rare, serotonin syndrome is a very serious condition that has a high mortality rate (5). Signs of serotonin syndrome include tachycardia, sialorrhea (excessive saliva production), hyperactive bowel sounds, mydriasis (sustained dilated pupils), hyperthermia, and diaphoresis (6). This condition is caused by combining monoamine oxidase inhibitors, tricyclic antidepressants, triptans, additional serotonin receptor modulators, or over-the-counter drugs such as St. John's Wort (6).
If a clinician is suspecting serotonin syndrome, the Sternbach and Hunter criteria can be useful to help arrive at a definitive diagnosis.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors or serotonergic neurotransmitter systems, including tricyclic antidepressants, fentanyl, buspirone, tramadol, amphetamines, and triptans, may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
PO (Adults): Tablets: 75 mg/day in 2-3 divided doses; may increase by up to 75 mg per day every 4 days, up to 225 mg/day. Do not exceed 375 mg per day in 3 divided doses.
Extended-release capsules: 75 mg once daily (some patients may be started at 37.5 mg once daily) for 4-7 days; may increase by up to 75 mg/day at intervals for no less than 4 days (not to exceed 225 mg/day).
ALERT: US Boxed Warning
Antidepressants increased the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders (10). Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults older than 24 years old (10).
Venlafaxine is not approved for use in pediatric patients.

Self Quiz
Ask yourself...
- What are the major mechanisms of action for Venlafaxine?
- Can you explain the side effects and contraindications of Venlafaxine?
- Are SNRIs currently recommended for pediatric patients?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
Duloxetine
Duloxetine (Cymbalta ™) has the most FDA-approved uses of any SNRI, and, unlike Venlafaxine, is proven to be effective in the treatment of other conditions, such as neuropathy, fibromyalgia, and osteoarthritis (9).
Drug Class
Selective Serotonin/Norepinephrine Reuptake Inhibitors
Uses
Duloxetine is used for the following conditions: (9)
- Major depressive disorder
- Diabetic peripheral neuropathic pain
- Generalized anxiety disorder
- Chronic musculoskeletal pain (including chronic lower back pain and chronic pain from osteoarthritis)
- Fibromyalgia
Mechanism of Action
Duloxetine inhibits serotonin and norepinephrine reuptake, and also enhances dopamine levels within the prefrontal cortex. This increase in dopamine levels involve the inhibition of norepinephrine transporters, which are particularly attracted to dopamine, making it effective in transporting dopamine and norepinephrine (2). Essentially, inhibition of norepinephrine transporters can cause an increase in dopamine.
The secondary mechanisms of action, in increasing dopamine, is helpful in pain reduction. This occurs due to increased activity of noradrenergic and serotonergic neurons in the descending spinal pathway on the dorsal horn and suppression of excessive input from reaching the brain (2). The perception of pain can be reduced as these signals are interrupted.
Pharmacokinetics
Absorption: Well-absorbed following oral administration.
Distribution: Unknown.
Protein Binding: >90%.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP2D6 and CYP1A2 isoenzymes. Excretion: Fecal is 20%; renal is 70% as metabolites.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
| ROUTE | ONSET | PEAK | DURATION |
| PO | Unknown | 6 hr. | 12 hr. |
Contraindications:
Contraindications in the use of duloxetine include: (9)
- Hypersensitivity
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Severe renal impairment (CCr <30 mL/min).
- Hepatic impairment or substantial alcohol use (increased risk of hepatitis).
Use cautiously in:
- History of suicide attempt or ideation.
- History of mania (may activate mania/hypomania).
- History of seizure disorder.
- Diabetes (may worsen glycemic control).
- Angle-closure glaucoma.
OB/ Lactation: Use while pregnant or breastfeeding only if potential maternal benefit justifies potential risk to infant.
Geriatric Precaution: Appears on Beers list. May worsen or cause syndrome of inappropriate antidiuretic hormone (SIADH) secretion and/or hyponatremia in older adults; closely monitor sodium concentrations.
Adverse Reactions / Side Effects
CV: Hypertension, orthostatic hypotension.
EENT: Increased intraocular pressure, blurred vision.
Endocrine: SIADH.
Fluids and Electrolytes: Hyponatremia.
GI: Decreased appetite, constipation, dry mouth, nausea.
GU: Dysuria.
Neurological: Drowsiness, fatigue, insomnia.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitor before initiation of levomilnacipran (9). Concurrent use with MAO-inhibitor like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
- Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Route and Dosage in Depression
PO (Adults): 40-60 mg/day (as 20 mg or 30 mg twice daily or as 60 mg once daily) as initial therapy, then 60 mg once daily as maintenance therapy (9).


