Course
Non-Opioid Chronic Pain Management
Course Highlights
- In this Non-Opioid Chronic Pain Management course, we will learn about FDA-approved and off-label indications for non-opioid medications and other various therapies for chronic pain management.
- You’ll also learn the pharmacokinetics of non-opioid medications, including antiepileptics, antidepressants, and topical medications.
- You’ll leave this course with a broader understanding of the pathophysiology of neural pathways in relation to chronic pain.
About
Contact Hours Awarded: 2
Course By:
Abbie Schmitt
RN, MSN-Ed
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Chronic pain is common and debilitating condition, affecting about one in five people globally (6). Musculoskeletal conditions such as back pain are typically the most common conditions leading to chronic pain, followed by headache, orofacial pain, and visceral pain (6). Fibromyalgia and neuropathic pain are also prevalent.
About one-third of people with chronic non-cancer-related pain are prescribed opioid analgesics; however, long-term research finds that the potential harms likely outweigh the benefits.
Chronic pain can be difficult to treat, and management is often suboptimal. The most common non-opioid drug treatment is paracetamol (acetaminophen) and non-steroidal anti-inflammatory drugs, but they need to be used with caution and for short periods because of the risk of serious adverse events with long-term use (6)
We will dive into the pharmacokinetics of various non-opioid pain management options and explore this option for optimal outcomes for patients with chronic pain.
Definition
Pain is a subjective term. The one experiencing the pain is responsible fordescribing and rating it. Pain can be associated with actual or potential tissue damage or abnormal functioning of nerves. It may be classified as acute, chronic, or cancer pain. Pain may also be categorized as adaptive or maladaptive.
- Adaptive (Physiologic) Pain
-
- Nociceptive Pain – Examples include touching something hot or sharp.
- Inflammatory pain – Examples include trauma or surgery.
- Maladaptive (Pathologic) Pain
-
- Pathophysiologic pain (e.g., postherpetic neuralgia, diabetic neuropathy, fibromyalgia, irritable bowel syndrome, chronic headaches) is often described as chronic pain.
- It results from damage or abnormal functioning of nerves in the central nervous system (CNS) or peripheral nervous system (PNS). Pain circuits sometimes rewire themselves anatomically and biochemically, resulting in chronic pain, hyperalgesia, or allodynia (condition in which the pain stimulus typically should not cause pain, like “touch” on sunburned skin).
The steps in processing pain are: (10)
- Transduction – Stimulation of nociceptors.
-
- Nociceptors, located in somatic and visceral structures, are activated by mechanical, thermal, and chemical stimuli.
- Noxious stimuli may cause release of cytokines and chemokines that sensitize and/or activate nociceptors.
- Conduction – Receptor activation leads to action potentials that continue along afferent fibers to the spinal cord.
-
- Stimulation of large-diameter, sparsely myelinated fibers stimulate sharp, wider spread pain.
- Stimulation of small-diameter, unmyelinated fibers produce aching, poorly localized pain.
- Transmission – Synapse occurs in the spinal cord’s dorsal horn, releasing excitatory neurotransmitters.
-
- The signal to the brain’s higher cortical structures.
- Perception – Experience of pain happens when signals reach higher cortical structures.
-
- Relaxation, meditation, and distraction can lessen pain.
- Anxiety and depression can exacerbate pain.
- Modulation – Attributing factors can possibly include glutamate, substance P, endogenous opioids, γ-aminobutyric acid (GABA), norepinephrine, and serotonin.
The margin between neurons and immune cells within the CNS may facilitate chronic pain (10).
Somatosensation encompasses sensations such as touch, pressure, temperature, itch, and pain. Somatosensory information is transmitted from primary afferent fibers in the periphery into the central nervous system via the dorsal horn of the spinal cord (9). There are many therapies that target the dorsal horn functions as an intermediary processing center for this information, comprising a complex network of excitatory and inhibitory interneurons.

Self Quiz
Ask yourself...
- How would you describe the factors associated with chronic pain?
- Do you have experience with patients that have various forms of chronic pain?
- Can you describe the involvement of neurotransmitters in the signaling of nerve impulses?
- How does conduction vary between sparsely myelinated and unmyelinated fibers within the nervous system?
Overview of Non-Opioid Therapies for Chronic Pain Management
|
Non-Opioid Therapies for Chronic Pain Management (** Pharmacokinetics discussed in this course) |
|
|
Non-Opioid Drug Class |
Description |
|
Acetaminophen |
|
|
Nonsteroidal Anti-Inflammatory Drugs |
|
|
Anticonvulsants** |
|
|
Antidepressants** |
|
|
Topicals: Medicated Creams, Foams, Gels, Lotions, Ointments, Patches |
|
|
Interventional Pain Management |
|
|
Chronic Pain Therapies |
Description |
|
Self-Care |
|
|
Complementary Therapies |
|
|
Rehabilitation Therapies |
|
|
Behavioral And Mental Health Therapies |
Psychiatrists, clinical social workers, and mental health counselors provide therapies that identify and treat mental disorders or substance abuse problems that may serve as barriers to pain management. |

Self Quiz
Ask yourself...
- Can you name the benefits of using non-opioid pain management therapies when compared with opioid medications?
- Are you familiar with complementary chronic pain therapy?
- What are some common roadblocks to therapeutic pain management for those with opioid addictions?