Self Quiz
Ask yourself...
- What are the major mechanisms of action for Duloxetine?
- Can you explain the side effects and contraindications of Duloxetine?
- Which of these patient symptoms do you think should be a priority: mild drowsiness or tachycardia?
- Does an increased dosage of Venlafaxine result in different neurotransmitter activity?
Desvenlafaxine
Desvenlafaxine (Pristiq ™) received approval from the FDA in 2008. The drug is similar to Venlafaxine structurally and 10 times more potent in inhibiting serotonin reabsorbing than norepinephrine (6). It has been observed weakly blocking dopamine reuptake, similar to Venlafaxine and Duloxetine.
Uses
Desvenlafaxine is an antidepressant that is an FDA-approved drug to treat major depressive disorder in adults. For healthy women who have contraindications to estrogen, desvenlafaxine has an off-label use to treat hot flashes during menopause (6).
Mechanisms of Actions
Desvenlafaxine is the primary active metabolite of venlafaxine. Therefore, the two agents are structurally similar in that they both contain two chemical rings that are not next to each other. In vitro studies have shown that desvenlafaxine is ten times more selective for serotonin than for norepinephrine, making it similar to the drug duloxetine (6).
Pharmacokinetics
Absorption: 80% absorbed following oral administration.
Distribution: Widely distributed to tissues.
Metabolism and Excretion: 55% metabolized by the liver, 45% excreted unchanged in urine.
Half-life: 10 hr.
Time / Action Profile (Plasma Concentrations):
|
ROUTE |
ONSET |
PEAK |
DURATION |
|
PO |
Unknown |
7.5 hr. |
24 hr. |
Contraindications/Precautions
- Hypersensitivity to venlafaxine or desvenlafaxine.
- Concurrent use of MAO inhibitors or MAO-like drugs (linezolid or methylene blue).
- Should not be used concurrently with venlafaxine.
Use cautiously in:
- Untreated cerebrovascular or cardiovascular disease, including untreated hypertension (control BP before initiating therapy).
- Bipolar disorder (may activate mania/hypomania).
- Moderate or severe renal impairment.
- History of seizures or neurologic impairment.
- Moderate or severe hepatic impairment.
- Angle-closure glaucoma.
OB: Safety is not established in pregnancy. Desvenlafaxine is excreted into breast milk, with one study reporting that peak levels are 3.3 hours after a dose (6).
Adverse Reactions / Side Effects
There is a broad range of general adverse effects that patients have reported with desvenlafaxine. A study comparing desvenlafaxine to a placebo in treating major depressive disorder in adolescents noted the most common side effects to be abdominal pain, decreased appetite, headache, and nausea (6).
Less common side effects were diarrhea, dizziness, and cough.
Abruptly stopping the use of desvenlafaxine can cause irritability, nausea, and headaches (6). Gradually tapering the medication is recommended to avoid such side effects.
Drug-Drug Interactions
- The concurrent use of all SNRIs and MAO inhibitors may result in serious and potentially fatal reactions (it may increase the risk of serotonin syndrome) (9). A period of at least two weeks is recommended between stopping MAO inhibitors and initiating venlafaxine (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.
Increased risk of hepatotoxicity in patients who abuse alcohol or have alcohol use disorder (9).
Increased risk of serious arrhythmias when used with thioridazine; avoid concurrent use (9).
Route and Dosage in Depression
PO (Adults): 50 mg once daily (range = 50-400 mg/day).
Renal Impairment:
PO (Adults): CCr 30-50 mL/min: 50 mg once daily; CCr <30 mL/min: 50 mg every other day or 25 mg once daily.
Hepatic Impairment:
PO (Adults): 50 mg once daily (not to exceed 100 mg/day).

Self Quiz
Ask yourself...
- What are adverse effects for desvenlafaxine?
- Can you explain why SNRI and MAO inhibitors are contraindicated to use together?
Levomilnacipran
Levomilnacipran (Fetzima ™) is the most recent medication approved for treating major depression.
Levomilnacipran is different from other SNRIs because it has greater inhibition of norepinephrine than serotonin; essentially, it has twice the potency of norepinephrine reuptake inhibition compared to serotonin (3). The more recent FDA approval and relatively lower number of prescriptions may be contributing factors to an overall low volume of research and evidence on this drug.
Levomilnacipran has a one-a-day extended-release form, which makes it easier for patients to stick to its regimen. The drug has no effect on dopamine and affects reuptake inhibition of serotonin and norepinephrine steadily at any dose (3).
Uses
- Major Depressive Disorder
Mechanisms of Actions
Levomilnacipran enhances the amount of serotonin (5-HT) and norepinephrine (NE) activity in the central nervous system by inhibition of reuptake at 5-HT and NE transporters (9). Levomilnacipran has shown to be a more potent inhibitor of NE versus 5-HT transporter (9).
Pharmacokinetics
Absorption: Well-absorbed (92%) following oral administration.
Metabolism and Excretion: Primarily metabolized in the liver via the CYP3A4 isoenzyme. 58% eliminated unchanged in urine; 42% metabolized; metabolites are renally eliminated.
Half-life: 12 hr.
Time/Action Profile (Plasma Concentrations):
|
ROUTE |
ONSET |
PEAK |
DURATION |
|
PO |
unknown |
6-8 hr. |
unknown |
Contraindications/Precautions
- Hypersensitivity to Levomilnacipran or milnacipran.
- Uncontrolled narrow-angle glaucoma.
- Use Cautiously in:
- Hypertension, cardiovascular or cerebrovascular disease (blood pressure should be controlled prior to treatment).
- Bipolar disorder (may activate mania/hypomania).
- Renal impairment.
OB: Use only if potential maternal benefit justifies potential fetal risk.
Geriatric: Consider age-related decrease in renal function, chronic disease state and concurrent drug therapy in older adults; increased risk of hyponatremia.
Adverse Reactions/Side Effects
CV: Hypertension, hypotension, palpitations, tachycardia.
Dermatology: Hyperhidrosis (excessive sweating), rash.
EENT: Mydriasis.
Fluids and Electrolytes: Hyponatremia (in association with syndrome of inappropriate antidiuretic hormone [SIADH]).
GI: Nausea, constipation, decreased appetite, vomiting.
GU: Sexual dysfunction, testicular pain, urinary hesitation, and retention.
Drug-Drug Interactions
- Concurrent use with MAO inhibitors is contraindicated; wait at least 14 days following discontinuation of MAO inhibitor before initiation of levomilnacipran (9). Concurrent use with MAO-inhibitor like drugs, serotonergic neurotransmitter systems, including tricyclic antidepressants, SNRIs, fentanyl, buspirone, tramadol, amphetamines, and triptans may increase the risk of serotonin syndrome (9).
- Concurrent use of NSAIDs, aspirin, warfarin or other drugs that affect coagulation may increase the risk of bleeding.
- Concurrent use of other medications that may increase the risk of hypertension.
- Concurrent use with alcohol may cause a rapid release of drug and should be avoided.
- Monitor closely for any changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression.

Self Quiz
Ask yourself...
- What are the major mechanisms of action for Levomilnacipran?
- Does an increased dosage of Levomilnacipran result in different neurotransmitter activity?
Review of SNRI Pharmacokinetics
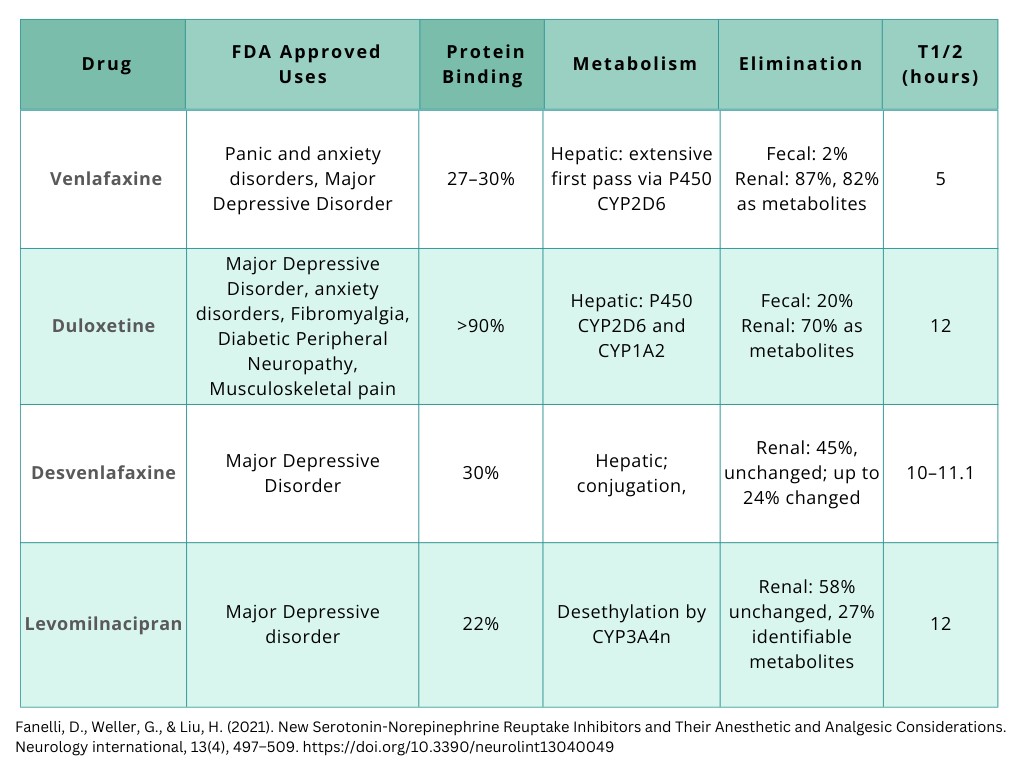
Figure 1. Pharmacokinetics of SNRIs [Created by course author].