- Can you name other medical professions that can become involved in the care planning for patients experiencing chronic pain?
Pharmacokinetics of Anticonvulsants
Anticonvulsants
Gabapentin and pregabalin are the most common anticonvulsants, or anti-epileptics, used for the treatment of chronic pain. These medications inhibit the alpha-2-delta subunit of voltage-gated calcium channels, which are involved in releasing nociceptive neurotransmitters. A number of systematic reviews strongly recommended using gabapentin for neuropathic pain and was backed by high-quality evidence.
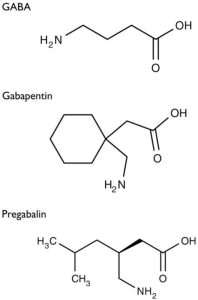
Figure 1. Structure of GABA, Gabapentin, and Pregabalin (1)
Gabapentin
Gabapentin [1-(aminomethyl)cyclohexane acetic acid] is an anti-epileptic agent and was originally developed as a gamma-aminobutyric acid (GABA)-mimetic compound to treat spasticity (1). Research found that it also has potent anticonvulsive effects. Initially approved only for use in partial seizures, it has evolved into an effective treatment agent for chronic pain syndromes, especially neuropathic pain.
Gamma-aminobutyric acid (GABA) was known to be a key inhibitory neurotransmitter, whose inhibition could cause seizures. Lipophilic groups were added to the carbon backbone to increase the bioavailability of GABA, as it does not penetrate the blood–brain barrier.
Gabapentin, available only as oral preparations, is absorbed in the small intestine by a combination of diffusion and facilitated transport. Its transport from the gut, following oral administration, is facilitated by its binding to a receptor (not yet identified) linked to a saturable l-amino acid transport mechanism (1).

Self Quiz
Ask yourself...
- Can you explain what Gabapentin was originally developed as?
- What is the cellular makeup of a GABA compound?
Gabapentin Drug Class
Gabapentin drug classes include: (16)
- Anticonvulsant, Miscellaneous
- GABA Analog
Gabapentin Uses
Gabapentin can be used in the management of postherpetic neuralgia (PHN) in adults, fibromyalgia, and various neuropathic pain (16).
Gabapentin Mechanism of Action
Gabapentinoids inhibit calcium-mediated neurotransmitter release through effects on α2δ-1 subunits, which inhibit forward movement of α2δ-1 that originates in the dorsal root ganglion within their endosomal compartments, causing processes that stimulate glutamate uptake by excitatory amino acid transporters (EAATs) (1). Na+-dependent excitatory amino acid transporters (EAATs) are the major transport mechanisms for extracellular glutamate removal in the CNS. EAATs-mediated clearance of amino acid glutamate released by neurons is vital to signaling and to prevent toxic accumulation of this amino acid in the extracellular space (11).
Glutamate is an amino acid and excitatory neurotransmitter that can stimulate all the CNS neurons— a capability that is unique to glutamate and explains why it is commonly known as the “master switch” (3). During this reuptake process, the cells can either reuse the glutamate or synthesize it back to glutamine, which is the form for storing for future use. Many different types of glutamate receptors exist, though they are classified into just two main categories: metabotropic and ionotropic. Ionotropic receptors are further divided into three main types of receptors: AMPA, harmac, and NMDA (3). Drugs can target these receptors to reduce glutamate release. Among the types of drugs that do so are anticonvulsants, mood stabilizers, and N -methylD-aspartate (NMDA) receptor antagonists (3).
Gamma-aminobutyric acid (GABA) is a powerful inhibitory neurotransmitter in the CNS. Glutamate and GABA work together to aid in the regulation of neurotransmitters and coordination with voltage-gated ion channels and G protein– coupled receptors (GPCRs) in the CNS (3).
GABA is synthesized from glutamate-by-glutamate decarboxylase (GAD) and is stored in synaptic vesicles. GABA-A receptors are the primary effector of the GABA-mediated inhibitory postsynaptic potential (IPSP). GABA-B receptors are responsible for the metabotropic effects of GABA and for the inhibition of voltage-gated calcium channels, the opening of potassium channels, and the release of glutamate and monoamines (3). Two main neurotransmitters regulate the sleep and wake function switch: histamine and GABA. The sleep encourager releases GABA, while the wake promoter, located within the tuberomammillary nucleus (TMN) of the hypothalamus, releases histamine (3). This is essentially why antihistamines can result in drowsiness.
Mechanisms not directly related to neurotransmitter release at dorsal horn include inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory actions, and influence on the affective component of pain.
Gabapentinoids do not bind to plasma proteins, and they are actively transported across the blood–brain barrier by LAT-1. The peak level in cerebrospinal fluid levels take significantly longer than the peak plasma levels, with a median time of 8 hours (1). Both are highly water-soluble and the volume of distribution of each is 0.8 and 0.5 L/kg for gabapentin and pregabalin, respectively.
Essential mechanisms of actions:
- α2δ-1 subunits are transported to the dorsal horn from their site of production in DRG (dorsal root ganglion) cell bodies. Elevated levels in the dorsal horn are associated with the development of neuropathic pain (1).
- Gabapentinoids inhibit the accumulation of α2δ-1 in the pre-synaptic terminals in the dorsal horn and reduce response to painful stimuli.
- α2δ-1 allows enhanced neurotransmitter release at decreased calcium influx.
- Analgesic effects are mediated by the facilitation of descending noradrenergic inhibition, decrease of descending serotonergic facilitation, and by cortical mechanisms affecting the limbic system (1).
- Stimulation of the uptake of glutamate by the excitatory amino acid transporters (EAAT).
- Suppression of the inflammatory response to injury.
- Modulation of the affective component of pain.

Self Quiz
Ask yourself...
- How would you describe the role of Na+-dependent excitatory amino acid transporters (EAATs) within the CNS?
- How do the gabapentinoids travel across the blood-brain barrier?
- Can you describe how analgesic effects can impact the limbic system?
- Can you name certain chronic conditions that may be associated with chronic inflammation?
Gabapentin Pharmacodynamics/Kinetics
The pharmacodynamics/kinetics of gabapentin is as follows: (17)
- Absorption: Variable
-
- Proximal small bowel by L-amino transport system
- Saturable process
- Dose-dependent
- Protein binding: <3%
- Metabolism: Not metabolized
- Immediate release:
-
- 900 mg/day: 60%
- 1,200 mg/day: 47%
- 2,400 mg/day: 34%
- 3,600 mg/day: 33%
- 4,800 mg/day: 27%
- Extended release: Variable; increased with higher fat content meal
- Half-life elimination:
-
- Adults, normal: 5 to 7 hours
- Increased half-life with decreased renal function
- Anuric adult patients: 132 hours
- Adults during hemodialysis: 3.8 hours
- Time to peak
-
- Adults: 2 to 4 hours
- Extended release: 8 hours
- Excretion: Proportional to renal function; urine (as unchanged drug)
- Clearance: Apparent oral clearance is directly proportional to CrCl
Gabapentin Adverse Effects
Adverse effects are common with gabapentinoids resulting in a discontinuation rate of at least 11%, but serious adverse events are uncommon. The substitution of gabapentin with pregabalin in gabapentin responders resulted in improved pain relief and fewer adverse events.
Central nervous system effects
Dizziness, somnolescence, and gait disturbances are the most common adverse effects. The effects often occur during the initiation of treatment and can diminish after several weeks of treatment. Visual blurring can also occur (1).
Other common side effects affecting the central nervous system (CNS) include impaired concentration, confusion, memory loss, altered mood, movement disorders, sleep disorder, speech impairment, and vertigo (1).
Most adverse reactions have a clear dose–response relationship with increased risk of complications with higher doses. However, ocular adverse effects such as amblyopia and blurred vision appeared at lower doses of pregabalin (1)
Respiratory depression
Respiratory depression has been reported when used in combination with opioids, resulting in an increased risk of accidental opioid-related mortality (1). Clinicians should be aware of this prior to prescribing multiple medications. A large primary care database review showed that 21.8% of patients with a new prescription for gabapentin and 24.1% of patients with a new prescription for pregabalin received additional prescriptions, primarily for opioids (1). Dose adjustments are recommended for patients with compromised respiratory function, respiratory or neurological diseases, renal impairment, and elderly people due to the higher risk of experiencing severe respiratory depression.
Weight gain
Weight gain is common with gabapentinoids and can affect up to one-fourth of all patients treated (This is a common reason for patients to stop taking it). However, the extent of weight gain appears to be moderate. The majority of patients treated with pregabalin for one year maintain weight within ±7% of their baseline or initial weight (1). Weight gain is related to dose and duration of use but not to body mass index, gender, age, and development of edema (1).
Gastrointestinal effects
Gastrointestinal adverse effects such as abdominal distension, abnormal appetite, constipation, dry mouth, and nausea are common side effects and are dose-related, all except constipation (1).
Misuse
There is potential for abuse of gabapentinoids, particularly in individuals with a history of opioid abuse (1). Both gabapentinoids have been reported to stimulate feelings of sociability, euphoria, calm and relaxation, which can lead to misuse. Abuse potential of pregabalin is higher as compared to gabapentin due to its pharmacokinetic properties (1). All anticonvulsants are associated with increased risk for suicidal thoughts and behavior (12).
Withdrawal
Withdrawal symptoms are common and appear between 12 hours and 7 days after cessation of use, with most cases occurring between 24 and 48 hours (1). Other symptoms include tachycardia, palpitations, anxiety, sweating, restlessness, hypertension, tremor, gastrointestinal symptoms, paranoia, auditory hallucinations, and suicidal ideation – all similar to the withdrawal effects of benzodiazepines and alcohol (1). Patients with psychiatric comorbidities and the elderly may be at an increased risk of withdrawal. Clinicians should do a slow tapering schedule for patients at higher risk for withdrawal. A slower tapering schedule such as a twice-weekly reduction of 10–25% of the dose has been suggested to minimize the risk of withdrawal effects (1).
Toxicity
The risk for toxicity is higher in patients with chronic kidney disease and those on dialysis (1). Toxicity may present with increased sedation, confusion, unsteady gait, myoclonus, ataxia, episodic leg spasm, asterixis and tremulousness (1).
Guidelines on recommendations for dose reductions based on creatinine clearance are available for prescribers. Patients on hemodialysis might require supplemental doses following dialysis due to it removing approximately 35% of gabapentin and 50–60% of pregabalin (1).
Gabapentin Dosing
Fibromyalgia
Initial: Oral: 100 to 300 mg once daily at bedtime; increase dose gradually based on response and tolerability every 1 to 2 weeks to a target dose of 1.2 to 2.4 g/day in divided doses (17)
Neuropathic pain
Note: For chronic use, an adequate trial with gabapentin may require two months or more. For critically ill patients with neuropathic pain, gabapentin may be a useful component of multimodal pain control (17).
Immediate release: Oral: Initial: 100 to 300 mg 1 to 3 times daily; increase dose based on response and tolerability to a target dose range of 300 mg to 1.2 g 3 times daily (17).
Extended release: Oral: Initial: 300 mg at bedtime; increase dose based on response and tolerability to a target dose of 900 mg to 3.6 g once daily (17).
Postherpetic neuralgia
Immediate release: Oral: 300 mg once on day one, 300 mg twice daily on day two, and 300 mg 3 times daily on day two, then increase as needed up to 1.8 to 3.6 g/day in divided doses (17).
Extended release: Oral: Initial: 300 mg once daily; increase by 300 mg each day up to 900 mg once daily. Further increase as needed up to 1.8 g once daily (17).


Self Quiz
Ask yourself...
- Can you discuss the side effects of gabapentin?
- How is gabapentin excreted?
- Can you name the contraindications for taking this medication, specifically associated with respiratory distress?
- Do you have experience with educating patients on a tapered dosing schedule?
Pregabalin
Pregabalin was originally approved by the U.S. Food and Drug Administration (FDA) as an anti-epileptic drug, also called an anticonvulsant. It works by reducing the conduction of impulses in the brain that cause seizures. Pregabalin also affects chemicals in the brain that send pain signals across the nervous system.
Pregabalin Drug Class
Pregabalin is classified as a Schedule V prescription drug (16). Schedule V drugs are defined as drugs with lower potential for abuse than Schedule IV by the U.S. Drug Enforcement Administration (DEA).
Pregabalin Uses
Pregabalin is used to treat pain caused by neuropathic pain associated with spinal cord injury, fibromyalgia, diabetic neuropathy, post-herpetic neuralgia, and management of postherpetic neuralgia (1, 12).
Pregabalin Mechanism of Action
Pregabalin binds to alpha-2-delta subunit of calcium channels within the CNS and lowers calcium influx at the nerve terminals, which inhibits excitatory neurotransmitter release (16). These excitatory neurotransmitters including glutamate, norepinephrine (noradrenaline), serotonin, dopamine, substance P, and calcitonin gene-related peptide (16).
Glutamate was discussed earlier in the course, but it is important to closely examine norepinephrine, as it relates to pain. Norepinephrine is another monoamine neurotransmitter in the catecholamine family, and it serves as both a neurotransmitter and a hormone (3). The sympathetic nervous system, which becomes activated during stressful or painful events, activates norepinephrine as the neurotransmitter primarily responsible for the “fight or flight” response (3). The sympathetic system is highly influenced by changes in the serum norepinephrine concentration and is associated with the regulation of heart rate and blood pressure.
By binding presynaptically to the alpha2-delta subunit of voltage-gated calcium channels in the CNS, it is essentially thought to calm the conduction of pain impulse. In addition, pregabalin prevents the alpha2-delta subunit from being transported from the dorsal root ganglia to the spinal dorsal horn, which may also contribute to the mechanism of action and use in chronic pain in those with spinal cord injuries (1).
Although structurally related to GABA, it does not bind to GABA or benzodiazepine receptors. Pregabalin may also affect descending noradrenergic and serotonergic pain transmission pathways from the brainstem to the spinal cord.
Alpha (α 2δ )-ligands modulate neurotransmitter release and increase membrane hyperpolarization and the seizure threshold.
Pregabalin Pharmacodynamics/Kinetics
The pharmacodynamics/kinetics of gabapentin is as follows: (16)
- Onset of action: Effects may be noted as early as the first week of therapy.
- Absorption
-
- Extended release: Approximately 30% lower when administered while fasting.
- Distribution: Vd: 0.5 L/kg
- Protein binding: 0%
- Bioavailability: ≥90%
- Half-life elimination: Adult: 6.3 hours
- Time to peak, plasma:
-
- Extended release: Median is 8 hours with food (Average 5 to 12 hours)
- Immediate release, Adults: Within 1.5 hours fasting; 3 hours with food
- Excretion: Urine (90% as unchanged drug; minor metabolites)
Pregabalin Adverse Effects
Central nervous system effects
Dizziness (3% to 45%), drowsiness (≤36), fatigue (4% to 11%) is included among adverse effects (16).
Ataxia, balance impairment, abnormal gait, euphoria, confusion, disturbance in attention abnormal thinking, neuropathy, myasthenia, insomnia, memory impairment, vertigo, speech disturbance, anxiety, paresthesia, intoxicated feeling, lethargy, and nervousness have also been reported (16).
Cardiovascular
Peripheral edema (4% to 16%), facial edema (1% to 3%), chest pain (2%), hypertension (2%), hypotension (2%) has been reported.
Endocrine & metabolic
Weight gain (2% to 14%) is noted.
Ophthalmic
Visual field loss (13%) and blurred vision (≤12%) have been noted.
Gastrointestinal
Constipation, increased appetite, nausea, flatulence, vomiting, abdominal distension, and abdominal pain are among the reported adverse effects.
Endocrine & metabolic: Fluid retention (2% to 3%), hypoglycemia (2% to 3%), decreased libido (≥1%)
Hematologic & oncologic
Thrombocytopenia (3%) has occurred.
Pregabalin Dosing
Fibromyalgia
Immediate release: Oral: Initial: 75 mg twice daily; may increase to 150 mg twice daily within one week based on response and tolerability; maximum dose: 450 mg/day (manufacturer’s labeling). Note: A lower initial doses of 25 to 50 mg at bedtime is suggested by many experts (8).
Neuropathic pain
Immediate release: Oral: Initial: 25 to 150 mg/day once daily or in two divided doses; may increase in increments of 25 to 150 mg/day at intervals ≥1 week based on response and tolerability up to a usual dose of 300 to 600 mg/day in two divided doses (16).
Postherpetic neuralgia
Immediate release: Oral: Initial: 150 mg/day in divided doses (75 mg twice daily or 50 mg three times daily); may increase to 300 mg/day within one week based on response and tolerability; after 2 to 4 weeks, may further increase up to the maximum dose of 600 mg/day (16).
Extended release: Oral: Initial: 165 mg once daily; may increase to 330 mg once daily within one week based on tolerability; after 2 to 4 weeks, may further increase up to the maximum dose of 660 mg/day (16).

Self Quiz
Ask yourself...
- Can you discuss the side effects of pregabalin?
- What is the half-life for this medication?
- Can you describe the mechanism of action of this medication?
- How can the classification or schedule of a drug impact prescribing?
Warnings for Gabapentin and Pregabalin
Warnings for gabapentin and pregabalin include the following risk factors, dosing and monitoring considerations, and patient education needs (1).
The FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Risk factors include the use of opioid pain medicines, and conditions that reduce lung function, such as chronic obstructive pulmonary disease (COPD) (14). The elderly are also at higher risk.
Health care providers should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation. Patients with underlying respiratory disease and elderly patients are also at increased risk and should be managed similarly.
Clinicians should assess the risk of misuse, dependence, and diversion.
Patients should be made aware of the importance of dosage titration, the titration process, and the requirement to take a stable regime for a few weeks before assessing for improvement in pain. Patients often misunderstand that gabapentinoids cannot be taken as needed and that taking an additional dose does not result in improved pain (1)
Patients must be warned about the potential alteration in concentration for certain tasks, such as using heavy equipment or driving.
Pharmacokinetics of Antidepressants
Antidepressants
Antidepressants, including tricyclic antidepressants (TCAs) and selective norepinephrine reuptake inhibitors (SNRIs), have shown to have analgesic effects by primarily blocking the reuptake of norepinephrine, thereby enhancing the pain-modulating pathway activity. TCAs also block peripheral sodium channels, which can also help reduce pain.
Serotonin and norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) are the most common classes of antidepressants used to manage chronic neuropathic pain.
All currently available antidepressants enhance monoamine neurotransmission by one of several mechanisms; the most common mechanism is inhibition of the activity of SERT, NET, or both. The serotonin transporter (SERT) is a glycoprotein with 12 transmembrane regions embedded within the axon terminal and cell body membranes of serotonergic neurons (2). As serotonin outside of the cells bind to receptors on the transporter, changes occur in the transporter and serotonin (2). Na+, and Cl− then are transported into the cell. Binding of intracellular K+ results in the release of serotonin inside the cell. The transporter is released into its original state.
At therapeutic doses, about 80% of the activity of the transporter is inhibited (2).
Ultimately, the increased availability of monoamines for binding in the synaptic cleft results in a cascade of events that enhance the transcription of some proteins and the inhibition of others (2). The inhibiting effects are most desirable for reduction of chronic pain.
We will use the pharmacokinetics of the tricyclic antidepressant (TCA), Amitriptyline, and selective norepinephrine reuptake inhibitor (SNRI), Duloxetine, to explore the research on use.
The SNRIs differ from the TCAs in that they lack the potent antihistamine, α-adrenergic blocking, and anticholinergic effects of the TCAs. As a result, the SNRIs tend to be favored over the TCAs in the treatment of pain syndromes because they are often tolerated better (2).
- Tricyclic antidepressant (TCA): Pharmacokinetics of Amitriptyline
- Serotonin norepinephrine reuptake inhibitors (SNRIs): Pharmacokinetics of Duloxetine
Tricyclic Antidepressants (TCA)
The first indication that tricyclic antidepressants (TCAs) may help with neuropathic pain came from a 1960 study that found that patients treated with imipramine experienced chronic pain relief. There have been many studies since that support the effectiveness of TCAs on chronic pain.
Amitriptyline
Example of tricyclic antidepressant (TCA) used in chronic pain management.
Amitriptyline Drug Class
Antidepressant, Tricyclic (Tertiary Amine)
Amitriptyline Uses
Used to treat major depressive disorder (unipolar), unipolar major depressive disorder, and management of chronic neuropathic pain.
Amitriptyline Mechanism of Action
The N-type calcium ion channel is an established target for the treatment of neuropathic pain. The channel consists of a unique α1 pore-forming subunit and auxiliary α2-δ and β subunits (6). The general structure of the α1 subunit is similar to that of other voltage-gated ion channels.
The mechanism of action involves increasing the synaptic concentration of serotonin and/or norepinephrine in the central nervous system by stopping their reuptake by the presynaptic neuronal membrane pump (Wil-A).
Amitriptyline is a tertiary amine with strong binding affinities for alpha-adrenergic, histamine (H1), and muscarinic (M1) receptors (13). Chronic treatment with amitriptyline desensitizes presynaptic receptors, producing long-lasting changes in monoaminergic neurotransmission (13).
Amitriptyline Pharmacodynamics/Kinetics
- Onset of action: Responses may vary per individual; however, 4 to 8 weeks of treatment are needed before determining if a patient is partially or non-responsive; desired therapeutic effect for pain reduction may take as long as 1 to 3 weeks.
- Absorption: Rapid, well absorbed.
- Distribution: Vd: 18 to 22 L/kg
- Protein binding: >90%
- Metabolism: Rapid; hepatic N to demethylation to nortriptyline (active), hydroxy derivatives and conjugated derivatives
- Bioavailability: 43% to 46%
- Time to peak, serum: 2 to 5 hours
(Wil-A)
- Amitriptyline has a half-life of 10 to 28 hours (13)
- Excretion: Amitriptyline and its metabolites are primarily excreted by the kidney (13)
- Special Populations: Elderly: May have increased plasma levels
- Amitriptyline can cross the placental barrier (13)
Amitriptyline Dosage Formulations
Amitriptyline dosage formulations come in oral tablets of 10 mg, 25 mg, 50 mg, 75 mg, 100 mg, and 150 mg.
Adult Dosing: For chronic pain, therapy can initiate a dose of 10 to 20 mg daily. The dose can be increased by 25 mg every 3 to 7 days, with a maximum of 150 to 300 mg/day. If the dose requires adjustment, it is preferable to change the bedtime dose. In cases of therapy cessation, the clinician should gradually taper to avoid withdrawal (13).
Plasma Levels: It is difficult to directly correlate plasma levels with desired, therapeutic effects. However, determining plasma levels might be useful in identifying toxicity with excessively high levels or in whom noncompliance is suspected.
Older adult patients have decreased hepatic metabolism and increased intestinal transport time, so plasma levels are usually higher for any given oral dose of amitriptyline for this population.
Amitriptyline Side Effects
Amitriptyline, due to its alpha-adrenergic receptor blockade, can cause orthostatic hypotension, dizziness, and sedation. Anticholinergic side effects include blurred vision, dry mouth, urinary retention, tachycardia, acute angle glaucoma, constipation, and confusion (13). Antihistamine side effects secondary to its histamine (H1) receptor binding property include sedation, increased appetite, weight gain, confusion, and delirium (13).
It can increase the risk of bone fracture and bone marrow suppression.
Amitriptyline Warnings
Anticholinergic Effects
May cause anticholinergic effects (constipation, xerostomia, blurred vision, urinary retention); use with caution in patients with decreased gastrointestinal motility, increased intraocular pressure (IOP), narrow-angle glaucoma, paralytic ileus, urinary retention, BPH, xerostomia, or visual problems (Wil-A).
CNS Depression
May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks that require mental alertness (e.g., operating machinery or driving).
Fractures
Bone fractures have been associated with antidepressant treatment (Wil-A)
Hematologic Effects
TCAs may rarely cause bone marrow suppression; monitor for any signs of infection and obtain CBC if symptoms are evident (e.g., fever, sore throat).
Orthostatic Hypotension
May cause orthostatic hypotension; use with caution in patients at risk of this (cerebrovascular disease, cardiovascular disease, hypovolemia, or concurrent medication use which may predispose to hypotension/bradycardia).
Therapy is relatively contraindicated in patients with symptomatic hypotension.
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and Hyponatremia
Associated with the development of syndrome of inappropriate antidiuretic hormone secretion (SIADH) and hyponatremia, predominately in the elderly (Wil-A).
Cardiovascular Risk
In a scientific statement from the American Heart Association, amitriptyline may exacerbate underlying myocardial dysfunction (WIL-A). Amitriptyline can also cause heart rate variability, slow intracardiac conduction, induce various arrhythmias, and cause QTc (corrected QT) prolongation (13).
Use with caution in patients with diabetes mellitus, hepatic impairment, or renal impairment (Wil-A).
A major contraindication is the coadministration with or within 14 days of Monoamine oxidase inhibitors (MAOIs); avoid coadministration with cisapride and avoid during the acute recovery phase following myocardial infarction (Wil-A).
Clinicians must monitor elderly patients carefully and obtain amitriptyline serum levels based on their clinical assessment. Clinicians should adjust amitriptyline dose according to the patient’s clinical response and not based on plasma levels (13).
Alternatives to Amitriptyline
Other TCAs that may be helpful in chronic pain management is Desipramine, Imipramine, and Nortriptyline.
Amitriptyline is more sedating and has increased anticholinergic properties than other TCAs (13).


Self Quiz
Ask yourself...
- Can you discuss the mechanism of action for Amitriptyline?
- What would you consider the most worrisome adverse effects?
- What are some common anticholinergic properties and effects?
- Why is the half-life and peak time important in the administration and prescribing of a medication?
Selective Norepinephrine Reuptake Inhibitors (SNRIs)
As mentioned earlier, serotonin and norepinephrine reuptake inhibitors (SNRIs) are one of the most common classes of antidepressants used to manage chronic neuropathic pain.
Duloxetine
Example of selective norepinephrine reuptake inhibitor (SNRI) used in chronic pain management.
Duloxetine Drug Class
Antidepressant, Serotonin/Norepinephrine Reuptake Inhibitor.
Duloxetine Uses
Duloxetine can be used to manage major depressive disorder (MDD), generalized anxiety disorder (GAD), fibromyalgia, diabetic peripheral neuropathy, and chronic musculoskeletal pain.
Duloxetine Mechanism of Action
Duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a weak inhibitor of dopamine reuptake.
Serotonin is another monoamine neurotransmitter derived from tryptophan; the majority of the serotonin found in the body is located in the enterochromaffin cells of the gastrointestinal tract, with the rest within in the CNS, where it regulates mood, sleep, and appetite (3).
Duloxetine inhibits serotonin and norepinephrine reuptake and enhances dopamine levels within the prefrontal cortex (5). The mechanism of action behind the increase in dopamine levels involves the inhibition of norepinephrine transporters; the transporters have an attraction for dopamine. Therefore, inhibition of norepinephrine transporters can lead to an increase in dopamine. This increase in dopamine specifically occurs in the prefrontal cortex, where dopamine transporters are scarce, and reuptake relies more heavily on norepinephrine transporters (5).
Duloxetine works to reduce neuropathic and chronic pain states by increasing the activity of noradrenergic and serotonergic neurons in the descending spinal pathway of the dorsal horn (5). These descending neurons decrease the activity of dorsal horn neurons, which essentially suppresses excessive input from reaching the brain. The hypothesis is that a deficiency in these inhibitory signals resulting in less signals perceived as pain being delivered.
Duloxetine has no significant activity for muscarinic cholinergic, H1-histaminergic, or alpha2-adrenergic receptors. Duloxetine does not possess MAO-inhibitory activity.
Duloxetine Pharmacodynamics/Kinetics
- Absorption: Well-absorbed.
- Protein binding: >90%; primarily to albumin and alpha1-acid glycoprotein.
- Metabolism: Hepatic, via CYP1A2 and CYP2D6; forms multiple metabolites (inactive).
- Half-life elimination (adults): ~12 hours (range: 8 to 22 hours); ~4 hours longer in elderly women.
- Time to peak: 5 to 6 hours; food delays by 1.7 to 4 hours (15).
Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are reliant on the dose (18). Plasma concentrations are typically stable after three days of dosing.
Elimination of duloxetine is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP1A2 (18). Orally administered duloxetine hydrochloride is well absorbed. There is a median two-hour lag until absorption begins with maximal plasma concentrations occurring six hours post dose (18).
Food does not affect the maximal concentration of duloxetine, but it delays the time to reach peak concentration from 6 to 10 hours and it marginally decreases the extent of absorption (AUC) by about 10% (18). There is a three-hour delay in absorption and a one-third increase in apparent clearance of duloxetine after an evening dose as compared to a morning dose (18).
Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and α1-acid glycoprotein (18). The plasma protein binding of duloxetine is not changed by renal or hepatic impairment. About 70% is excreted and found in the urine as metabolites of duloxetine; about 20% is excreted in the feces (18).
Duloxetine Dosing for Management of Chronic Pain
Low back pain: 30 mg can be given once daily for one week and increased up to 60 mg once daily as tolerated as an adjunct therapy. Maximum dose: 60 mg/day.
Neuropathy: 60 mg can be administered initially; however, lower starting doses may be appropriate depending on patient tolerance. Maximum dose: 60 mg/day.
Duloxetine Side Effects
Side and adverse effects of the cardiovascular, gastrointestinal, central nervous system, such as headaches and drowsiness, and fatigue, are common (5). Duloxetine has a very low anticholinergic impact.
Common adverse effects of duloxetine include: (5)
- Headache
- Drowsiness
- Fatigue
- Nausea
- Xerostomia
- Abdominal pain
- Weight loss
- Weakness
- Insomnia
- Dizziness
- Change in libido
- Diaphoresis
- Tremor
Serious adverse effects of duloxetine include:
- Suicidality
- Serotonin syndrome
- Hepatoxicity
- Mania
- Syncope
- Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
- Hyponatremia
Duloxetine Warnings
Warnings for duloxetine include the following information (5).
Duloxetine is contraindicated in patients with concurrent or recent (within 14 days) use of monoamine oxidase (MAO) inhibitors, uncontrolled angle-closure glaucoma, or hypersensitivity to duloxetine.
Duloxetine should also be avoided in patients with liver failure or severe renal dysfunction (5). Also avoid using duloxetine in patients receiving treatment with linezolid or intravenous methylene blue due to an increased risk of serotonin syndrome (5)
Precautions and Warning in Special Population:
- Duloxetine is FDA pregnancy category C, which means risk to fetal development cannot be ruled out.
- Caution is necessary when prescribing duloxetine in the geriatric population.
Monitor for suicidal ideation, especially when starting treatment, altering the dose, and after discontinuation of therapy.
Caution should be exercised when using anticoagulants or antiplatelet medications along with duloxetine therapy.
Laboratory workup should include monitoring serum creatinine, blood urea nitrogen (BUN), transaminase levels, blood glucose levels, and HgbA1c in diabetic patients.
Sodium levels require follow-up when prescribing duloxetine in the geriatric population.
Duloxetine Toxicity
Signs and symptoms of toxicity include serotonin syndrome, seizures, somnolence, syncope, tachycardia, diarrhea, and vomiting (5).
Signs of serotonin syndrome include agitation, disorientation, diaphoresis, hyperthermia, tachycardia, nausea, vomiting, myoclonus, dilated pupils, dry mucous membranes, and increased bowel sounds; clonus and hyperreflexia are particularly common in serotonin syndrome (5).
There is no antidote to duloxetine overdose; cyproheptadine and cooling measures may be considered for toxicity or overdose (5).

Self Quiz
Ask yourself...
- Can you discuss the mechanism of action of duloxetine?
- Can you name the signs of serotonin syndrome?
- How can inhibiting neuronal serotonin and norepinephrine reuptake improve symptoms of pain or depression?
- Is there an antidote to duloxetine overdose?
Nursing Considerations
It is meaningful to remember that pain is subjective and should be determined by each patient. Those with chronic pain spend a significant amount of time in out-of-hospital environments, in their homes and in their community settings.
There is no “one size fits all” care plan for those with chronic pain. Personalized care is a collaborative process that should be used in chronic condition management in which patients, caregivers, and healthcare providers can identify and discuss problems and develop plans and goals to empower them in their own care. Individualized care can improve aspects of physical health, mental health, and the ability to self-manage conditions.
Nursing should utilize appropriate pain assessment tools and identify changes in levels of pain. Pain intensity, pain relief, and medication side effects must be assessed regularly. The frequency of assessment depends on the type of pain, analgesic used, route of administration, and concomitant medications.
Current Research and Opportunities
Chronic pain affects more than 100 million people in the United States (4). The U.S. is currently in the midst of an opioid epidemic with increased opioid use over the last two decades. Research is extensively focused on treatment alternatives, including medications, physical therapy, exercise, injections, and neuromodulation.
Conclusion
Clinicians face difficult decisions when managing chronic pain in their patients. There is an increasing need for meaningful use of non-opioid options. Research supports the positive outcomes of therapies such as anticonvulsants, antidepressants, and various other alternatives. Knowledge of the unique pharmacokinetics of each therapy is essential for clinicians.
References + Disclaimer
- Chincholkar M. (2020). Gabapentinoids: pharmacokinetics, pharmacodynamics, and considerations for clinical practice. British journal of pain, 14(2), 104–114. https://doi.org/10.1177/2049463720912496
- DeBattista C (2024). Antidepressant agents. Vanderah T.W.(Ed.), Katzung’s Basic & Clinical Pharmacology, 16th Edition. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3382§ionid=281751777
- Demler, T. L., & Rhoads, J. (Eds.). (2018). Pharmacotherapeutics for advanced nursing practice. Jones & Bartlett Learning.
- Dey S, Vrooman BM. Alternatives to Opioids for Managing Pain. [Updated 2023 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574543/
- Dhaliwal JS, Spurling BC, Molla M. (2023). Duloxetine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Ferreira G E, Abdel-Shaheed C, Underwood M, Finnerup N B, Day R O, McLachlan A et al. Efficacy, safety, and tolerability of antidepressants for pain in adults: overview of systematic reviews BMJ 2023; 380 :e072415 doi:10.1136/bmj-2022-072415
- Fraser G.L., & Riker R.R. (2020). Critical care: pain, agitation, and delirium. DiPiro J.T., & Yee G.C., & Posey L, & Haines S.T., & Nolin T.D., & Ellingrod V(Eds.), Pharmacotherapy: A Pathophysiologic Approach, 11e. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2577§ionid=234136370
- Goldenberg, D.L. (2019). Treatment of fibromyalgia in adults not responsive to initial therapies. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com.
- Harding, E. K., Fung, S. W., & Bonin, R. P. (2020). Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches. Frontiers in neural circuits, 14, 31. https://doi.org/10.3389/fncir.2020.00031
- Macintyre, P. E., & Schug, S. A. (2021). Acute pain management : a practical guide (Fifth edition.). CRC Press.
- Malik, A. R., & Willnow, T. E. (2019). Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System. International journal of molecular sciences, 20(22), 5671. https://doi.org/10.3390/ijms20225671
- Schwinghammer T.L., & DiPiro J.T., & Ellingrod V.L., & DiPiro C.V.(Eds.), (2021). Pharmacotherapy Handbook, 11e. McGraw Hill. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3012§ionid=253436360
- Thour A, Marwaha R. Amitriptyline. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537225/
- U.S. Food and Drug Administration (FDA). (2020). FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). Retrieved from https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Amitriptyline. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426455#monoNumber=426455§ionID=243191387&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Pregabalin. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426994#monoNumber=426994§ionID=243281218&tab=tab0
- Wolters Kluwer Clinical Drug Information, Inc. (2024). Gabapentin. Retrieved from Access Pharmacy. https://accesspharmacy.mhmedical.com/drugs.aspx?GbosID=426662#monoNumber=426662§ionID=243246336&tab=tab0
- Accessdata.fda.gov (FDA). (n.d.). Cymbalta. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021427s009s011s013lbl.pdf
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click Complete
To receive your certificate