Nursing Considerations
It is vital to recognize how each patient is unique and different in their journey of depression treatment. Nurses play a key role in assessing and providing advocacy for these patients.
The following are important nursing considerations:
- Thorough and focused mental status assessment
- Assess level of consciousness and orientation, appearance and general behavior, speech, motor activity, affect and mood, thought and perception, attitude and insight, and cognitive abilities.
- Assess status and mood changes.
- Assess suicidal tendencies, especially in early beginning of SNRI (9).
- Monitor BP before and periodically during therapy. Sustained hypertension may be dose-related; decrease dose or discontinue therapy if this occurs.
- Assess for serotonin syndrome.
- Provide patient education on medication use, dosage, and side effects. Provide patient education on support and resources available.
Nursing Diagnosis: Disturbed Thought Processes
Nursing Diagnosis: Ineffective Coping
Lab Assessments
Monitor CBC with differential and platelet count periodically during SNRI therapy. These medications can cause anemia, leukocytosis, leukopenia, thrombocytopenia, basophilia, and eosinophilia (9).
May cause an increase in serum alkaline phosphatase, bilirubin, AST, ALT, BUN, and creatinine, and serum cholesterol.
SNRIs can also cause electrolyte abnormalities (hyperglycemia or hypoglycemia, hyperkalemia or hypokalemia, hyperuricemia, hyperphosphatemia or hypophosphatemia, and hyponatremia).
Conclusion
SNRIs are an effective method of treatment for those suffering from depression. The effectiveness of SNRIs is dependent on a variety of factors like dosage and time spent in the body. Although the mechanisms of actions and makeup of SNRIs are consistent, each drug has differences on how much reuptake they prevent and the side effects of each type. Prescribers must be aware of these differences, the uses, mechanisms of action, side effects, and warnings.
Conclusion
SNRIs are an effective method of treatment for those suffering from depression. The effectiveness of SNRIs is dependent on a variety of factors like dosage and time spent in the body. Although the mechanisms of actions and makeup of SNRIs are consistent, each drug has differences on how much reuptake they prevent and the side effects of each type. Prescribers must be aware of these differences, the uses, mechanisms of action, side effects, and warnings.
References + Disclaimer
- Bamalan, O.A., Moore, M.J., Al Khalili Y. (2024). Physiology, serotonin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK545168/
- Dhaliwal JS, Spurling BC, Molla M. (2023). Duloxetine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Fanelli, D., Weller, G., & Liu, H. (2021). New Serotonin-Norepinephrine Reuptake Inhibitors and Their Anesthetic and Analgesic Considerations. Neurology international, 13(4), 497–509. https://doi.org/10.3390/neurolint13040049
- Hussain LS, Reddy V, Maani CV. (2023) Physiology, Noradrenergic Synapse. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK540977/
- Li, J., Lu, C., Gao, Z, Feng, Y., Luo, H., Lu, T., Sun, X., Hu, J., & Luo, Y. (2020). SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology, 177, 108237, ISSN 0028-3908. https://doi.org/10.1016/j.neuropharm.2020.108237.
- Naseeruddin, R., Rosani, A., & Marwaha, R. (2023). Desvenlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534829/
- National Institute of Mental Health (NIH). (2022). Depression. Retrieved from https://www.nimh.nih.gov/health/publications/depression.
- Singh, D., & Saadabadi, A. (2022). Venlafaxine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Vallerand, A. H., & Sanoski, C. A. (2023). Davis’s drug guide for nurses (Eighteenth edition.). Philadelphia, PA. F. A. Davis Company. ISBN-10: 1-7196-4640-6. eISBN-10: 1-7196-4811-5.
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Venlafaxine. Access Pharmacy. Retrieved from https://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=426975.
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate
